ABSTACT
Wnt/β-catenin signaling pathway through Frizzled receptors has been shown to play a key role in both normal development and tumorigenesis. Overexpression of Wnt pathway genes, such as Fzd7 in several malignancies is well-documented. Therefore, targeting of Fzd7 and its ligand inhibits cancer cells proliferation metastasis. In the present study we isolated single chain variable fragments (scFvs) against Fzd7 receptor using phage display method. Semi-synthetic human naive antibody libraries (Tomlinson I + J) was employed in panning procedure to isolate specific scFv against specific peptide from extracellular domain of Fzd7 receptor. The reactivity and growth inhibition effects of the selected antibodies was evaluated using enzyme-linked immunosorbent assay (ELISA), MTT and annexin V assays, respectively. Seven scFvs reactive to Fzd7 were selected following 4 rounds of panning. The results showed that the selected scFvs inhibits cell growth through apoptosis cell death in a triple negative breast cancer cells, MDA-MB-231. Given that Fzd7 and Wnt pathway plays a critical role in tumor progression, selected blocking scFvs represent significant potential for immunotherapy of breast cancer cells.
KEYWORDS: breast cancer, Fzd7, phage display, scFv, Wnt
Introduction
Breast cancer is a heterogeneous disease that comes in various clinical, histological and molecular forms and all of these features together determine the risk of disease progression and therapeutic resistance. Breast cancer patients usually have the expression of progesterone receptor (PR), estrogen receptor (ER), and increase of HER-2/Neu. These markers allow classification of breast cancer tumors and those tumors which do not express PR, ER, and do not have HER-2/Neu amplification are known as triple-negative breast cancer (TNBC).1 TNBC is an aggressive form of breast cancer which consists about 15 percent of all breast cancer and associated with poor outcome. Upregulation of Wnt pathway genes such as low density lipoprotein receptor-related protein 6, frizzled homolog 7 (Fzd7), and transcription factor 7 (TCF7) is well documented in TNBC.2 Over the past decade, our understanding and treatment of several types of breast cancers have improved but treatment of TNBC remained as a clinical unmet need Period3
Frizzled (Fzd) proteins are receptor family of 7-pass transmembrane molecules comprising 10 members in humans. Fdzs consist of the N-terminal extracellular called cysteine-rich domain (CRD)4,5 and intracellular C-terminal domain, including PDZ domain. Wnt protein family directly bind to Fzd CRD domain as well as PDZ domain, interacts with disheveled (Dvl) protein and begins Wnt/ß catenin signaling pathway.6 Wnt family is a group of signal transduction pathway proteins that induce signals through cell surface receptors including Fdz receptor.7 Wnt signaling initiates other transcription factor activities which are necessary for polarity,8 differentiation and cell proliferation.9,10 Fzd7, consists of 574 amino acids.11 Wnt-Fzd ligation through LRP (low density lipoprotein receptor-related protein) co-receptor (LRP5 or LRP6)12 leads to activate both canonical and non-canonical β-catenin pathways.13 In canonical pathway, Wnt binds to Fzd receptors and triggers Dvl activation and β-catenin releases. As a result, β-catenin conciliates and aggregates in cytoplasmic region. and enters to nucleus and activates TCF/LEF (T-cell factor/lymphoid enhancer factor) transcription factors and initiates downstream target genes,14 c-myc and cyclin D.15,16
The SOX family of transcription factors can serve as the modulators of Wnt signaling pathway. The vertebrate genome encodes over 20 different types of SOX proteins and it found recently that many of them interact physically with β-catenin and modulate the transcription of Wnt-target genes.17 Recently, the overexpression of SOX4 has been reported in several tumors, including prostate cancer, breast cancer and colon cancer.18-20 It has well documented that SOX4 stabilizes β-catenin, and increases Wnt signaling pathway in many types of cancers.21 Taking together it seems that SOX4 transcription factor plays an vital role in Wnt signaling pathways in TNBC14,22
Aberrant Fzd7 expression is found in different types of human cancers.23 Recently, it has been shown that up-regulating of Fzd7 receptor correlate with tumor formation and metastasis mainly through the activation of the canonical signaling pathway, genetic mutation and aberrant Wnt signaling via Fzd7 in multiple tumor types, such as triple negative breast cancer,2 gastric cancer,24 hepatocellular carcinoma25 and colorectal cancer.26 Therefore, Wnt/Fzd7 is a promising target for the development of new anticancer agents. Recently, several small molecules that interfere with Wnt pathway have been developed. Suppression of Fzd7 with RNA interference decreases the invasion and metastasis of tumor cells.11 However, these candidates also interfere with other signaling pathways beyond the Wnt-Fdz.
Phage display is a novel methods for the monoclonal antibody production, as well as, rapid generation of antibody against antigen without immunization.27 The advent of antibody phage display technology has authorized to quickly isolate recombinant antibodies against small self and non-self-antigens such as cancer associated antigens.28,29
Single-chain fragment variable (scFv) antibody includes variable domains of heavy (VH) and light (VL) chains connected through an artificial flexible linker.30 These small molecules can be applied to produce anti-small biomarkers for different purposes both in vitro and in vivo. In addition, it shows enhanced availability to tumor cell in vivo, and in radionuclide, drug and hormone delivery system.31
In the present study we employed the human naive antibody libraries to isolated scFvs against specific peptide from extracellular domain of Fzd7 receptor. The reactivity and growth inhibition properties of the selected antibodies was evaluated using ELISA, MTT and annexin V assays, respectively.
