Abstract
The demand for small-diameter blood vessel substitutes has been increasing due to a shortage of autograft vessels and problems with thrombosis and intimal hyperplasia with synthetic grafts. In this study, hybrid small-diameter vascular grafts made of thermoplastic polyurethane (TPU) and silk fibroin, which possessed a hybrid fibrous structure of an aligned inner layer and a random outer layer, were fabricated by the electrospinning technique using a customized striated collector that generated both aligned and random fibers simultaneously. A methanol post-treatment process induced the transition of fibroin protein conformation from the water-soluble, amorphous, and less ordered structures to the water-insoluble β-sheet structures that possessed robust mechanical properties and relatively slow proteolytic degradation. The methanol post-treatment also created crimped fibers that mimicked the wavy structure of collagen fibers in natural blood vessels. Ultrafine nanofibers and nanowebs were found on the electrospun TPU/fibroin samples, which effectively increased the surface area for cell adhesion and migration. Cyclic circumferential tensile test results showed compatible mechanical properties for grafts made of a soft TPU/fibroin blend compared to human coronary arteries. In addition, cell culture tests with endothelial cells after 6 and 60 days of culture exhibited high cell viability and good biocompatibility of TPU/fibroin grafts, suggesting the potential of applying electrospun TPU/fibroin grafts in vascular tissue engineering.
1 Introduction
31% of global deaths–an estimated 17.5 million people–were caused by cardiovascular diseases (CVDs) in 2012, especially coronary heart disease and stroke (World Health Organization, 2015; Mendis et al., 2011). As the population ages and grows, it is anticipated that the need of treatments and healthcare for CVDs will increase substantially year by year (Heidenreich et al., 2011). Clinically, to treat severe cardiovascular diseases, arterial bypass surgeries are conducted to create a passage rerouting the blood flow around the clogged artery to maintain the normal function of the circulation system. Vascular substitutes are required in the operations and the two main sources are autologous vessels, such as saphenous veins or internal mammary arteries, and synthetic prosthetic grafts. Unfortunately, suitable autologous vessels are not always available for patients with previous harvest, amputation, or vascular disease (Isenberg et al., 2006). Although artificial grafts made of polyethylene terephthalate (PET, Dacron) and expanded poly-tetrafluoroethylene (ePTFE, Teflon) have been used to fill the shortage, the occurrence of thrombosis, intimal hyperplasia, and immune rejection have limited the adaptability of these prostheses in replacing small-diameter (<6 mm) blood vessels (Baguneid et al., 2006; Wise et al., 2011).
In an attempt to find effective alternatives, the development of small-diameter vascular tissue engineering has advanced quickly over the past decades. Generally, current approaches focus on the regeneration of vascular tissue, which includes self-assembled cell sheets and scaffold-guided vascular reconstruction. The latter is an extension of synthetic vascular graft research incorporating in vitro cell expansion and maturation on the scaffold before implantation (Seifu et al., 2013). To create a proper template for tissue remodeling, natural-origin polymers such as collagen and fibrin were first used as the materials for tissue-engineered blood vessels (TEBVs) due to their biologically analogous nature (Cummings et al., 2004; Seifu et al., 2013; Swartz et al., 2005; Weinberg and Bell, 1986; Wu et al., 2007). However, the poor mechanical strength and compliance mismatch made them unfavorable in clinical applications. Compared to natural polymers, synthetic polymers have tunable mechanical properties, degradation rates, and microstructures, which can be controlled by material compositions, copolymer ratios, and fabrication methods. However, the low bioactivity and cellular toxicity of the by-products of some of the polymers are still of concern (Catto et al., 2014; Ravi and Chaikof, 2010; Seifu et al., 2013; Zhang et al., 2007).
Recently, many studies have combined natural and synthetic polymers to form hybrid scaffolds by different methods in the hope of taking advantage of the positive aspects of various materials (Catto et al., 2014). Among all of the candidate polymers, natural silk fibroin from silkworm cocoons (Bombyx mori) and synthetic thermoplastic polyurethane (TPU) were selected in this research for further investigation for fabricating TEBVs using the electrospinning method.
Fibroin is the main component of silk fiber, accounting for 75% of its weight, and is comprised of at least two fibroin proteins–light chains (25 kDa) and heavy chains (325 kDa) (Horan et al., 2005). The β-sheet structure provides the fibroin filaments with robust mechanical properties and relatively slow proteolytic degradation, making it an advantageous biomaterial compared to other natural polymers. Also, the characteristics of biocompatibility, low- or non-immunogenicity, ease of chemical modification, and low thrombogenicity raise the potential of applying fibroin to vascular grafts (Altman et al., 2003; Matsumoto et al., 2006; Wang et al., 2006). Several studies have been conducted to fabricate vascular-related products with silk fibroin by dipping (Lovett et al., 2007), gel spinning (Lovett et al., 2008, 2010), braiding (Enomoto et al., 2010; Nakazawa et al., 2011), and electrospinning (Liu et al., 2011; Soffer et al., 2008; Zhang et al., 2008).
