Abstract
Introduction:
Aim of this paper is to describe some of models of outsourcing (numerous and response to different types of risks and increment of quality is based on individual problem and situation). Defining whether to outsource or not and whether to build or buy new information technology (IT) is question for contract research organization (CRO) and Pharma companies dealing with clinical trials, so the aim of this paper is to show business model that could make process of decision making less time consuming, less segmented and more efficient.
Material and methods:
This paper has a descriptive character, and represents a review of the literature that deals with the described issues.
Results:
Outsourcing should enable optimal capacity flexibility (technology that is outsourced should be done only optimally not entirely). The goal with CRO partners is to establish equivalent levels of global quality, as extensions of other research and development activities (by unification of standards of performance of alliance partners with best standards of industry). IT is gaining greater significance at each stage of clinical study and represent an inevitable element of the quality of a clinical study (for the purpose of monitoring of clinical site activities, data collection and management, medical monitoring, statistical programming, statistical analysis, clinical study reporting).
Conclusion:
CROs are able to maximize work within the CRO global development, to support the notion of a fully integrated outsourced company; facilitate the use of similar business processes and norms, reusing established CRO standards and improve CRO operational decision making within outsourced studies by providing consistent and current information across outsourced and in-house activities.
Keywords: clinical trials, outsourcing, information technologies, CRO
1. INTRODUCTION
Global pharmaceutical companies and contract research organizations are more globally connected than ever before. According to European Federation of Pharmaceutical Industries and Associations (hereinafter EFPIA) report (Figure 1) pharmaceutical and biotechnology industries are leading industries today (analysis is made in accordance with data of the top 1,500 companies with registered offices in the EU, Japan, The USA and the Rest of the World, ranked by total worldwide Research & Development (R&D)) (1, 2).
Figure 1.
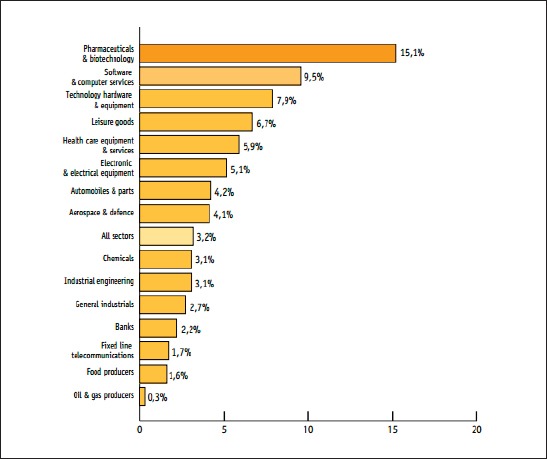
Ranking of industrial sectors by overall sector R&D intensity (EFPIA, 2013) (1)
Emerging market of biotechnology and pharmacy lead worldwide companies in creation of diversified interactions in order to keep up with modern science and business effectiveness. Pharmaceutical companies have developed an overall strategic approach in working with contract research organization (CRO) in Clinical Development, and recognizes that there are considerable efficiencies to be obtained by doing so. As such, Alliance Partnerships is one example model that could be formed to allow continuous access to clinical service providers who can support Sponsors in clinical research activities and add a competitive advantage and efficiencies to the business. In addition, it is anticipated that the partnerships will be beneficial to the Contract research organizations as they gain experience and success with Pharma companies that initiate projects (sponsors).
When discussing outsourcing of medical technology, we are considering different clinical trial management solutions (CTMS) that might include electronic trial master file (eTMF), electronic case report form (eCRF), Pharmacovigilance software’s and documents management systems. CRO’s and Pharma companies always have dilemma in front themselves whether to build or buy (outsource) software they need to manage project. Cost-effectiveness in innovative technologies is becoming important segment and necessity for implementation of clinical trials (1). Reason for this could be found in fact that quality and efficiency on long term are affected with lack of technology. For example, in the Institute of Clinical Excellence in UK, cost per quality is criteria for providing recommendations to National Health Service.
Moreover, in order to understand development and importance of electronic systems in clinical trials, it is important to mention that in 2012 Global clinical trial management system was estimated on 543.2 and in 2013 607.5 million USD (3, 4). Increased research and development spending has high impact on the market. In addition, increasing incidences in chronic diseases will influence furtherly on market growth. As road Map of EMA development has set goal of achieving wider access of information, we could connect this goal with e-solutions development as well.