Results
Selection of anti-Fzd7 receptor scFv antibodies
Human single fold scFv libraries I + J (Tomlinson I + J), was amplified and employed to isolate scFvs against Fzd7 specific peptide. The titer of 4 outputs and inputs were determined by counting the colony-forming units (CFU) of the infected E. coli TG1. The titers of input phages were approximately 104 cfu/ml. The results showed that the number of eluted phages was increased from 104 to 1013 cfu/ml following 4 rounds of panning (Fig. 1), indicating enrichment of clones specific to Fzd7.
Figure 1.
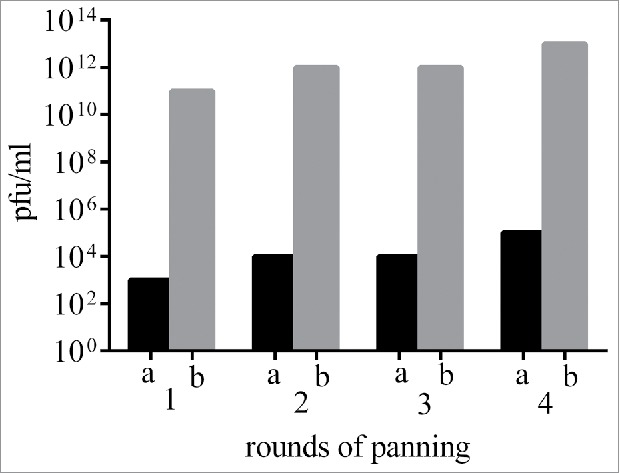
Outputs and inputs phage titer from 4 rounds of panning. cfu: colony-forming unit. Tomlinson I+J naïve scFv library was employed in panning procedures to select reactive phages to Fzd7 peptide.
Polyclonal and monoclonal ELISA
Outputs from round 2, 3 and 4 were evaluated for Fzd7 binding properties. As shown in Fig. 2A, the OD values of wells coated with Fzd7 peptide were higher than uncoated wells and increased round by round, indicating the effectiveness of panning strategy.
Figure 2.
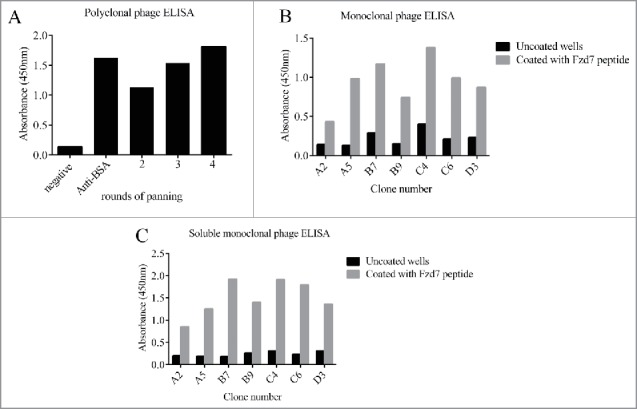
Polyclonal phage (A), monoclonal Phage (B) and soluble preparation (C) ELISA of the selected scFvs against Fzd7 peptides. No reactivity was detected for scFvs with unrelated peptide. Data are presented as mean ± SD of OD from duplicate experiments. Positive control: anti BSA scFv, negative control: BSA coated wells.
Sixty-five individual clones from the 3 and fourth rounds of panning were randomly selected and screened by monoclonal phage ELISA, of them 7 clones represented positive reactivity to Fzd7 peptide (Fig. 2B). Criteria for positive reaction was considered as absorbance of Fzd7 peptide/absorbance of uncoated wells >2.5.
Soluble scFv ELISA
An arbitrary cut-off OD 2.5 folds above negative control was considered as positive reaction. As shown in Fig. 2C, Fig. 3 out of 7 preparations of soluble scFv, C6, C4 and B7 met the criteria for positive reactivity to Fzd7 peptide (Fig. 2C).
Figure 3.
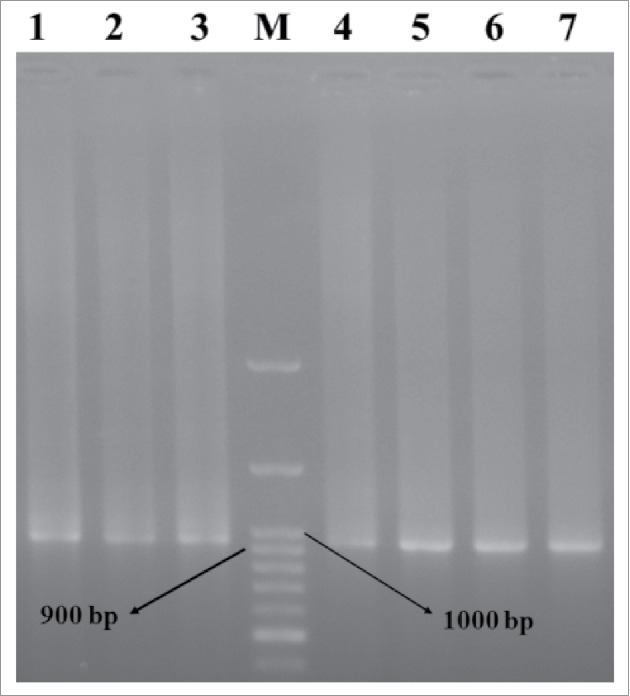
Electrophoresis of PCR products of scFv genes. Lane M: DNA marker. Lanes 1–7: PCR products of the selected clones.
Colony PCR
Seven positive clones from the soluble monoclonal ELISA were selected and amplified by PCR, to investigate the presence of full length VH and Vκ inserts (950 bp). A single band corresponded with scFv gene was detected in all clones (Fig. 3).
Specificity
As shown in Table 1 the cross reactivity of soluble monoclonal scFvs to irrelevant peptide ROR1, IGF-1 receptor and BSA was investigated using ELISA assay. ELISA plates were coated with 50 μg/ml of each 3 different antigens. Our results showed the specific binding of soluble monoclonal scFvs to Fzd7 peptide.