The early applications of TPUs in medical fields can be traced back to the 1970s as the biomaterial for vascular catheters, blood bags, implants targeting both soft and hard tissues, and other medical device components (Zdrahala and Zdrahala, 1999). TPU is a flexible linear polymer consisting of soft and hard segments that exhibits good biocompatibility, manufacturability, and mechanical properties. By altering the ratio of the soft and hard segments, TPU can be modified to reach the required elasticity and strength for vascular tissue engineering (Mi et al., 2015a; Jing et al., 2015). Conventional TPU is nonbiodegradable but a biodegradable TPU can be synthesized with its electrospun samples achieving satisfying biomechanical behaviors as vascular substitutes (Baudis et al., 2012; Bergmeister et al., 2015). Also, the in vivo trials of biodegradable TPU grafts show promising results with long-term patency and remodeling (Bergmeister et al., 2015). Although several vascular grafts made of polyurethane (PU) or its blends have been developed and evaluated, only a few studies specifically assessed electrospun TPU blends (Grasl et al., 2010; Huang and Chen et al., 2011; Jing et al., 2015; Tiwari et al., 2002; Wang et al., 2012; Williamson et al., 2006).
Depending on the materials and process settings, electro-spinning is able to fabricate fibers with diameters ranging from 3 nm to over 5 μm, which can provide the high interconnectivity and surface areas needed for cell activity, as well as adjustable mechanical properties (Pham et al., 2006; Hasan et al., 2014). In this study, both aligned and randomly oriented fiber matrices of TPU/fibroin were collected to produce a hybrid vascular scaffold with an aligned structure at the inner layer and a random structure at the outer layer. Since the fiber orientation plays an important role in cell growth and tissue formation (Lee et al., 2005; Chew et al., 2007), the purpose of this design was to provide a directional microenvironment for cells to attach to and migrate to in the lumen to form the desired morphology. Followed by a methanol treatment, the fibroin in the hybrid scaffold became water-insoluble. Furthermore, the shrinkage effect caused the fibers to develop a crimped structure that mimicked the wavy structure of natural collagen fibers; this structure largely determines the mechanical properties and elastic stability of natural blood vessels (Rezakhaniha et al., 2012).
2 Materials and Methods
2.1 Materials
Medical grade hard and soft TPU, Tecoflex SG-93A and SG-80A (TPU-93A and TPU-80A), respectively, were provided by Lubrizol, Countryside, USA. Silk fibroin was extracted from the cocoons of Bombyx mori silkworms following the procedure described below. The solvent used to dissolve TPU and fibroin was hexafluoroisopropanol (HFIP) purchased from CovaChem, Loves Park, USA.
2.1.1 Preparation of Silk Fibroin
Natural cocoons of Bombyx mori silkworms (Shiaying Farmers Association, Tainan, ROC) were boiled in a 0.02 M Na2CO3 aqueous solution for 30 min, then rinsed with distilled water several times to remove the glue-like silk sericin, and dried at room temperature to obtain pure fibroin fibers. To prepare the fibroin aqueous solution, fibroin fibers were first dissolved in a 9.3 M LiBr solution at 60°C for 4 to 6 h. The fibroin–LiBr solution was then dialyzed against distilled water for 3 days at room temperature using a cellulose membrane (MWCO 14,000, Sigma-Aldrich, St. Louis, USA) to remove the LiBr. Later, the dialyzed solution was centrifuged twice, each for 20 min at 4°C, to remove any impurities and aggregates. The final concentration of the fibroin aqueous solution was 6 to 8% (w/v). Lastly, the clear fibroin solution was frozen overnight at −40°C and lyophilized by a freeze dryer (Labconco, Kansas City, USA) to obtain the dry fibroin for further usage. The preparation procedure followed Rockwood’s protocol with partial adjustments (Rockwood et al., 2011). All of the chemicals used were purchased from Sigma-Aldrich, St. Louis, USA.
2.1.2 Fabrication of Small-Diameter Vascular Grafts
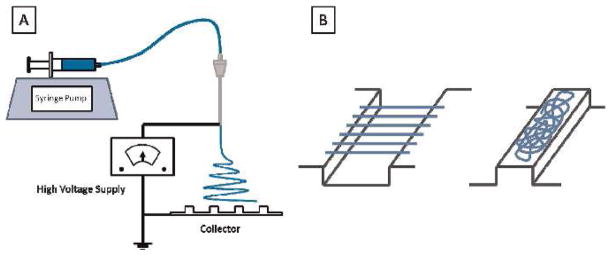
The TPU-93A/fibroin blends were dissolved in HFIP at 12 w/ v% with various ratios of polymers (1:1, 1:2, and 2:1). The TPU-80A/fibroin solution was prepared at a 1:1 ratio for comparison. All of the mixtures were stirred at room temperature for 12 h before electrospinning. The solution was then infused through a 10 ml syringe and tubing by a syringe pump (Harvard Apparatus 11 Plus, Harvard Apparatus, Holliston, USA) to an 18 gauge metal needle. The feeding flow rate was 0.5 ml/h, the applied voltage 15 kV using a high voltage power supply, and the humidity was maintained at 35 to 40%. Located 18 cm downward from the needle tip, a customized striated ground collector was used to collect randomly oriented and aligned fibers at the same time to form a continuous TPU/ fibroin sheet with both structures, as depicted in Fig. 1. The length of the groove, where the aligned fibers were collected, was adjustable from 1 cm to 3 cm depending on the dimensions required for the graft, and the length of the ridge was kept at 1 cm. The whole electrospinning process was conducted at room temperature for 6 h. Later, the collected continuous electrospun sheet was rolled around a mandrel to form a multilayered tube, and treated with methanol to induce the conformational transition of less ordered fibroin proteins to the β-sheet structure, which made it water-insoluble and fixed the shape of the graft.
Fig. 1.
