1. Introduction
Temporomandibular disorder involves the muscles of mastication, the temporomandibular joint (TMJ), and/or associated orofacial structures [42]. Clinical manifestations include limited jaw movement, noises or locking in the TMJ, and pain aggravated by jaw function, the latter being a necessary criterion for diagnoses of myalgia and TMJ arthralgia [51]. TMD affects 6–10% of women and 3% of men in the US population [24,25], making it the most common type of orofacial pain. Chronic TMD produces substantial disability and suffering and diminishes quality of life [9]. In addition, it is associated with increased unemployment and decreased work effectiveness [11,37,60]. This societal burden potentially could be prevented if risk factors for TMD were better understood.
TMD is often comorbid with headache although the estimated degree of overlap varies among studies. In a recent analysis of the US National Health Interview Survey, the population prevalence of TMD-related pain was much higher among individuals with severe headache (15.6%) than those without severe headache (2.6%) [44]. Among patients seeking treatment for headache, Korean and Danish studies reported a strikingly high rate of TMD: 51.6% and 56.1%, respectively [7,27]. While TMD symptoms in a large Brazilian population study were more common in individuals with all types of headache compared to headache-free people, the highest association was observed with migraine [17]. Similarly, several reports confirmed that particular types of headache are frequently comorbid with TMD. Specifically, among orofacial pain patients in a US study, 61.3% reported any headache and 38% reported migraine with or without aura [10]. In an Italian population-based study, headache prevalence was significantly higher among individuals with TMD relative to TMD-free persons (27.4% vs. 15.2%) [8] while a greater difference was observed in a US study that compared TMD patients with general dental patients (72.7% vs. 31.9%) [40]. The variability in TMD and headache prevalence rates across studies is likely due to methodologic factors such as the methods for ascertaining the diagnostic criteria and sample characteristics such as population or clinic settings.
Although many reports demonstrate a relation between headache and TMD, most evidence comes from studies using cross-sectional designs which cannot determine a causal relation. Only prospective cohort studies offer an opportunity to measure exposure to a hypothesized risk factor (in this instance, headache) prior to onset of an index disease (in this case, TMD) and therefore establish a temporal sequence between putative cause and effect, necessary for confirming causal inference [22]. To overcome limitations of the previous cross-sectional reports, the present manuscript used data from the large-scale prospective cohort study of first-onset TMD. This study was a part of the project entitled “Orofacial Pain: Prospective Evaluation and Risk Assessment” (OPPERA). The objectives of our study were to: 1) Describe the prevalence of headache in a community-based sample of U.S. adults at their time of enrollment; 2) Evaluate contribution of headache to the risk of developing first-onset TMD during prospective follow-up of the participants; and 3) Describe the dynamic patterns of headache types at baseline and during follow-up of the participants.
2. Methods
This paper reports findings from the multisite OPPERA project. Institutional Review Boards at each OPPERA study site approved the study and each enrollee signed informed consent to participate. Study methods have been described in detail elsewhere [6,55,56] and are summarized below.
2.1. Study design and setting
Data in this paper are from two study designs used to investigate incidence of first-onset temporomandibular disorder (TMD) in a sample of U.S. adults: 1) the OPPERA prospective cohort study enrolled community-based volunteers who had no history of TMD and followed them for up to five years in order to identify incident cases of TMD; and 2) the OPPERA nested case-control study of TMD incidence collected additional follow-up data from those incident cases and from a similar number of controls selected at random from members of the prospective cohort study who remained free of TMD.
2.2. Study participants and measurements
The prospective cohort study enrolled 3,263 volunteers between May 2006 and November 2008 at four U.S. study sites: Baltimore, MD; Buffalo, NY; Chapel Hill, NC; and Gainesville, FL. Volunteers were recruited from communities surrounding each study site using advertisements, flyers, and email. To be eligible for enrollment, volunteers had to satisfy selection criteria determined during telephone screening and at the baseline clinic visit.