Table 1.
Specificity of soluble monoclonal scFvs to a variety of antigens by ELISA.
| Clones | C1 | C2 | C3 | C4 | C5 | C6 | C7 | |
|---|---|---|---|---|---|---|---|---|
| Antigens | Fzd7 | 0.85 | 1.25 | 1.92 | 1.40 | 1.91 | 1.79 | 1.36 |
| ROR1 | 0.21 | 0.19 | 0.17 | 0.19 | 0.13 | 0.19 | 0.23 | |
| IGF-1 R | 0.19 | 0.14 | 0.12 | 0.13 | 0.21 | 0.17 | 0.17 | |
| BSA | 0.11 | 0.27 | 0.25 | 0.16 | 0.17 | 0.12 | 0.18 |
ROR1, receptor tyrosine kinase-like orphan receptor 1; IGF-1 receptor, insulin-like growth factor-1 receptor; BSA, bovine serum albumin.
Cell proliferation assay
From all reactive clones, clone C4 represented the most binding property (OD = 1.38 and 1.91 in monoclonal phage and soluble ELISA) and was selected for further evaluation. To investigate growth inhibitory effect of anti-Fzd7 scFv on MDA-MB-231 cells MTT assay was done. The cells were treated with anti-Fzd7 scFv at different concentrations for 24 and 48 h.
No significant growth inhibition was observed in the cells treated with anti BSA scFv. However, treatment with 5 × 103 and 104 anti Fzd7 scFvs/cell showed significant growth inhibition of the cells after 24 h, (P value < 0.05). The growth inhibition of cells treated with 5 × 103 and 104 scFvs/cell were 8% and 20%, respectively (Fig 4A). As shown in Fig. 4B, treatment with 2.5 × 103, 5×103 and 104 anti Fzd7 scFvs/cell resulted in significant growth inhibition of the cells after 48 h (P value < 0.05). The growth inhibitions of cells treated with 2.5 × 103, 5 × 103 and 104 scFvs/cell were 16%, 17% and 24%, respectively. No significant inhibitory effects were observed from Anti-BSA scFv (as negative control) (P value > 0.05).
Figure 4.
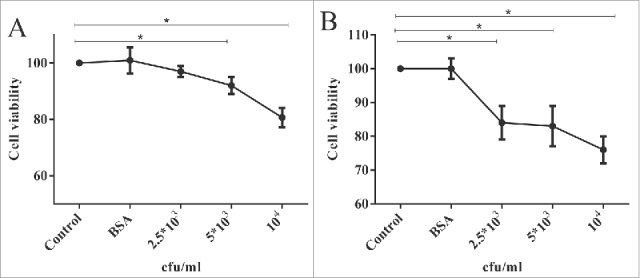
MDA-MB-231 Proliferation assay using MTT assay following (A) 24 and (B) 48 h treatment with anti Fzd7 scFvs. Anti-Fzd7 scFv significantly inhibited cell growth in MDA-MB-231, breast cancer cells. Treatment with anti BSA scFvs had no significant effect on cell growth. Data are shown as mean ± SD based on 4 independent experiments. (*) represents P-value < 0.05.
Apoptosis cell death assay
Apoptosis was assessed by Annexin V-FITC/PI staining. Fig. 5A shows that following 24 h, the majority of untreated MDA-MB-231 cells (51 %) of were alive (Annexin V−/PI−), 7 % and 36 % of the cells identified as early apoptotic (Annexin V+/PI−) and late apoptotic (Annexin V+/PI+) cell populations, respectively (Fig 5A). However, following 24 h treatment, 42 % of the cells treated with anti-Fzd7 scFv (104 scFvs/cell) identified as apoptotic cells. In the cells treated with anti-BSA scFv (as negative control), 13 % of the cells identified by apoptosis cell death. Data analysis indicated significant differences in percentage of apoptotic cell population between cells treated with anti-Fzd7 scFv and untreated (cell control) and also cells treated with equivalent number of anti BSA scFv (scFv control) (*P value < 0.05).
Figure 5.
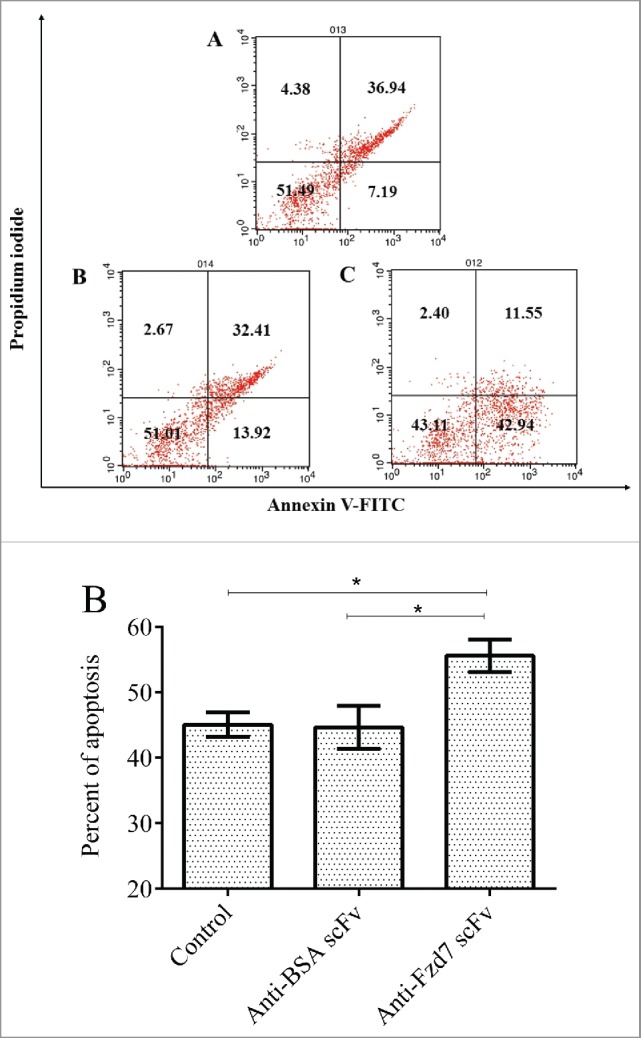
(A) Annexin V/PI apoptosis assay in MDA-MB-231 cells. Apoptosis cell death was analyzed in cells treated with unrelated scFv (negative control) and anti Fzd7 scFv for 24 h. A) untreated, B) Anti-BSA treated and C) anti-Fzd7 scFv treated cells. (B) Present of apoptotic MDA-MB-231 cell line vs. control. (*) represents P-value < 0.05.