An Alliance partnership (is any type of cooperative relationship among different firms, where an international alliance is comprised of two or more firms from different countries) is one in which companies work closely together to share systems and processes in an effort to reduce the costs and challenges of working together.
The objective of the outsourcing strategy is to establish a consistent, proactive approach that creates optimal capacity flexibility within Global Development.
However, there are numerous scientific papers explaining that outsourcing strategy could brought more harm than benefit to company. Potential disadvantages include: “chemistry” between vendor and client, danger on depending on third party, loss of control in IT assets, possible treat for opportunism, loss of flexibility, potential loss of competitive advantage in operational management, it can cause a decline of morale and performance of the remaining employees, long term cost savings are not guaranteed.
2. AIM
Aim of this paper is to describe some of models of outsourcing (response to different types of risks and increment of quality is based on individual problem and situation). Defining whether to outsource or not and whether to build or buy new IT technology is question for CRO and Pharma companies dealing with clinical trials, so the aim of this paper is to show business model that could make process of decision making less time consuming, less segmented and more efficient.
3. METHODS
This paper has a descriptive character, and represents a review of the literature that deals with the described issues. Method of analysis of content, comparative method, method of induction, method of deduction and research method were used. In addition, dialectic method is used in process of understanding management of clinical trials as appearance in constant change and improvement.
4. RESULTS
4.1. Considerations of outsourcing cro management and it services in clinical trials
When deciding which projects and services should be outsourced to the Sponsor CRO Alliance Partners, Sponsor could intend to adhere to several guiding principles in order to be compliant with the Code of Federal Regulations title:
Outsourcing should enable optimal capacity flexibility within Global Development (in further text GD), meaning also that technology that is outsourced should be done only optimally not entirely
We may assume that observed CRO intends that 30% (or more) of Full Time Equivalent workload globally will be performed by CRO partners. The reason for such decision could be found in allowing CRO to maximize internal resources. By internal resources, CRO could consider already developed software which is company brand. From the other hand any partnership and contractual obligation of working on clinical trial project could potentially include use of outsourced services in terms of technology e.g. CTMS.
Next assumption could be that CRO intends to manage external work with greater effectiveness by prioritizing and selecting substantial parts of programs and protocols for outsourcing - which could further include the potential to outsource full programs; This is case related to specialization of outsourced services in which basically, CRO is able to choose only product of interest for successful development of project. This could be potentially software that originated CRO do not possess, but would like to gain experience working on it as this kind of system would add additional value and provide higher quality of data (e.g. Response Evaluation Criteria In Solid Tumors (RECIST) in Oncology trials). Also, we can add that CRO will strive to make a decision to outsource an entire program rather than fragments of a program.
In general, the objective of this alliance approach is not to move to a model where entire functions are routinely outsourced, nor to routinely outsource work in specific Therapeutic Areas (i.e. areas of new drug development research) or geographies across all programs and protocols. However, CRO will consider partnering with local and specialty CROs that can fill specific geographic and therapeutic expertise needs, to supplement the Alliance relationships (as per specific requirements).
4.2. Reasons for Outsourcing to Alliance Partners
The goal with CRO partners is to establish equivalent levels of global quality, as extensions of other research and development activities (already utilized within observed company). Only by unification of standards of performance of alliance partners with best standards of industry (considering that our observed organization is following best industry standards and principles) - alliance partnerships (and outsourcing activities) could be acceptable and would represent strategic advantage as CRO intends to enable its partners to deliver the same standards of work as it performs itself.
This will in turn results in following benefits (strengths):
provide CRO with an additional pool of resources;
provide continuous access to experienced clinical service providers;
achieve financial, service level and quality improvements;
help obtain delivery, reduced cycle times and lower administrative costs;
provide consistency in contractual terms and conditions, working relationships and processes, tools, templates, work practices, SOPs and clinical systems.
From the side of technology, there is wide sector of services that could be outsourced trough component (software, hardware, services), type (enterprise based, site based), delivery mode (web, cloud, on premise), end use (CRO, Pharma companies, healthcare providers).
4.3. Outsourced Services: Resources and IT
Project scheduling is used to plan and control a project efficiently and must include:
Determining earliest and latest stage of each separate activity within scope of project;
Calculating likelihood that a project will be completed within projected period of time;
Finding minimum costs for completion of project in defined time;
Monitoring of project activities.
Determining allocation of resources trough out the project.