Schematic of the customized electrospinning device (A). TPU/fibroin fibers were collected by a striated collector to form a fibrous sheet comprised of alternatively aligned and randomly oriented structures (B). Aligned fibers were collected in the grooves, while randomly oriented fibers fell on the ridges (B)
3 Characterization
Fourier transform infrared spectroscopy (FTIR) was used to verify the compositions of the electrospun sheets. Both random and aligned samples were surveyed using a FTIR instrument (Bruker Tensor 27, Bruker, Manning Park, USA) in transmittance mode. The range of wavenumbers was from 500 to 4000 cm−1, with a 4 cm−1 resolution and 60 scans for each measurement.
The microstructure and morphology of the electrospun TPU/ fibroin fibers were observed by scanning electron microscopy (Leo Gemini 1530 SEM, Zeiss, Oberkochen, Germany) with an accelerating voltage of 8 kV. The samples were gold sputter coated for 45 s before imaging.
The tensile strength of the electrospun TPU/fibroin samples with a random fiber orientation was measured on a testing frame (Instron 5967, Instron, Norwood, USA) with a 50 N load cell and Biopuls pneumatic clamps as a preliminary reference (n = 6 for each group). The dimensions of all of the specimens were a 10 mm·35 mm rectangle, with a 20 mm gauge length and measured thickness. A micrometer was used to measure the thickness before every trial. The specimens were stretched at a crosshead speed of 5 mm/min until rupture. The circumferential cyclic tensile test was performed on selected tubular grafts. Tube segments with a 3.18 mm inner diameter, 5 mm length, and a measured wall thickness were held by two L-shape grips, stretched in the transverse direction to 20% strain, and then released to 0% strain, at a speed of 5 mm/min, repeatedly, for 400 cycles.
4 Cell Culture and Seeding
Human endothelial cells derived from embryonic stem cells were prepared for cell viability testing. The differentiated endothelial cells were cultured in E7V media for another three days before seeding on the scaffolds. Before cell seeding, the scaffolds were coated with or without Matrigel (0.5 mg in 9 ml DF12 media) for 1 day. At the day of cell seeding, cells were digested with Accutase for 3 min and then resuspended in E7V media (107 cells/ml). The cell suspension was loaded onto the scaffolds using a 200 μl pipet. The scaffolds were carefully transferred on a V-shaped notch in a cell plate filled with E7V media. The V-shaped notch was made by polydimethylsiloxane (PDMS). The plates were then transferred into a cell culture incubator and cultured for two hours. Two hours later, cell seeding was repeated, but the scaffold was turned 180 degrees to make sure that the cells were seeded on all inner surfaces of the scaffolds. Cell morphologies were observed with a Nikon Ti-E confocal microscope at day 6 and day 60 of culturing.
5 Results and Discussion
5.1 Fourier Transform Infrared Spectroscopy
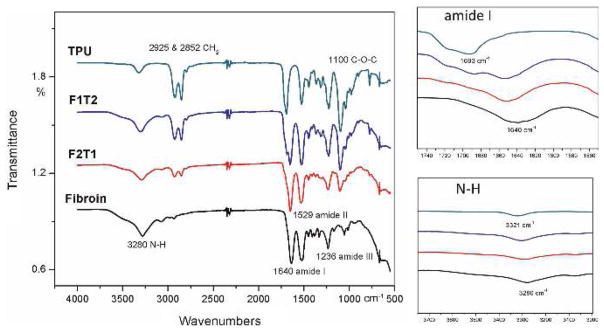
The purpose of using FTIR was to confirm the existence of both TPU and fibroin in the electrospun matrices. FTIR spectra of TPU-93A, fibroin, and TPU-93A/fibroin at 2:1 and 1:2 ratios with methanol treatment are shown in Fig. 2.
Fig. 2.
FTIR test results for electrospun TPU-93A, fibroin, and TPU-93A/fibroin at 2 : 1 (F1T2) and 1 : 2 (F2T1) ratios. The peak shifts and the variation of intensities indicate a change in the material composition
The characteristic absorption bands of fibroin appeared at 1640 cm−1 (amide I C=O stretching), 1529 cm−1 (amide II N–H in-plane bending), 1236 cm−1 (amide III C–N stretching), and 3280 cm−1 (amine N–H stretching) (Alessandrino et al., 2008; Huang and Liu et al., 2011). In the samples combined with TPU, shifts could be observed at amide I and N–H groups toward 1693 cm−1 and 3321 cm−1, respectively, indicating a change in their compositions. Also, the presence of CH2 symmetric stretching vibrations at 2925 and 2852 cm−1, and the stretching of ether groups C–O–C at 1100 cm−1, demonstrated the incorporation of TPU with fibroin (Mi et al., 2015a). When the content ratio of TPU increased, the intensities of these three peaks increased obviously.
5.2 Structure and Morphology
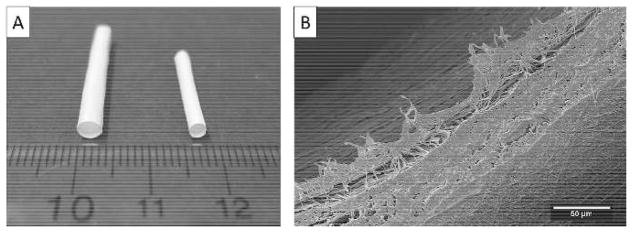
Electrospun TPU/fibroin sheets with a continuous aligned–random fibrous structure were collected on a customized striated collector and then rolled around a mandrel to produce a tubular graft. Figure 3 shows finished vascular grafts with 3.18 and 2.38 mm diameters as well as the morphology of inner and outer surfaces and the layered structure. By using different sizes of mandrels and adjusting the groove and ridge lengths of the collector, the inner diameter of the graft, the wall thickness, and the layered structure are all tunable. Moreover, the fiber structure of the TPU/fibroin sheets can be changed by modifying the material compositions and the process parameters for the electrospinning process, such as the concentration of the solution, the injecting flow rate, the applied voltage, the distance between the needle tip and the collector, and the humidity, to produce the required morphology.