Discussion
Targeting of tumor associated receptors using monoclonal antibodies is one of the effective strategies in cancer therapy, due to specificity and low toxicity. Anti-breast tumor activity of several scFvs specific for HER2,32 EGFR,33 Muc-134 have been proven so far. Recently, we also reported success in selection and characterization of a cocktail of anti-IL-25R scFvs.35 It has been shown that Fzd7 is upregulated in cancers24 and inhibition of Fzd7 in cancer cells leads to decrease of cell growth36 and tumor transformation.2 Recognition of the importance Wnt signaling pathway mutations in some cancers has provided strong evidence that the pathway plays a major role in the genesis of some types of human cancers.37 The findings suggested that targeting Fzd7 receptor and related signaling pathway molecules can be considered as a novel pharmacologic tool for caners therapy.2
The aim of the present study was to isolate and characterize human single scFv against Fzd7 receptor using phage display. Tomlinson library I + J was employed to isolate human single scFv against the Fzd7 epitope. Following 4 rounds of panning, 7 positive reactive clones were obtained, A2, A5, B7, B9, C4, C6 and D3, using monoclonal Phage ELISA. For testing positive clones was selected to be produced as soluble scFv using E. coli HB2151. The results of the soluble scFv ELISA were consistent with monoclonal phage ELISA. Three scFvs C6, C4 and B7 represented higher binding reactivity to Fzd7 in both soluble and phage ELISA. The PCR result performed to confirm positive clone in ELISA that indicated both VH and VL gens insert binding to Fzd7 antigen. The growth inhibition and apoptotic properties of anti-Fzd7 scFv were assessed with MTT assay. Latter indicate that targeting Fzd7 by specific scFv could antagonize blockade of Wnt and inhibit growth in human breast cancer cells. Annexin V/PI assay indicates apoptosis cell death as mechanisms underlying growth inhibition of MDA-MB-231 treated by anti-Fzd7 scFv.
Interaction between Wnt and Fzd7 receptor results in releasing of β-catenin from the complex and its transportation to the nucleus and finally expression the genes related to cell proliferation such as cyclin D and c-myc. Our finding revealed that the selected scFvs may block Wnt and Fzd7 interaction and lead to tumor growth inhibition through apoptotic cell death. The structure of Wnt has not yet been reported, but both Wnt and the scFv are substantially larger proteins than the relatively small cysteine-rich domain of Fzd7, thus it is possible that steric hindrance prevents simultaneous binding of Wnt and scFv to Fzd7. Due to high affinity property of selected scFv using phage display technology, It is also predicted that anti Fzd7 scFv binds to Fzd7 with a higher affinity than Wnt proteins. A complementary binding study to determine whether the selected scFvs directly block the ability of Wnt to interact with Fzd, is being conducted in our lab.
Most recent studies have revealed that targeting Wnt signaling pathway could result in decreasing cyclin D proteins which involve in cell proliferation.38,39 Birdal Bilir et al showed that blocking the Wnt signaling pathway could result in apoptosis induction and reducing the cell proliferation through SOX4 and cyclin D levels in TNBC cells.40 The studies have shown that the decrease of SOX4 leads to apoptosis induction in several cancer cells.41 SOX4 upregulates an inhibitor of apoptosis protein (IAP) called survivin. Survivin is undetectable in normal human tissue, but is overexpressed in most human cancers.42 Inhibition of Wnt/Fzd signaling pathway induces apoptosis through the releasing of cytochrome c, downregulation of survivin, and subsequent activation of caspase-3.43 Austin Gurney et al. showed that Fzd7 targeting with a monoclonal antibody, OMP-18R5 (vantictumab), inhibits Wnt signaling pathway and tumor cell proliferation.44 Interestingly, OMP-18R5 (vantictumab) interact to an epitope that is similar to the peptide in the present study. Moreover, residues lining the base of this site are highly conserved across the Fzd family, supporting that this region is functionally important and appropriate for targeting.
In the aspects of cell surface expression and correlation with aggressiveness and poor prognosis of breast cancer, Fzd7 could be compared to HER2/neu. Targeting HER2/neu by means of a humanized monoclonal antibody, trastuzumab has become the first-line strategy for breast cancer therapy. However, HER2 is overexpressed on barely 30% of patients and resistance to trastuzumab often occurs.45 Little or none of the other alternative promising target was discovered. Fzd receptors overexpression has a strong correlation with tumor progression and are also expressed in HER2 negative breast cancer cell lines.46 As Wnt targeting is a new candidate for breast cancer immunotherapy, anti-Fzd7 antibodies could be new alternatives in this regard.