2.2.1. Telephone screening
Potential participants were interviewed by telephone to establish that they met all nine of the following screening eligibility criteria: 1) aged 18–44 years; 2) no significant history of TMD symptoms; 3) never diagnosed with TMD; 4) no use of an occlusal splint during sleep; 5) no recent history of facial injury or surgery; 6) no significant medical illnesses; 7) not pregnant or nursing; 8) not receiving orthodontic treatment; and 9) <5 headaches per month within each of the preceding three months. The latter was determined by asking “Considering headaches of all types in the past 3 months, how many headaches on average per month have you had?” People who reported ≥5 headaches per month were excluded because there was a suspected higher likelihood that they would have failed to meet the clinical examination criterion of no significant TMD-related pain history, described below. Potential study participants who satisfied all screening criteria were mailed questionnaires and they were invited to attend their local study site for a baseline clinic visit.
2.2.2. Baseline headache questionnaire
One of the mailed questionnaires was the Comprehensive Pain Symptom Questionnaire (CPSQ) which asked detailed questions about headaches (see Appendix A of [41]). The CPSQ was completed at home, prior to the baseline clinic visit, either on paper or online. The three pages of questions about headache first asked “In the past year, how many different types of headaches have you had (e.g., stress or tension-type, migraine, hunger headache, sinus headache)?” Details of up to three different types of headaches were then reported in separate sections. Each section asked about location, intensity, characteristics, duration, frequency, and aggravating factors associated with each type of headache.
2.2.3. Baseline clinic visit
Trained examiners applied the Research Diagnostic Criteria for TMD (RDC/TMD) [12] to exclude anyone who had myalgia and/or arthralgia in the masticatory structures. The two criteria required for TMD classification were: 1) history of pain in examiner-verified masticatory tissues on ≥5 days of the preceding 30 days; and 2) pain in masticatory tissues evoked by standardized jaw movements or examiner-palpation of the masticatory muscles and temporomandibular joints. Also during the visit, participants completed questionnaires, underwent quantitative sensory testing, provided cardiovascular measures of autonomic function, and provided a blood sample.
2.2.4. Quarterly follow-up questionnaires and second clinic visit
At quarterly (i.e., three-monthly) intervals after baseline, participants completed a questionnaire that included a single question about headache frequency: “In the last 30 days, how many headaches of any type have you had?” and they recorded a number or marked “Don’t know.” The questionnaire also asked about TMD symptoms, and those responding positively were invited to return to study site clinics for a follow-up examination. Before the second clinic visit, questionnaires used at enrollment were mailed for completion at home, including the CPSQ that was used to classify headache again. During the second clinic visit, examiners used the same RDC/TMD criteria, and incident TMD was classified when examiners classified myalgia and/or arthralgia.
Follow-up data collection continued for each participant until clinical TMD was classified or until censoring (i.e., the study closeout date of May 31, 2011, or, for people lost to follow-up, the date of the final follow-up questionnaire).
2.2.5. Nested case-control study of TMD incidence
As each incident TMD case was identified, one TMD-free control was sampled at random from the cohort and invited to visit the study site clinic for follow-up data collection. This included the CPSQ and the RDC/TMD examination to verify absence of clinical TMD. Controls were selected to match cases according to study site, gender, and time-in-study [56].
2.3. Variables used in this analysis
For demographic group comparisons, responses to the screening interview were used to create three age groups (18–24, 25–34, and 35–44 years), two genders (male, female), and five race-ethnicity groups (White, African-American, Hispanic, Asian, and Other).