The above could be calculated using PERT (Program evaluation and review technique) or CPM (Critical path Method). However, based on analytics in literature and deduction, bellow are defined some of the project activities which are highly influenced by outsourcing activities (allocation of resources trough out the project). Also, the steps in project management are as follows:
Establish goals and scope.
Establish teams.
Set timelines and budget.
Decide on how to implement the study.
Implement and monitor the study.
Respond to unexpected or expected challenges, deviations, and problems.
Perform assessment after the project on lessons learned.
The services that CRO intends to outsource include (but not limited to) Project Management and Tracking - This refers to general clinical study project management activities. This includes the management and tracking of status, timelines and issues, as well as training and communication aspects.
Clinical Site Activities - This refers to activities related to the investigative Sites, including ensuring that the required Site documentation is in place, investigator meetings are conducted, recruitment is managed and investigator payments are being issued.
Data Collection and Management - This includes study data management activities ranging from set up tasks, such as the creation of the Input/Output plan and design to the ongoing entry and cleaning of clinical data in the Clinical Data Management System.
Medical Monitoring - This refers to the ongoing monitoring of the investigative Sites during the conduct of the clinical trial.
Statistical Programming - This includes development of the statistical programs for data extraction and analysis, as well as generation of report data tables, listings and graphs.
Statistical Analysis - This includes conduct of interim and final statistical analyses and generation of study report tables, listings and graphs.
Clinical Study Reporting - This includes the creation of the clinical study report from the first report outline to the final draft of the report.
5. DISCUSSION
Outsourcing is described as “the process by which a user employs the supplier, under a contract, to perform a function, which had previously been carried out in-house; and transfer to that supplier assets, including people and management responsibility” (5).
In deciding what to outsource lies with those elements that distinguish the organizations, especially in value and quality. Any activities that bring competitive advantage and are thus critical to the organization should be kept in-house (6, 7).
Processes, technology and people are all components of the outsourcing model. As there is present trend of outsourcing services in clinical trials, hardware and software market is going to show significant growth in future.
Decision making in outsourcing takes place at strategic, tactical and operational levels within an organization, but also decision making is a linchpin between the CEOs power, the delegation of authority and the performance of an organization. If a project is to be outsourced, the decision making group must agree to the scope of services to be outsourced, whether there is budget available to conduct the project and timing for the key deliverables.
When making these decisions, the group should consider the following criteria:
The size of the project;
CRO’s projected capacity to perform the work;
Any internal objectives to build or maintain specific therapeutic area or related competencies;
The Alliance Partners’ competency to perform the project;
Any clinical design complexities that may require exceptionally high levels of internal oversight and hands-on attention; and
Any business or strategic factors that may require high levels of internal oversight and hands-on attention.
5.1. Evaluation of performance
CRO intends to evaluate CRO performance across a number of areas. The most important of these - is project delivery. However, in order for CRO to evaluate the real value of its CRO alliances, performance should also be assessed in the areas of cost management, innovation and learning and overall satisfaction on the part of both CRO and CRO outsourced project teams.
The objectives of CRO performance management are to provide:
framework for measurement of key drivers of strategic goals, as they apply to working with CROs;
common language for discussing the achievement of performance goals in internal and Joint Project Team meetings;
standard approach to managing CRO performance across multiple projects and Therapeutic Areas;
and an early warning system that allows timely corrective action to be taken and ensures that goals are met.
Processes, technology and people are all components of the outsourcing model. The full benefit of this model is realized when these components interact.
Before a team can be formed and work can commence in collaboration with a CRO, the Scope and Award process must be completed. This ensures that a clear definition of the project is produced, which defines the organization responsible for each activity and ensures that the right CRO is chosen to execute the project and that the budget is defined and agreed upon. Selection of outsourced CRO is part of this process as well as finalizing Contracting and signing specific work orders:
Scope and Award of the Outsourced Project.
Approximation of Process of Start-Up and Management of Outsourced Project based on initial assumptions
Once the Work Order is signed by both companies, the Start-up phase can commence.
This phase continues until the first subject is treated and/or the first data is entered into the database. The Start-up phase could involve three main steps based on project perspectives (9).
Mobilize Joint Project Team: The project team is mobilized at a ‘Kick-Off’ meeting, the objectives of which are: to ensure that all team members understand the objectives of the project and feel committed to achieving project goals; to define an optimized plan for the project to meet clinical, regulatory and commercial requirements; and to identify the key challenges and start to develop solutions for proactive management of these challenges. The agenda of the Kick-Off meeting is designed to provide the required information for the Joint Project Team Project Management Group which is produced by the Project Director following the meeting. Clinical investigators participating in the clinical studies and the study sponsors must document every phase of the study planning, data generation, and data management (10).