Fig. 3.
Small-diameter vascular grafts with 3.18 and 2.38 mm inner diameters made of electrospun TPU/fibroin sheets (A). The graft contains an aligned fibrous layer for the inner surface, upper-left, and a random-distributed layer at the outside, lower-right, (B)
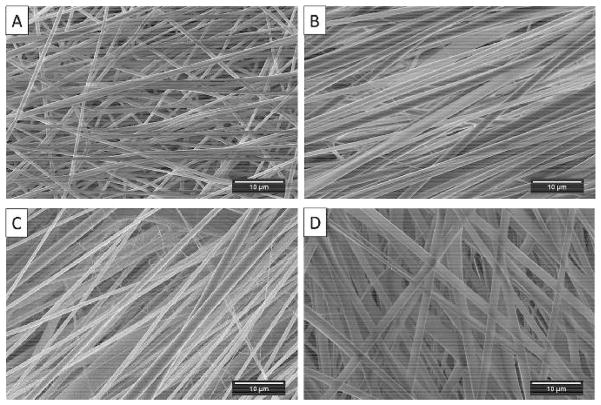
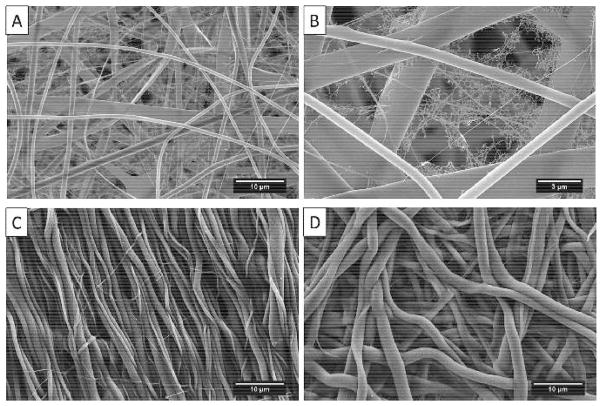
TPU/fibroin solutions in various compositions were prepared and electrospun to investigate the resulting fiber morphology. Compared to the pure TPU SG-93A sample, it was found that the addition of fibroin could improve the alignment of the fibers and create larger fibers (Fig. 4A to D). However, when the ratio of fibroin was higher than TPU, the fiber orientation became less aligned (Fig. 4D). Adding fibroin also induced the formation of ultrafine nanofibers and nanowebs widely distributed on the regular fibers (Fig. 5A). Figure 5B shows a nanoweb observed on the random region of an electrospun sheet with a 1:1 TPU/fibroin composition. After methanol treatment, it was obvious that the fibers swelled and changed to a crimped structure, which was regarded as the effects of retraction of the elongated polymer chains in each fiber (Fig. 5C, D) (Liu et al., 2015).
Fig. 4.
Electrospun samples of (A) pure TPU-93A, (B) TPU-93A/fibroin = 2 : 1, (C) TPU-93A/fibroin = 1 : 1, and (D) TPU-93A/ fibroin = 1 : 2
Fig. 5.
Ultrafine nanofibers and nanowebs distributed on the regular electrospun TPU/ fibroin fibers (A). A nanoweb at a higher magnification (B). The crimped structure of the methanol-treated aligned- (C) and (D) randomly-oriented electrospun fibers
The improved fiber alignment and the formation of ultrafine fibers are considered to be due to the increased conductivity of the solution. Previous studies have shown that the low conductivity of the pure TPU solution was not able to generate enough electric force for alignment in the electric field, and the high flexibility of TPU molecules also increased the bending instability of the fibers (Mi et al., 2015b). By increasing the conductivity, more charges can be carried by the solution, which enhances the electrostatic force on the electrodes and the elongational force applied to the electrospinning jet, leading to a better fiber alignment and ultrafine fiber formation (Pham et al., 2006). Conventional TPU has a volume resistivity that exceeds 108 Ohm·cm; namely, a conductivity lower than 10−8 S·cm−1 (BASF, 2015). The conductivity of a 20% fibroin–formic acid mixture was measured to be 2.07×10−3 S·cm−1 from other research, which was much higher than the 7.5×10−7 S·cm−1 of a 10% TPU-dichloromethane solution (Huang and Liu et al., 2011; Mi et al., 2015b). At the same time, the viscosity of the solution increased visibly with the proportion of fibroin. The higher viscosity of the TPU/fibroin solution with a 1:2 ratio could be the cause of the larger fiber diameter.
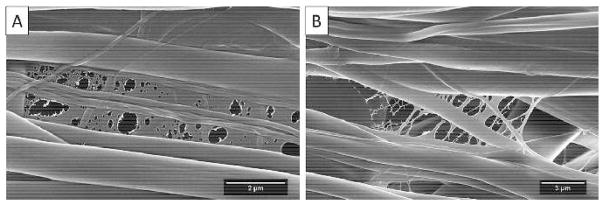
For the nanoweb structure, a similar phenomenon was seen in previous research by Ding et al. (2006) on electrospun poly(-acrylic acid) (PAA) and polyamide-6 mats. It is believed that the fast phase separation of TPU/fibroin and HFIP is the reason for the formation of the nanowebs. The evaporation of HFIP and the rapid solidification of TPU/fibroin during the electrospinning process induced the formation of the 2D porous structure. Figure 6A shows a film with different sizes of circular defects among the random fibers. A finer nanoweb structure can be found in other regions of the same electrospun sheet (Fig. 6B). These nanowebs effectively increased the surface area and were favorable for cell attachment and spreading.