In summary, the present study illustrated that isolated scFv-antibodies against Fzd7 represented binding to specific peptide from extracellular domain of Fzd7. Selected scFv can be employed as the best property for progress in treatment associated with this antigen in the triple negative breast cancer cells. Possible synergistic response to the selected scFv and breast cancer chemotherapy e.g gemcitabine and taxol and capability of the scFv to reduce tumor-initiating cell frequency are worth investigating.
Materials and methods
Libraries and bacterial strains
Tomlinson I+J single chain fragment variable (scFv) libraries, (containing synthetic V-gene of human immunoglobulin inserted in the pIT2 phasmid vector), positive control anti-BSA (Bovine Serum Albumin) scFv in E. coli strain TG1 (labeled TG1-anti-BSA), E. coli strains HB2151 and TG1 were obtained from the medical research council (MRC, Cambridge, England). Tomlinson library has diversity approximately I+J of 1.47 ×108 pfu ml1. KM13 helper phage and Isopropyl-β-D-thiogalactopyranoside (IPTG and DAB (3, 3′-diaminobenzidine)) were purchased from Sigma (St. Louis, MO). Maxi Sorptm immune tubes and MaxiSorp ELISA plates were purchased from Nunc (Denmark). Tryptone, yeast extract, NaCl and European bacteriological agar were purchased from Laboratories Conda (Madrid, Spain). Peptide (QEDAGLEVHQFYPLV) was obtained from JPT (Germany).
Phage library preparation
Briefly, 500 μl of the library stock (Tomlinson I + J) was incubated in 200 ml pre-warmed 2xYT (16gTryptone,5 g NaCl and 10 g Yeast Extract in 1 L) containing 1 % glucose and 120 μg/ml of ampicillin at 37°C with shaking (250 rpm) until OD600 reached 0.4-0.5 (1–2 h). Then, about 5 × 1011 plaque-forming units (PFU) of M13K07 helper phage (New England Biolabs Inc., USA) were added to each ml of cell suspension, incubated without shaking for 30 min at 37°C and for another 30 min in a shaker. Subsequently, infected bacteria were collected (centrifuged at 4000 rpm at 4°C for 10 min), resuspended in100 ml 2xYT containing 50 µg/ml kanamycin (Sigma, USA), 120 µg/ml ampicillin and 1 % glucose and incubated overnight at 30°C with shaking (200 rpm) and centrifuged at 4000 rpm at 4°C for 20 min. Phages were precipitated from supernatant with PEG/NaCl (20 % Polyethylene glycol 6000, 2.5 M NaCl) and collected by centrifugation at 14000 rpm at 4°C for 20 min. Phages were stored at −70°C in 15 % glycerol or at 4°C for longer term and short-term storage, respectively.
Selection of anti-Fzd7 scFv clones
In order to isolate specific phage-scFv antibodies against synthetic oligopeptide from the extracellular domain of Fzd7, panning technique was performed. Ten μg/ml of peptide in PBS (for positive screening) and 10 µg/ml of bovine serum albumin (BSA) in PBS (for negative screening) were coated on maxisorp immuno-tubes (Nunc, Denmark) for 14 h at 4°C. The coated immuno-tubes were washed 3 times with PBS and blocked with 5 % MPBS (5 % Skim milk powderin PBS) at 37°C for 90min. Subsequently, immuno-tubes were washed 3 times with PBS and 1012 phage from the library in 4 ml of 5 % MPBS (input 1) were added to the immuno-tube coated with BSA and incubated at 37°C for 60 min on a rotator (negative panning). Afterward, supernatants containing phages not bound to BSA were added to the immuno-tube coated with specific peptide and incubated at 37°C for 30 min with shaking (50 rpm) and a further 30 min without shaking (positive panning). The immune-tubes were washed 10 times with PBST 0.5% (PBS containing 0.5 % Tween 20), 5 times with PBS and 2 times with distilled water to remove the unbound phages, and the binders were eluted from the immune-tube by adding 2 ml of triethylamine (TEA, 100 mM, pH 10.0). Following 10 min at RT, 2 ml of Tris–HCl (0.5 M, PH 7.4) was added to neutralize the eluted phages solution (output 1). In order to propagate eluted phages, E. coli TG1 cells (OD 600 = 0.5) were infected with eluted phages and M13K07 helper phage. Subsequently, propagated phages were collected from supernatant as described above and named input 2. The titer of outputs and inputs were also determined.35,47 Five rounds of panning were performed for isolation of specific scFv-phage clones (Fig. 2).
Expression of Soluble Fzd7 scFvs
In order to production soluble scFvs, E. coli HB2151 was infected with the selected phages. Briefly, 15 µL of competent HB2151 were incubated in 7 mL of LB broth until OD600 = 0.6. Subsequently, 50 µL of the purified scFv-phages were added and incubated for 30 min at 37°C without agitation and another 30 min at 37°C in a shaker (rpm: 150). 7 mL LB broth containing (120 mg/mL) ampicillin were added until OD600 reached to 0.9. Subsequently, 200 µL of IPTG (isopropyl β-D-thiogalactoside, 1 mmol/L) were added and incubated for 18 h at 37°C in a shaker (200 rpm). Afterwards, the culture was centrifuged at 3700 rpm for 20 min and the pellet was dissolved in 10 µL PMSF (phenylmethanesulfonyl fluoride) (1 mmol/L) to produce periplasmic extract. TES buffer (Tris-Hcl 30 mM, EDTA 1 mM, Sucrose 20%, pH = 8.0) was added and incubated for 60 min at 4°C in a shaker (150 rpm) then TES 20% buffer was added for 120 min at 4°C in a shaker (150 rpm). Bacterial extract was centrifuged at 10000 rpm for 20 min and supernatant was collected and used for ELISA.