For headache data collection, CPSQ questions were based on the diagnostic criteria specified in the 2nd edition of the International Classification of Headache Disorders (ICHD-2) [21] which was current when the OPPERA project began (Supplementary Table 1). However, for this publication, we developed a new algorithm that, to the extent possible, used responses from the existing CPSQ questions to classify study participants according to the latest ICHD-3 beta criteria [1]. This algorithm updates the one used previously in the OPPERA study [49] for a three-level classification of headache. The current algorithm is restricted to classification of only primary headaches; an adequate inquiry to exclude secondary headaches of all causes was not part of the CPSQ. Self-reported headaches were classified into one of 7 types: no headache, unclassified headache, probable tension-type headache (TTH), definite TTH, probable migraine, definite migraine, and mixed headache. The “no headache” type comprised participants who did not report any headache in the preceding year. “Unclassified headache” included participants who reported headache in the last year but did not provide a complete set of data necessary for classification in the other types or did not meet the minimum ICHD criteria for even probable TTH or migraine. “Probable TTH” was determined when all but one ICHD criterion for TTH were satisfied, and “definite TTH” was identified when all ICHD criteria for TTH were met. As migraine with aura is difficult to determine by questionnaire [58], migraine classification was limited to migraine without aura. “Probable migraine” was defined when all but one ICHD criterion for migraine without aura were fulfilled, and “definite migraine” was classified if all ICHD criteria for migraine without aura were met. When a single headache type met more than one set of criteria, classification was made according to the hierarchical sequence recommended by ICHD-3 beta [21], namely: precedence was given to definite migraine when all criteria were met for this headache type, then to definite TTH over probable migraine and probable TTH, and, finally, to probable migraine over probable TTH. When participants reported more than one type of headache among the three types that could be reported, a similar hierarchical approach was applied to these multiple classifications, except when ICHD criteria, for each of two headache types, were satisfied for both definite TTH and definite migraine without aura, in which case the classification was “mixed headache” (Supplementary Table 2). For some analyses, headache types were collapsed into 4 broader categories by combining 2 similar types together, e.g., probable migraine and definite migraine were combined into a category of “migraine.”
For analysis of TMD incidence, the binary outcome variable was the clinical classification of incident TMD, and time-in-study was measured in days as the period from baseline to either censoring or the visit when incident TMD was classified.
2.4 Test-retest reliability of headache classification
The test-retest reliability of the headache classification was assessed in a separate methodological study in which the CPSQ questionnaire was administered twice to each of 108 study participants. These participants were selected from the OPPERA baseline case-control study based on either presence (n=52) or absence of chronic TMD (n=56). The median interval between questionnaire administrations was 3 days (range = 1 to 8 days). Given that there are 250 data items in the CPSQ, we reasoned that this interval represented a sufficient period for participants to avoid recalling their first set of answers while minimizing the possibility that their symptom status of any type might change. The algorithm described above was used to make six binary classifications, one for each type of ICHD headache. Kappa values of test-retest reliability for the binary classifications ranged from 0.50 (migraine) to 0.54 (TTH). The weighted kappa statistic for the 7-category hierarchical headache classification was 0.62. These all represent acceptable reliability [14]. The exception was the binary classification of mixed headache where the kappa value of 0.33 signified poor reliability. Kappa for the binary classification of any headache was 0.81 indicating excellent reliability for this global classification.
2.5. Statistical methods
Data from baseline and follow-up were used to address the descriptive objectives of the study. At baseline, annual TMD incidence rate were calculated for all participants and for each demographic group, headache category, and headache frequency. The annual TMD incidence rate was calculated as the number of participants with first-onset TMD divided by person-years of follow-up. It was estimated using a Poisson regression model that adjusted for OPPERA study site.
The second aim was addressed using data from baseline and quarterly follow-up questionnaires in the prospective cohort study. Because there were censored observations and different follow-up periods, Cox proportional hazard models were used to evaluate contributions of headache to risk of developing first-onset TMD. Repeated measurements of headache frequency in quarterly questionnaires were analyzed as time-varying covariates, and the effects of time were investigated using two strategies for time-to-event data [4]:
In the concurrent method, the time-varying predictor variable was headache frequency reported in the questionnaire completed at the same quarterly follow-up at which time and censoring status (i.e., TMD incidence) were recorded. For the last quarter among incident cases, the 30-day reference period of the headache frequency question overlapped the 30-day reference period used to determine clinical TMD at that follow-up. This creates potential for reverse causation (i.e., clinical TMD causing headache).