Fig. 6.
The formation of 2D nanowebs was due to the fast phase separation of TPU/fibroin and HFIP. The formed bubbles were larger in (B) than (A) which led to a finer porous structure
5.3 Mechanical Properties
Compatible mechanical properties of natural blood vessels are an essential requirement for vascular grafts. In natural blood vessels, blood flow continuously induces a dynamic mechanical environment with shear stress, pulsatile pressure, and cyclic stretching that highly influences the maintenance and regeneration of vascular tissue (Sonoda et al., 2001). It was reported that the compliance mismatch between the native vessel and implanted vascular graft caused turbulent blood flow and platelet activation, and led to the development of intimal hyperplasia and thrombogenicity which was especially severe for small-diameter grafts (Seifu et al., 2013). Besides compliance, vascular grafts also need to maintain sufficient mechanical support during tissue formation without losing their strength due to material degradation. That means that a structure with higher strength is preferred.
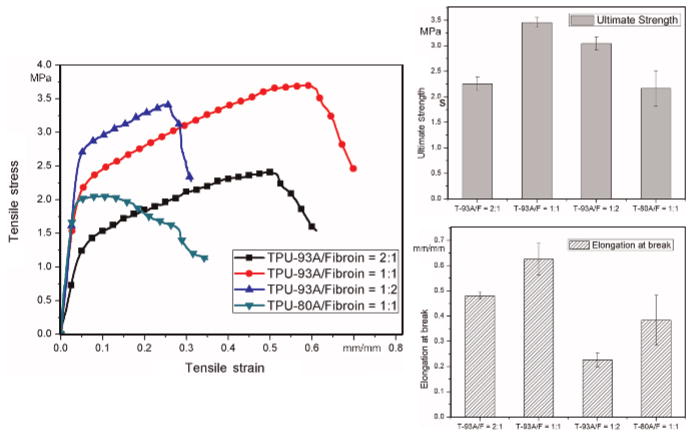
To investigate the characteristics of electrospun TPU/fibroin matrices, tensile tests for randomly-oriented samples were performed to understand how the different compositions affected the mechanical properties. The obtained data provides a preliminary perspective on determining the adequate TPU/fibroin ratio for fabricating vascular grafts. Figure 7 presents the experimental results of the representative stress–strain curve, ultimate tensile strength, and elongation-at-break for each blend. Comparing the first three TPU-93A/fibroin groups, it can be seen that adding fibroin effectively reinforced the strength of the sample, but also reduced the ductility when its content was higher than that of TPU. The slightly lower strength of the TPU-93A/fibroin = 1:2 sample could be contributed to its brittleness; namely, the lower extensibility that hindered it from being stretched farther. TPU-80A is a soft TPU possessing an intrinsically lower strength than TPU-93A. The testing results exhibited these differences distinctly.
Fig. 7.
Mechanical properties of various TPU/fibroin blends including representative stress–strain curves, ultimate tensile strengths, and elongation-at-break values
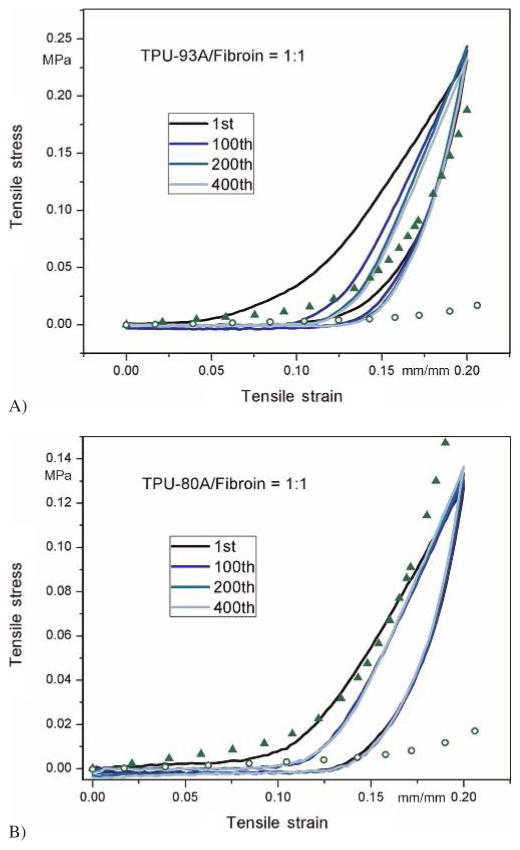
According to the observations above, it was decided that the graft comprised of TPU-93A/fibroin at a 1:1 ratio might be suitable for vascular applications and a further examination of its cyclic mechanical behavior in the circumferential direction was required. This formula displayed better mechanical properties while containing a high amount of fibroin. Grafts made of TPU-80A/fibroin were also inspected for comparison. The stress–strain curves of the 1st, 100th, 200th, and 400th cycles are depicted in Fig. 8. Scatter data points represent the experimental stress–strain range of human coronary arteries from other research (van Andel et al., 2003). In the first cycle, similar to natural soft tissues, the electrospun fibrous structure stretched and loosened without reaching the plastic region. In the rest of the cycles, the mechanical behavior of the tubular sample was almost the same, which ensured its ability for long-term use. However, compared to human coronary arteries, grafts made of TPU-93A/fibroin had a shorter toe region and the curves did not completely fall within the range, indicating that it could have a mismatch compliance problem. For TPU-80A/fibroin, the curves exhibited similar behaviors to human coronary arteries, making it a suitable choice as a TEBV from mechanical aspects.