Polyclonal phage ELISA
Polyclonal phages isolated at the final round of panning were screened for specific binding using ELISA. Briefly, MaxiSorp ELISA plates were coated with 100 µl per well of Fzd7 peptide (10 μg/mL) in the PBS buffer and incubated overnight at 4°C. BSA protein was also coated as negative control in the ELISA plates. The wells were washed for 2 times with PBS. 250 µl per well of 3% bovine serum albumin (blocking buffer) was added and incubated for 90 min at 37°C. The wells were washed 2 times with PBS and 100 µl of rescued phages (1012 per well) diluted in 3% BSA-PBS were incubated for 60 min with shaking at 37°C. The wells were washed 4 times with PBS-0.1 % Tween 20. 1:5000 dilutions of HRP-anti-M13 in 0.1% BSA-PBS was added and incubated for 60 min at 37°C with shaking. Following washing 3 times with PBS-0.05% Tween 20 and 1 times with PBS, 100 µl/well of TMB substrate were added and incubated at room temperature (RT) in a dark place for 2–20 min. The reactions were stopped by 50 µl/well of sulfuric acid (1M). ODs were read at 450 nm by ELISA plate reader (Bio-Rad, Hercules, California, USA). All experiments were carried out in duplicate.
Monoclonal -phage and soluble scFv ELISA
The output Phages from last round of panning were used to infect exponentially growing TG1 by incubating for 30 min with shake (150 rpm) and 30 min without shake at 37°C. Infected cells were spread on a LB plate containing ampicillin (120 µg/mL), subsequently, the plate was incubated overnight at 37°C. Selected phage clones were picked, phage rescued and evaluated for binding to Fzd7 peptide by phage ELISA as described in polyclonal phage ELISA. An anti BSA specific scFv clone was employed as positive control of ELISA procedure. Wells coated with BSA but not specific Fzd7 peptide were included as negative control. Soluble scFv ELISA protocol was performed similar to phage ELISA except using bacterial periplasmic extracts and 1:2000 dilution of anti C-myc-HRP were used as conjugate.
Colony PCR
Colony PCR was performed to investigate the presence of the desired band corresponding to the full length VH and VL genes using phagmid specific primer pHEN [5′CAG GAA ACA GCT ATG AC3′] and LMB3 [5′ CTA TGC GGC CCC ATT CA 3′]. The annealing temperature for PCR reaction was 55°C for 2 min extension for VH and VL together.
Specificity
The specificity of soluble scFvs to Fzd7 was assayed by ELISA. Briefly, ELISA plates were coated with 50 μg/ml of various antigens including receptor tyrosine kinase-like orphan receptor 1 (ROR1), IGF-1 (insulin-like growth factor-1) receptor and BSA. Cross reactivity of soluble monoclonal scFvs to were evaluated by ELISA as described above.
MTT cell proliferation assay
Triple negative human breast carcinoma cell line MDA-MB-231 was employed in the present study. Seven × 103 cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), penicillin (100IU) and streptomycin (100ug/mL). The cells were treated with of antibody fragments scFv phage (104 cfu/cell) from positive clones and incubated for 24 and 48 h at 37°C, 5% CO2. Cells were washed with PBS and 200 μl of 5 mg/ml MTT solution was added to each well and incubated for 4 hrs at 37°C. Subsequently, MTT solution was aspirated off and 150 μl DMSO was replaced to dissolve formazin crystals. Finally, the plates were incubated in a shaker at RT for 20 min. The colorimetric evaluation was performed using ELISA reader (Bio-Rad, Hercules, California, USA) at 570 nm. Each assay was repeated 3 times.
Annexin V-PI apoptosis assay
Apoptosis was assessed using Annexin V-FITC apoptosis detection kit (BD PharMingen, CA, USA) according to the manufacturer's protocol. Briefly, the MDA-MB-231 cells at a density of 300 × 103 cells were seeded per culture plate overnight at 37°C. Medium was replaced with fresh medium containing scFv (104 scFvs/cell) or anti-BSA scFv for 24 h at 37°C. Cells were harvested and washed twice with cold PBS and stained with Annexin V-FITC and PI in 1 × binding buffer for 15 min at RT in the dark. Finally, the cells were analyzed by FACSCalibur (Becton Dickinson, Mountain View, CA, USA).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis 2010; 32:35-8; PMID:21778573; http://dx.doi.org/ 10.3233/BD-2010-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan Y, Deng X, Chen L, Kim CC, Lau S, et al.. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene 2011; 30:4437-46; PMID:21532620; http://dx.doi.org/ 10.1038/onc.2011.145 [DOI] [PubMed] [Google Scholar]
- [3].Gluz O, Liedtke C, Gottschalk N, Pusztai L, Nitz U, Harbeck N. Triple-negative breast cancer—current status and future directions. Ann Oncol 2009; 20:1913-27; PMID:19901010; http://dx.doi.org/ 10.1093/annonc/mdp492 [DOI] [PubMed] [Google Scholar]
- [4].Dann CE, Hsieh JC, Rattner A, Sharma D, Nathans J, Leahy DJ. Insights into Wnt binding and signalling from the structures of two Frizzled cysteine-rich domains. Nature 2001; 412:86-90; PMID:11452312; http://dx.doi.org/ 10.1038/35083601 [DOI] [PubMed] [Google Scholar]