The “lagged” method addresses that problem by using headache frequency reported in the questionnaire that preceded the quarter used in the concurrent method. Because all participants, including incident cases, were TMD-free in the lagged quarter, this variable precludes the possibility of reverse causation.
Separate Cox models were created for each method, and each model included time-constant covariates of demographics and study site. Overall model fit was judged using the likelihood ratio test, while effects of individual predictors were quantified as hazard ratios and their 95% confidence interval (95% CI). In these models, time-varying headache frequency was transformed from the count of headaches to z-scores. This allowed hazard ratios to be interpreted consistently as the relative effect on the TMD incidence rate associated with a one standard deviation increase in headache frequency. Because lagged quarters could be computed only for participants who completed at least two quarterly follow-up questionnaires, all models were limited to participants who completed ≥2 follow-up questionnaires.
Follow-up data from the nested case-control study were used to address the third aim of the study. Headache frequency was plotted at four time points: 1) the first quarter; 2) intermediate quarters, defined as follow-up questionnaires completed after the first quarter but before the penultimate quarter; 3) the penultimate quarter, defined as the follow-up questionnaire completed three months before the final quarter; and 4) the final quarter, terminated by TMD onset or censoring. At each time point, mixed models for repeated measures were used to estimate odds of reporting any headache vs. none and the mean number of headaches per month. Predictor variables were time (4 categories), incident TMD case-classification (two categories), and their two-way interaction. Covariates were study site and the demographic characteristics. Changes in headache types were analyzed using data from the CPSQ completed at baseline and the follow-up in the nested case-control study. Contingency tables cross-classified study participants according to the seven headache types reported at baseline and follow-up. The degree of change was expressed as the percentage of participants whose headache type had changed, and Bowker’s test of symmetry for matched data (baseline and follow-up) was used to evaluate the null hypothesis of no change.
When headache types were collapsed to dichotomies, the degree of change was expressed as the odds ratio for matched pairs and McNemar’s test was used to evaluate the null hypothesis of no change. This was done separately for incident TMD cases and controls using three dichotomies of headache types for each group. The dichotomies were based on the following thresholds, with the hierarchically-lower types of headache than the named type in each dichotomy forming the comparison group: a) probable TTH or worse (any ICHD-classified headache disorder), b) definite TTH or worse, and c) probable migraine or worse. The odds ratio represents the odds of headache at follow-up relative to baseline. Odds ratios exceeding 1.0 signify a net increase in occurrence at follow-up relative to baseline, whereas odds ratios less than 1.0 signify a net decrease in occurrence. For clarity, we therefore use the label “progression/remission” to describe this odds ratio, emphasizing that it quantifies temporal changes in headache over time, not an association between headache and incident TMD case status. Specifically, “progression” refers to a headache transition to any hierarchically-higher type, while “remission” refers to a change to any hierarchically-lower type including complete absence of headache. All analyses were performed using SAS, version 9.3(NC, USA).
2.5. Sample size justification
In the OPPERA prospective cohort study, the target sample size of 3,200 enrollees was expected to yield 196 first-onset TMD cases during a three-year follow-up period. Calculations made when designing the study indicated that those numbers would provide 80% statistical power to detect risk ratios of at least 1.8 for risk predictors with as few as 15% of people in the high-risk category [6].
3. Results
3.1. Characteristics of participants
Of 3,263 enrollees in the prospective cohort study, 2,410 participants provided at least two follow-up questionnaires, completing a median of 10 quarterly follow-up questionnaires during follow-up periods ranging from 0.34 to 5.2 years (Supplementary Figure 1). At follow-up examinations, 199 participants had examiner-verified TMD, representing an average incidence rate of TMD of 2.8% per year (Table 1). Although the incidence rate did not vary appreciably among demographic groups, it differed among categories of baseline headache and categories of headache frequency. Based on non-overlap of 95% CIs, the TMD incidence rate was higher for mixed headache (8.4% per year) and for a headache frequency category of 4 headaches per month (6.0% per year).