Fig. 8.
Cyclic tensile behaviors of vascular grafts made from TPU-93A/fibroin (A) and TPU-80A/fibroin (B) at a 1 : 1 ratio. Scatter data points represent the lower and upper bounds of human coronary arteries from experiments (van Andel et al., 2003)
5.4 Cell Culture Results
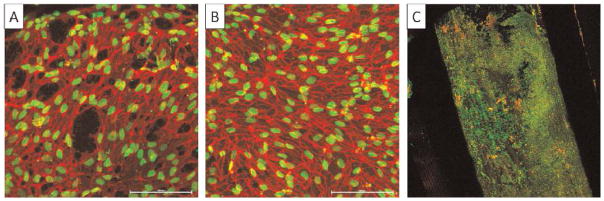
A preliminary assay of cell–matrix interactions was conducted by seeding human endothelial cells on small-diameter TPU/fibroin grafts with or without Matrigel coating. The purpose of applying Matrigel was to create different environments for comparing cell activity on a pure scaffold and one with more similar conditions to the natural extracellular environment. At day 6, although the sample with Matrigel coating had a more uniform cell distribution, the TPU/fibroin sample without Matrigel also showed high cell viability (Fig. 9A, B). At day 60, the pure sample was fully covered with cells and exhibited alignment in certain regions. These test results indicated that living cells were able to attach and migrate on the TPU/fibroin graft to form a thin cell layer, which yielded favorable cellular interactions in the long term.
Fig. 9.
Endothelial cell culture results on electrospun grafts made of TPU-93A/fibroin blends at (A) day 6, (B) day 6 with Matrigel coating, and (C) day 60 (dots: nuclei)
6 Conclusions
Synthetic polymer, TPU, and natural polymer, fibroin, were blended at different ratios and electrospun to fabricate small-diameter vascular grafts. By using a striated collector, an electrospun sheet with an alternatively aligned and random structure was created for producing tubular grafts with adjustable diameters and a layered structure. The morphology, mechanical properties, and biocompatibility were investigated to evaluate the feasibility of using TPU/fibroin grafts for vascular tissue engineering. SEM results showed that the composition of the solution highly influenced the fiber morphology due to variations in conductivity and viscosity. The crimped structure of the methanol-treated fibers could provide a similar microenvironment to natural collagens for cell activity. Mechanical tests revealed that the properties of the TPU/fibroin hybrid grafts were tunable by altering the composition ratio and type of TPU. It was found in the cyclic tensile test that the grafts made from the soft TPU/fibroin solution could achieve similar cyclic behavior to human coronary arteries in the circumferential direction, which is directly related to the pressure exerted on the vessels from blood flow in long-term usage. Endothelial cell culture results also exhibited promising cell viability and bio-compatibility for the TPU/fibroin grafts. Overall, electrospun small-diameter TPU/fibroin grafts have demonstrated the adaptability required in vascular tissue engineering applications. Future studies will focus on further improving the fiber morphology and mechanical properties to mimic natural blood vessels, and a more systematic assay of cell activities on grafts.
Acknowledgments
This research was supported by the Wisconsin Institute for Discovery (WID) and the Morgridge Institute for Research (MIR).
References
- Alessandrino A, Marelli B, Arosio C, Fare S, Tanzi MC, Freddi G. Electrospun Silk Fibroin Mats for Tissue Engineering. Eng Life Sci. 2008;8:219–225. doi: 10.1002/elsc.200700067. [DOI] [Google Scholar]
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-Based Biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/S0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Baguneid MS, Seifalian AM, Salacinski HJ, Murray D, Hamilton G, Walker MG. Tissue Engineering of Blood Vessels. Br J Surg. 2006;93:282–290. doi: 10.1002/bjs.5256. [DOI] [PubMed] [Google Scholar]
- BASF. 2015 Elastollan TPU Electric & Electronics http://www.elastollan.-basf.us/industries/electric.