- [5].Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 1996; 382:225-30; PMID:8717036 [DOI] [PubMed] [Google Scholar]
- [6].Schulte G. International union of basic and clinical pharmacology. LXXX. The class Frizzled receptors. Pharmacol Rev 2010; 62:632-67; PMID:21079039; http://dx.doi.org/ 10.1124/pr.110.002931 [DOI] [PubMed] [Google Scholar]
- [7].Miller JR. The wnts. Genome Biol 2002; 3:3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Saini S, Majid S, Dahiya R. The complex roles of Wnt antagonists in RCC. Nat Rev Urol 2011; 8:690-9; PMID:22025172 [DOI] [PubMed] [Google Scholar]
- [9].Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signalling: diseases and therapies. Nat Rev Genet 2004; 5:691-701; PMID:15372092; http://dx.doi.org/ 10.1038/nrg1427 [DOI] [PubMed] [Google Scholar]
- [10].Clevers H. Wnt/β-catenin signaling in development and disease. Cell 2006; 127:469-80; PMID:17081971; http://dx.doi.org/ 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- [11].King TD, Zhang W, Suto MJ, Li Y. Frizzled7 as an emerging target for cancer therapy. Cell Signal 2012; 24:846-51; PMID:22182510; http://dx.doi.org/ 10.1016/j.cellsig.2011.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 2000; 407:535-8; PMID:11029008; http://dx.doi.org/ 10.1038/35035124 [DOI] [PubMed] [Google Scholar]
- [13].He X, Saint-Jeannet JP, Wang Y, Nathans J, Dawid I, Varmus H. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science 1997; 275:1652-4; PMID:9054360; http://dx.doi.org/ 10.1126/science.275.5306.1652 [DOI] [PubMed] [Google Scholar]
- [14].Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer 2008; 8:387-98; PMID:18432252; http://dx.doi.org/ 10.1038/nrc2389 [DOI] [PubMed] [Google Scholar]
- [15].Röhrs S, Kutzner N, Vlad A, Grunwald T, Ziegler S, Müller O. Chronological expression of Wnt target genes Ccnd1, Myc, Cdkn1a, Tfrc, Plf1 and Ramp3. Cell Biol Int 2009; 33:501-8; PMID:19353769; http://dx.doi.org/ 10.1016/j.cellbi.2009.01.016 [DOI] [PubMed] [Google Scholar]
- [16].Zhang J, Gill AJ, Issacs JD, Atmore B, Johns A, Delbridge LW, Lai R, McMullen TP. The Wnt/β-catenin pathway drives increased cyclin D1 levels in lymph node metastasis in papillary thyroid cancer. Hum Pathol 2012; 43:1044-50; PMID:22204713; http://dx.doi.org/ 10.1016/j.humpath.2011.08.013 [DOI] [PubMed] [Google Scholar]
- [17].Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/â‐catenin signaling in development and disease. Dev Dyn 2010; 239:56-68; PMID:19655378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu P, Ramachandran S, Seyed MA, Scharer CD, Laycock N, Dalton WB, Williams H, Karanam S, Datta MW, Jaye DL, et al.. Sex-determining region Y box 4 is a transforming oncogene in human prostate cancer cells. Cancer Res 2006; 66:4011-9; PMID:16618720; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-3055 [DOI] [PubMed] [Google Scholar]
- [19].Song GD, Sun Y, Shen H, Li W. SOX4 overexpression is a novel biomarker of malignant status and poor prognosis in breast cancer patients. Tumor Biol 2015; 36:4167-73; PMID:25592378; http://dx.doi.org/ 10.1007/s13277-015-3051-9 [DOI] [PubMed] [Google Scholar]
- [20].Wang B, Li Y, Tan F, Xiao Z. Increased expression of SOX4 is associated with colorectal cancer progression. Tumor Biol 2016; 37:9131-7; PMID:26768610; http://dx.doi.org/ 10.1007/s13277-015-4756-5 [DOI] [PubMed] [Google Scholar]
- [21].Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, Jonatan D, Zorn AM, Wells JM. Sox17 and Sox4 differentially regulate β-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol 2007; 27:7802-15; PMID:17875931; http://dx.doi.org/ 10.1128/MCB.02179-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011; 121:2750-67; PMID:21633166; http://dx.doi.org/ 10.1172/JCI45014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol 2002; 2:1; PMID:11872147; http://dx.doi.org/ 10.1186/1471-213X-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kirikoshi H, Sekihara H, Katoh M. Up-regulation of Frizzled-7 (FZD7) in human gastric cancer. Int J Oncol 2001; 19:111-5; PMID:11408930 [PubMed] [Google Scholar]
- [25].Merle P, Kim M, Herrmann M, Gupte A, Lefrançois L, Califano S, Trépo C, Tanaka S, Vitvitski L, de la Monte S, et al.. Oncogenic role of the frizzled-7/β-catenin pathway in hepatocellular carcinoma. J Hepatol 2005; 43:854-62; PMID:16098625; http://dx.doi.org/ 10.1016/j.jhep.2005.05.018 [DOI] [PubMed] [Google Scholar]
- [26].Samaei NM, Yazdani Y, Alizadeh-Navaei R, Azadeh H, Farazmandfar T. Promoter methylation analysis of WNT/β-catenin pathway regulators and its association with expression of DNMT1 enzyme in colorectal cancer. J Biomed Sci 2014; 21:1; PMID:24397824; http://dx.doi.org/ 10.1186/s12929-014-0073-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Willats WG. Phage display: practicalities and prospects. Plant Mol Biol 2002; 50:837-54; PMID:12516857; http://dx.doi.org/ 10.1023/A:1021215516430 [DOI] [PubMed] [Google Scholar]