Table 1.
Characteristics of participants in the OPPERA prospective cohort study (n = 2,410) and nested case-control study (n = 439)
| Prospective cohort study | Nested case-control study | ||||
|---|---|---|---|---|---|
|
| |||||
| Number of subjects (column %) | Number of QHUs per person (median) | TMD incidence rate* (% of people per year) (95% CI) | TMD cases: number of subjects (column %) | TMD-free controls: number of subjects (column %) | |
| All subjects | 2410 (100.0) | 10 | 2.8 (2.4, 3.2) | 248 (100.0) | 191 (100.0) |
| Age (years) | |||||
| 18–24 | 1266 (52.5) | 10 | 2.1 (1.7, 2.7) | 107 (43.2) | 92 (48.2) |
| 25–34 | 654 (27.1) | 11 | 2.9 (2.3, 3.8) | 76 (30.7) | 49 (25.7) |
| 35–44 | 490 (20.3) | 9 | 3.2 (2.4, 4.3) | 65 (26.2) | 50 (26.2) |
| Gender | |||||
| Male | 967 (40.1) | 9 | 2.2 (1.7, 2.8) | 87 (35.1) | 69 (36.1) |
| Female | 1443 (59.9) | 10 | 2.8 (2.4, 3.4) | 161 (64.9) | 122 (63.9) |
| Race-ethnicity | |||||
| White | 1324 (54.9) | 11 | 2.5 (2.0, 3.1) | 135 (54.4) | 99 (51.8) |
| African-American | 627 (26.0) | 8 | 3.4 (2.5, 4.4) | 79 (31.9) | 47 (24.6) |
| Asian | 228 (9.5) | 10 | 1.1 (0.5, 2.1) | 9 (3.6) | 24 (12.6) |
| Hispanic | 157 (6.5) | 10 | 2.2 (1.3, 3.8) | 18 (7.3) | 17 (8.9) |
| Other | 74 (3.1) | 11 | 2.7 (1.3, 5.7) | 7 (2.8) | 4 (2.1) |
| Headache classification at baseline | |||||
| No/unclassified headache | 839 (34.8) | 10 | 2.1 (1.6, 2.8) | 75 (30.2) | 62 (32.5) |
| TTH | 1310 (54.4) | 10 | 2.6 (2.1, 3.2) | 129 (52.0) | 109 (57.1) |
| Migraine | 248 (10.3) | 10 | 3.7 (2.6, 5.3) | 40 (16.1) | 16 (8.4) |
| Mixed headache | 13 (0.5) | 12 | 8.4 (3.1, 22.5) | 4 (1.6) | 4 (2.1) |
| Headache frequency at baseline | |||||
| 0 headache per month | 1117 (46.4) | 10 | 2.1 (1.6, 2.6) | 94 (37.9) | 90 (47.1) |
| 1 headache per month | 610 (25.3) | 11 | 2.2 (1.6, 3.0) | 49 (19.8) | 48 (25.1) |
| 2 headache per month | 367 (15.2) | 10 | 3.2 (2.3, 4.4) | 46 (18.6) | 30 (15.7) |
| 3 headache per month | 173 (7.2) | 10 | 3.6 (2.3, 5.7) | 25 (10.1) | 12 (6.3) |
| 4 headaches per month | 143 (5.9) | 8 | 6.0 (4.0, 8.9) | 34 (13.7) | 11 (5.8) |
Abbreviations: TTH = tension-type headache, QHU = quarterly health update, TMD = temporomandibular disorder, 95% CI = 95% confidence interval.
TMD incidence rate is computed using Poisson regression adjusted for study site (4 categories).