- Baudis S, Ligon C, Seidler K, Weigel G, Grasl C, Bergmeister H, Schima H, Liska R. Hard-Block Degradable Thermoplastic Urethane-Elastomers for Electrospun Vascular Prostheses. J Polym Sci Part A: Polym Chem. 2012;50:1272–1280. doi: 10.1002/pola.25887. [DOI] [Google Scholar]
- Bergmeister H, Seyidova N, Schreiber C, Strobl M, Grasl C, Walter I, Messner B, Baudis S, Fröhlich S, Marchetti-Deschmann M, Griesser M, di Franco M, Krssak M, Liska R, Schima H. Biodegradable, Thermoplastic Polyurethane Grafts for Small Diameter Vascular Replacements. Acta Biomater. 2015;11:104–113. doi: 10.1016/j.actbio.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Catto V, Farè S, Freddi G, Tanzi MC. Vascular Tissue Engineering: Recent Advances in Small Diameter Blood Vessel Regeneration. ISRN Vascular Medicine. 2014;2014:1–27. doi: 10.1155/2014/923030. [DOI] [Google Scholar]
- Chew SY, Mi R, Hoke A, Leong KW. Aligned Protein–Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv Funct Mater. 2007;17:1288–1296. doi: 10.1002/adfm.200600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CL, Gawlitta D, Nerem RM, Stegemann JP. Properties of Engineered Vascular Constructs Made from Collagen, Fibrin, and Collagen-Fibrin Mixtures. Biomaterials. 2004;25:3699–3706. doi: 10.1016/j.biomaterials.2003.10.073. [DOI] [PubMed] [Google Scholar]
- Ding B, Li C, Miyauchi Y, Kuwaki O, Shiratori S. Formation of Novel 2D Polymer Nanowebs via Electrospinning. Nanotechnology. 2006;17:3685–3691. doi: 10.1088/0957-4484/17/15/011. [DOI] [Google Scholar]
- Enomoto S, Sumi M, Kajimoto K, Nakazawa Y, Takahashi R, Takabayashi C, Asakura T, Sata M. Long-Term Patency of Small-Diameter Vascular Graft Made from Fibroin, a Silk-Based Biodegradable Material. J Vasc Surg. 2010;51:155–164. doi: 10.1016/j.jvs.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Grasl C, Bergmeister H, Stoiber M, Schima H, Weigel G. Electrospun Polyurethane Vascular Grafts: in Vitro Mechanical Behavior and Endothelial Adhesion Molecule Expression. J Biomed Mater Res A. 2010;93:716–723. doi: 10.1002/jbm.a.32584. [DOI] [PubMed] [Google Scholar]
- Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A. Electrospun Scaffolds for Tissue Engineering of Vascular Grafts. Acta Biomater. 2014;10:11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the Future of Cardiovascular Disease in the United States: A Policy Statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. In Vitro Degradation of Silk Fibroin. Biomaterials. 2005;26:3385–3393. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Huang C, Chen R, Ke Q, Morsi Y, Zhang K, Mo X. Electrospun Collagen–Chitosan–TPU Nanofibrous Scaffolds for Tissue Engineered Tubular Grafts. Colloids Surf, B. 2011;82:307–315. doi: 10.1016/j.colsurfb.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu L, Yao J. Electrospinning of Bombyx Mori Silk Fibroin Nanofiber Mats Reinforced by Cellulose Nanowhiskers. Fibers Polym. 2011;12:1002–1006. doi: 10.1007/s12221-011-1002-7. [DOI] [Google Scholar]
- Isenberg BC, Williams C, Tranquillo RT. Small-Diameter Artificial Arteries Engineered in Vitro. Circ Res. 2006;98:25–35. doi: 10.1161/01.RES.0000196867.12470.84. [DOI] [PubMed] [Google Scholar]
- Jing X, Mi HY, Salick MR, Cordie TM, Peng XF, Turng LS. Electrospinning Thermoplastic Polyurethane/Graphene Oxide Scaffolds for Small Diameter Vascular Graft Applications. Mater Sci Eng, C. 2015;49:40–50. doi: 10.1016/j.msec.2014.12.060. [DOI] [PubMed] [Google Scholar]
- Lee CH, Shin HJ, Cho IH, Kang YM, Kim IA, Park KD, Shin JW. Nanofiber Alignment and Direction of Mechanical Strain Affect the ECM Production of Human ACL Fibroblast. Biomaterials. 2005;26:1261–1270. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- Liu H, Li X, Zhou G, Fan H, Fan Y. Electrospun Sulfated Silk Fibroin Nanofibrous Scaffolds for Vascular Tissue Engineering. Biomaterials. 2011;32:3784–3793. doi: 10.1016/j.biomaterials.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Liu W, Lipner J, Moran CH, Feng L, Li X, Thomopoulos S, Xia Y. Generation of Electrospun Nanofibers with Controllable Degrees of Crimping through a Simple, Plasticizer-Based Treatment. Adv Mater. 2015;27:2583–2588. doi: 10.1002/adma.201500329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett M, Cannizzaro C, Daheron L, Messmer B, Vunjak-Novakovic G, Kaplan DL. Silk Fibroin Microtubes for Blood Vessel Engineering. Biomaterials. 2007;28:5271–5279. doi: 10.1016/j.biomaterials.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett M, Eng G, Kluge JA, Cannizzaro C, Vunjak-Novakovic G, Kaplan DL. Tubular Silk Scaffolds for Small Diameter Vascular Grafts. Organogenesis. 2010;6:217–224. doi: 10.4161/org.6.4.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett ML, Cannizzaro C, Vunjak-Novakovic G, Kaplan DL. Gel Spinning of Silk Tubes for Tissue Engineering. Biomaterials. 2008;29:4650–4657. doi: 10.1016/j.biomaterials.2008.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Chen J, Collette AL, Kim UJ, Altman GH, Cebe P, Kaplan DL. Mechanisms of Silk Fibroin Sol-Gel Transitions. J Phys Chem B. 2006;110:21630–21638. doi: 10.1021/jp056350v. [DOI] [PubMed] [Google Scholar]
- Mendis S, Puska P, Norrving B. Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organization in Collaboration with the World Heart Federation and the World Stroke Organization; Geneva: 2011. [Google Scholar]