Perform Set-up Activities: The set-up phase is one of the most intensive phases of starting an outsourced project, and requires direct input from all Core Team members and some Full Team members depending on the scope of the project.
Perform Training: The Project Management Charter should include the training requirements for Core Team members and Full Team members. These requirements should be incorporated into a full training plan addressing the training needs of each team member and training should be provided according to the schedule defined and the needs of the project.
Once the first subject has been treated and data is entered into the data management system, the process progresses from the project start-up phase into the project management or maintenance phase, which continues until the final deliverable is received from the CRO, for example, the clean database or Clinical Study Report. This process consists of three steps:
Conducting Key Milestone Progress Reviews: In order to effectively manage the project, Joint Project Teams are expected to conduct review meetings at least every alternate week. In addition to regular review meetings, it is recommended that key milestone progress review meetings are held on a quarterly basis, if possible as face-to-face meetings.
Managing Payments: Payments are made to the CRO vendor on an ongoing basis throughout the project based on a set of deliverables defined in the Work Order. In addition to deliverable based payments, invoices will be received for expenses incurred by the CRO while working on the project. These expenses may be related to travel or couriers’ costs, for example, and are unlikely to occur on a fixed price basis. Investigator payments are also invoiced to CRO as a separate line item or invoice on the basis of a pre-determined payment schedule that is related to patient evaluability or number of patients.
Providing Ongoing Support and Training: Ongoing support and training may be identified via: the Project Management Charter; ad hoc requests identified at progress review meetings; findings either from co-monitoring visits or audits; or when a new user joins the team. In general, training of CRO personnel will be provided by their CRO trainer who has been trained. If, however, the CRO trainers have not received training themselves or if the requirements are outside the normal scope (for example, protocol or disease-area related), other members of the Core or Full Joint Project Team may need to be involved.
Start-up activities will continue well into the management phase of a project as additional sites are initiated and subjects enrolled, therefore these two phases should be considered to be overlapping.
5.1.3. Close of Outsourced Project
Once the Final CRO deliverable is available, the project progresses from the project management phase into the project close phase which continues until the final CRO payment has been made and all aspects of the project have been closed.
Archiving Data and Documents to the Appropriate CRO Repository is one of the requirements required by International Conference on Harmonisation - Good Clinical Practice (ICH-GCP) regulation. In order to accomplish what is required, CRO needs to use adequate services on its own or to outsource same.
Case Report Form copies held by the CRO should be transferred to the appropriate CRO repository according to the archiving standard operating procedure (SOP). To archive clinical study files, the guidelines found in the CRO training manual on the European Science Foundation (ESF) document submission by a CRO should be followed.
Conducting Final Project Reviews as a part of overall project evaluation is a key in understanding the events of a project and capturing lessons learned to assist future teams. It is a process owned and managed by the CRO Project Director and involves a meeting of the Joint Project Team (ideally face-to-face) during which a structured review is conducted concerning what went well; what issues were encountered; and what recommendations or solutions the team can make to improve CRO outsourcing practices in the future (11, 12, 13).
Following contractual obligations, final payments need to be reconciled. The final invoice from the CRO initiates the last step in the CRO management process. Once received from the vendor, Central Contracts and Grants reviews the final invoice and any applicable pass through costs in consultation with the Project Director and, if in agreement, approves the invoices for payment. Once the final payment has been generated, CRO closes the project in its IT and accounting systems.
5.2. Components of Outsourcing – People
Processes, technology and people are all components of the outsourcing model. In this section, people who will be working with the processes and technology are addressed.
A complete CRO team may not have been formed when the project is being scoped and awarded. A contact name and details of the Outsourced manager should be designated by Project Leader allocated to work to complete process steps. This designated leader should reside within the existing project team and is likely to be a Project Director, Operations Lead or, if none of those roles are in place, the Medical Lead.
Depending on the scope of the services to be outsourced, the following roles should be called upon to assist the Outsourcing Manager and the designated Project Leader throughout the process.
Once the project has been awarded, the Joint Project Team should be formed to conduct the project. Team member roles will be allocated either from CRO or from the outsourced company, depending on which services are outsourced. There should be little or no duplication of roles from the different organizations.