- [28].Liu R, Yuan B, Emadi S, Zameer A, Schulz P, McAllister C, Lyubchenko Y, Goud G, Sierks MR. Single chain variable fragments against β-amyloid (Aβ) can inhibit Aβ aggregation and prevent Aβ-induced neurotoxicity. Biochemistry 2004; 43:6959-67; PMID:15170333; http://dx.doi.org/ 10.1021/bi049933o [DOI] [PubMed] [Google Scholar]
- [29].Juarez-Gonzalez V, Riano-Umbarila L, Quintero-Hernandez V, Olamendi-Portugal T, Ortiz-Leon M, Ortiz E, Possani LD, Becerril B. Directed evolution, phage display and combination of evolved mutants: a strategy to recover the neutralization properties of the scFv version of BCF2 a neutralizing monoclonal antibody specific to scorpion toxin Cn2. J Mol Biol 2005; 346:1287-97; PMID:15713481; http://dx.doi.org/ 10.1016/j.jmb.2004.12.060 [DOI] [PubMed] [Google Scholar]
- [30].Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Pope SH, Riordan GS, Whitlow M. Single-chain antigen-binding proteins. Science 1988; 242:423-6; PMID:3140379; http://dx.doi.org/ 10.1126/science.3140379 [DOI] [PubMed] [Google Scholar]
- [31].Luo D, Mah N, Wishart D, Zhang Y, Jacobs F, Martin L. Construction and expression of bi-functional proteins of single-chain Fv with effector domains. J Biochem 1996; 120:229-32; PMID:8889803; http://dx.doi.org/ 10.1093/oxfordjournals.jbchem.a021402 [DOI] [PubMed] [Google Scholar]
- [32].Nejatollahi F, Ranjbar R, Younesi V, Asgharpour M. Deregulation of HER2 downstream signaling in breast cancer cells by a cocktail of anti-HER2 scFvs. Oncol Res 2013; 20:333-40; http://dx.doi.org/ 10.3727/096504013X13657689382734 [DOI] [PubMed] [Google Scholar]
- [33].Gupta P, Han SY, Holgado-Madruga M, Mitra SS, Li G, Nitta RT, Wong AJ. Development of an EGFRvIII specific recombinant antibody. BMC Biotechnol 2010; 10:72; PMID:20925961; http://dx.doi.org/ 10.1186/1472-6750-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Albrecht H, Denardo G, Denardo S. Development of anti-MUC1 di-scFvs for molecular targeting of epithelial cancers, such as breast and prostate cancers. Q J Nucl Med Mol Imaging 2007; 51:304-13; PMID:17464275 [PubMed] [Google Scholar]
- [35].Younesi V, Nejatollahi F. Induction of anti-proliferative and apoptotic effects by anti-IL-25 receptor single chain antibodies in breast cancer cells. Int Immunopharmacol 2014; 23:624-32; PMID:25466271; http://dx.doi.org/ 10.1016/j.intimp.2014.10.015 [DOI] [PubMed] [Google Scholar]
- [36].Bafico A, Liu G, Yaniv A, Gazit A, Aaronson SA. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol 2001; 3:683-6; PMID:11433302; http://dx.doi.org/ 10.1038/35083081 [DOI] [PubMed] [Google Scholar]
- [37].Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci 1995; 92:3046-50; http://dx.doi.org/ 10.1073/pnas.92.7.3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci 1999; 96:5522-7; http://dx.doi.org/ 10.1073/pnas.96.10.5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Su Y, Simmen RC. Soy isoflavone genistein upregulates epithelial adhesion molecule E-cadherin expression and attenuates β-catenin signaling in mammary epithelial cells. Carcinogenesis 2009; 30:331-9; PMID:19073877; http://dx.doi.org/ 10.1093/carcin/bgn279 [DOI] [PubMed] [Google Scholar]
- [40].Bilir B, Kucuk O, Moreno CS. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J Transl Med 2013; 11:280; PMID:24188694; http://dx.doi.org/ 10.1186/1479-5876-11-280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pramoonjago P, Baras A, Moskaluk C. Knockdown of Sox4 expression by RNAi induces apoptosis in ACC3 cells. Oncogene 2006; 25:5626-39; PMID:16636670; http://dx.doi.org/ 10.1038/sj.onc.1209566 [DOI] [PubMed] [Google Scholar]
- [42].Gehrke I, Gandhirajan RK, Kreuzer KA. Targeting the WNT/β-catenin/TCF/LEF1 axis in solid and haematological cancers: multiplicity of therapeutic options. Euro J Cancer 2009; 45:2759-67; PMID:19729298; http://dx.doi.org/ 10.1016/j.ejca.2009.08.003 [DOI] [PubMed] [Google Scholar]
- [43].He B, You L, Uematsu K, Xu Z, Lee AY, Matsangou M, McCormick F, Jablons DM. A monoclonal antibody against Wnt-1 induces apoptosis in human cancer cells. Neoplasia 2004; 6:7-14; PMID:15068666; http://dx.doi.org/ 10.1016/S1476-5586(04)80048-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et al.. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci 2012; 109:11717-22; http://dx.doi.org/ 10.1073/pnas.1120068109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Claret FX, Vu TT. Trastuzumab: updated mechanisms of action and resistance in breast cancer. Front Oncol 2012; 2:62; PMID:22720269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].King TD, Suto MJ, Li Y. The wnt/â‐catenin signaling pathway: A potential therapeutic target in the treatment of triple negative breast cancer. J Cell Biochem 2012; 113:13-8; PMID:21898546; http://dx.doi.org/ 10.1002/jcb.23350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sadreddini S, Seifi-Najmi M, Ghasemi B, Kafil HS, Alinejad V, Sadreddini S, Younesi V, Jadidi-Niaragh F, Yousefi M. Design and construction of immune phage antibody library against Tetanus neurotoxin: Production of single chain antibody fragments. Human Antibodies 2015; 23:73-9; PMID:27472865; http://dx.doi.org/ 10.3233/HAB-150287 [DOI] [PubMed] [Google Scholar]