- Mi HY, Jing X, Salick MR, Cordie TM, Peng XF, Turng LS. Properties and Fibroblast Cellular Response of Soft and Hard Thermoplastic Polyurethane Electrospun Nanofibrous Scaffolds. J Biomed Mater Res Part B. 2015;103:960–970. doi: 10.1002/jbm.b.33271. [DOI] [PubMed] [Google Scholar]
- Mi HY, Salick MR, Jing X, Crone WC, Peng XF, Turng LS. Electrospinning of Unidirectionally and Orthogonally Aligned Thermoplastic Polyurethane Nanofibers: Fiber Orientation and Cell Migration. J Biomed Mater Res Part A. 2015;103:593–603. doi: 10.1002/jbm.a.35208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y, Sato M, Takahashi R, Aytemiz D, Takabayashi C, Tamura T, Enomoto S, Sata M, Asakura T. Development of Small-Diameter Vascular Grafts Based on Silk Fibroin Fibers from Bombyx Mori for Vascular Regeneration. J Biomater Sci Polym Ed. 2011;22:195–206. doi: 10.1163/092050609X12586381656530. [DOI] [PubMed] [Google Scholar]
- Pham QP, Sharma U, Mikos AG. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- Ravi S, Chaikof EL. Biomaterials for Vascular Tissue Engineering. Regen Med. 2010;5:107–120. doi: 10.2217/rme.09.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezakhaniha R, Agianniotis A, Schrauwen JTC, Griffa A, Sage D, Bouten CVC, van de Vosse FN, Unser M, Stergiopulos N. Experimental Investigation of Collagen Waviness and Orientation in the Arterial Adventitia Using Confocal Laser Scanning Microscopy. Biomech Model Mechanobiol. 2012;11:461–473. doi: 10.1007/s10237-011-0325-z. [DOI] [PubMed] [Google Scholar]
- Rockwood DN, Preda RC, Yücel T, Wang X, Lovett ML, Kaplan DL. Materials Fabrication from Bombyx Mori Silk Fibroin. Nat Protoc. 2011;6:1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifu DG, Purnama A, Mequanint K, Mantovani D. Small-Diameter Vascular Tissue Engineering. Nat Rev Cardiol. 2013;10:410–421. doi: 10.1038/nrcardio.2013.77. [DOI] [PubMed] [Google Scholar]
- Soffer L, Wang X, Zhang X, Kluge J, Dorfmann L, Kaplan DL, Leisk G. Silk-Based Electrospun Tubular Scaffolds for Tissue-Engineered Vascular Grafts. J Biomater Sci Polym Ed. 2008;19:653–664. doi: 10.1163/156856208784089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda H, Takamizawa K, Nakayama Y, Yasui H, Matsuda T. Small-Diameter Compliant Arterial Graft Prosthesis: Design Concept of Coaxial Double Tubular Graft and its Fabrication. J Biomed Mater Res. 2001;55:266–276. doi: 10.1002/1097-4636(20010605)55:3<266::AID-JBM1014>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Swartz DD, Russell JA, Andreadis ST. Engineering of Fibrin-Based Functional and Implantable Small-Diameter Blood Vessels. Am J Physiol Heart Circ Physiol. 2005;288:H1451–H1460. doi: 10.1152/ajpheart.00479.2004. [DOI] [PubMed] [Google Scholar]
- Tiwari A, Salacinski H, Seifalian AM, Hamilton G. New Prostheses for Use in Bypass Grafts with Special Emphasis on Polyurethanes. Cardiovasc Surg. 2002;10:191–197. doi: 10.1016/S0967-2109(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Van Andel CJ, Pistecky PV, Borst C. Mechanical Properties of Porcine and Human Arteries: Implications for Coronary Anastomotic Connectors. Ann Thorac Surg. 2003;76:58–64. doi: 10.1016/S0003-4975(03)00263-7. Discussion 64–5. [DOI] [PubMed] [Google Scholar]
- Wang H, Feng Y, Fang Z, Yuan W, Khan M. Co-Electrospun Blends of PU and PEG as Potential Biocompatible Scaffolds for Small-Diameter Vascular Tissue Engineering. Mater Sci Eng, C. 2012;32:2306–2315. doi: 10.1016/j.msec.2012.07.001. [DOI] [Google Scholar]
- Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem Cell-Based Tissue Engineering with Silk Biomaterials. Biomaterials. 2006;27:6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Weinberg CB, Bell E. A Blood Vessel Model Constructed from Collagen and Cultured Vascular Cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- Williamson MR, Black R, Kielty C. PCL–PU Composite Vascular Scaffold Production for Vascular Tissue Engineering: Attachment, Proliferation and Bioactivity of Human Vascular Endothelial Cells. Biomaterials. 2006;27:3608–3616. doi: 10.1016/j.biomaterials.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Wise SG, Byrom MJ, Waterhouse A, Bannon PG, Weiss AS, Ng MK. A Multilayered Synthetic Human Elastin/Polycaprolactone Hybrid Vascular Graft with Tailored Mechanical Properties. Acta Biomater. 2011;7:295–303. doi: 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- World Health Organization. CVDs. 2015 http://www.who.int/mediacentre/factsheets/fs317/en/
- Wu HC, Wang TW, Kang PL, Tsuang YH, Sun JS, Lin FH. Coculture of Endothelial and Smooth Muscle Cells on a Collagen Membrane in the Development of a Small-Diameter Vascular Graft. Biomaterials. 2007;28:1385–1392. doi: 10.1016/j.biomaterials.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Zdrahala RJ, Zdrahala IJ. Biomedical Applications of Polyurethanes: A Review of Past Promises, Present Realities, and a Vibrant Future. J Biomater Appl. 1999;14:67–90. doi: 10.1177/088532829901400104. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Liu W, Cui L, Cao Y. Tissue Engineering of Blood Vessel. J Cell Mol Med. 2007;11:945–957. doi: 10.1111/j.1582-4934.2007.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Baughman CB, Kaplan DL. In Vitro Evaluation of Electrospun Silk Fibroin Scaffolds for Vascular Cell Growth. Biomaterials. 2008;29:2217–2227. doi: 10.1016/j.biomaterials.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]