Core team members share accountability for all team deliverables and are responsible for completing their specific deliverables on time and on budget to an appropriate standard of quality and completeness.
The Core Team may include: the CRO Project Director, the CRO Project Manager, a CRO Outsourcing Manager, a CRO Drug Supply Manager, a Project Clinical Programmer, Medical Monitors, a Project Data Manager and a Project Statistician.
The Full Team members are responsible for contributing to Joint Project Team deliverables that require their expertise and for providing a link between the joint project team and their functional areas.
6. CONCLUSION
One of the most important aspects of alliance (use of outsourcing resources) is related to transfer of obligations during merging of resources.
The applications to be used on each study are dependent on the services to be outsourced, which are detailed in the Transfer of Obligations (TOO). A TOO is required by Health Authorities to clearly allocate which activities in an outsourced project will be performed by CRO and which will be carried out by the outsourced company. This kind of authorization should be submitted to Regulatory Authorities including Ministry of Health in some countries.
There has been a great deal of confusion about tasks that should be performed by outsourcing management form one hand and operational management of CRO. Outsourced management takes care of day-to-day project-specific issues and global Operational management is concerned with master contract-related issues.
Today’s clinical trials are run and managed with the support of a tightly integrated set of clinical systems that support trial management and operational aspects such as data collection and tracking.
A number of CRO IT systems have been enabled at the CROs to allow the Alliance Partner CROs to work within the same environment as CRO, to provide a seamless service and to maintain project timelines.
With CRO clinical systems, CROs are able to: maximize work within the CRO global development, to support the notion of a fully integrated outsourced company; facilitate the use of similar business processes and norms, reusing established CRO standards; and improve CRO operational decision making within outsourced studies by providing consistent and current information across outsourced and in-house activities.
Footnotes
• Conflict of interest: None declared.
• Author statement: M.S. and A.D. made substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data. M.S. and A.D. participated in drafting the article or revising it critically for important intellectual content and give final approval of the version to be submitted.
REFERENCES
- 1.Ranking of industrial sectors by overall sector R&D intensity. [retrieved: 20.06.2017]. URL: https: //www.efpia.eu/media/219735/efpia-pharmafigures2017_statisticbroch_v04-final.pdf .
- 2.Adang M.M.E. The Implementation of innovative Technologies in Healthcare: Barriers and strategies. IGI Global. 2009:38. doi: 10.4018/978-1-60566-356-2.ch020. [Google Scholar]
- 3.Vukojevic P European clinical trial Site Options: an Insiders Analysis. Insight Pharma reports. Cambridge healthcare Istitute; [retrieved: 20.06.2017]. URL: http://www.insightpharmareports.com/reports_report.aspx?r=639&id=85738 . [Google Scholar]
- 4.Atanasov A. Quality and reliability Aspects in Evidence Based E-Medicine. Clinical technologies. IGI Global. :171. ISBN 978-1-60960-561-2. [Google Scholar]
- 5.Barrett P, Baldry D. In: Facilities Management: Towards Best Practice. 2nd edition. Drion B, Melissen F, Wood R, editors. Oxford: Blackwell; 2016. Facilities Management: Lost, or Regained? [Google Scholar]
- 6.Facilities Management. 2012;30(5/6):254–61. [Google Scholar]
- 7.Fill C, Visser E. The outsourcing dilemma: A composite approach to the make or buy decision. Management Decision. 2000;38(1):43–50. [Google Scholar]
- 8.Gottschalk P, Solli-Saether H. Critical success factors from IT outsourcing theories an empirical study, Industrial Management & Data Systems. 2005;105(6):685–702. [Google Scholar]
- 9.WMA decleration of Helsinki ethical principles for medical research involving human subjects. [retrieved on 21.06.2017]. URL: https: //www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/
- 10.SC Gad. Safety and Regulatory Requirements and Challenge for CNS Drug Development. Neurobiol Dis. 2013;61:39–46. doi: 10.1016/j.nbd.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Clinical trials. [retrieved on 21.06.2017]. URL: https: //www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0022681/
- 12.Consort statement. [retrieved on 21.06.2017]. URL: https: //www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0029908/
- 13.Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The Clinical Trials.gov Results Database - Update and Key Issues. The New England journal of medicine. 2011;364(9):852–60. doi: 10.1056/NEJMsa1012065. doi: 10.1056/NEJMsa1012065. [DOI] [PMC free article] [PubMed] [Google Scholar]