In the nested case-control study, there were 248 incident TMD cases and 191 TMD-free controls (Table 1). The number of incident cases in the nested case-control study is larger than analyzed for the prospective cohort study because additional cases that provided less than two valid follow-up questionnaires were included in the analysis for the case-control study. While cases and controls did not differ appreciably in distribution of demographic characteristics, migraine at baseline occurred more commonly in cases than controls. Likewise, cases were about twice as likely as controls to report 3 or 4 headaches per month at baseline. For mixed models presented in Figure 1, data analysis was restricted to 361 participants (196 incident TMD cases and 165 TMD-free controls) who completed at least two follow-up questionnaires and provided a valid report of headache frequency.
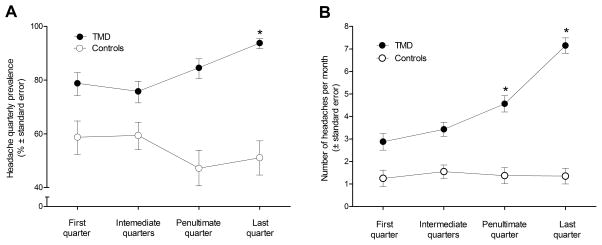
Fig. 1.
Headache characteristics at four time points prior to follow-up visit for incident TMD cases (●) and TMD-free controls (○) in the OPPERA nested case-control study (n=361): (1A) headache quarterly prevalence and (1B) adjusted means for headache monthly frequency were calculated from mixed models. Asterisks denote statistically significant difference (P<0.0001) between the first quarter and subsequent time points for TMD cases. TMD cases significantly differed from controls at all time points (P<0.0001 for headache prevalence and P≤0.0002 for headache frequency).
3.2. Headache as a risk factor for first-onset TMD in the prospective cohort study
Among the 2,410 participants in the prospective cohort study, headache at baseline was a significant predictor of TMD incidence (Table 2). Of the 4 baseline headache categories, migraine and mixed headache were significant predictors of TMD incidence in Model 1 (HR=1.67, 95% CI: 1.06, 2.62; and HR=4.11, 95% CI: 1.47, 11.46; respectively). When baseline headache frequency was instead used as the predictor, there was a dose-response gradient of HRs which increased monotonically from 1.11 (95% CI: 0.76, 1.63), for those with 1 headache per month, to 3.09 (95% CI: 1.94, 4.94), for those with 4 headaches per month. Addition of time-varying headache frequency calculated using the concurrent quarter dramatically increased the chi-square statistic of model fit from 74.3 (Model 2) to 263.5 (Model 3) and the corresponding hazard ratio for concurrent time-varying headache frequency was statistically significant (HR=1.53, 95% CI: 1.46, 1.6; Model 3). When lagged time-varying headache frequency was instead added to Model 2 in order to avoid potential for reverse causation, it too was a significant predictor (HR=1.36, 95% CI: 1.28, 1.45; Model 4).
Table 2.
Multivariable-adjusted* Cox regression models of time-constant and time-varying influences of headache on hazard of first-onset TMD in the OPPERA prospective cohort study (n = 2,410)
| Model | LR test chi-square | df | P value | Predictor | Hazard Ratio (95% CI) |
|---|---|---|---|---|---|
| 1. Headache type at baseline | 60.0 | 12 | <0.0001 | No and unclassified headache (reference) | - |
| TTH | 1.20 (0.87, 1.66) | ||||
| Migraine | 1.67 (1.06, 2.62) | ||||
| Mixed headache | 4.11 (1.47, 11.46) | ||||
| 2. Headache frequency at baseline (number of headaches per month) | 74.3 | 13 | <0.0001 | 0 headache (reference) | - |
| 1 headache | 1.11 (0.76, 1.63) | ||||
| 2 headaches | 1.57 (1.04, 2.37) | ||||
| 3 headaches | 1.80 (1.08, 3.01) | ||||
| 4 headaches | 3.09 (1.94, 4.94) | ||||
| 3. Headache frequency at baseline + time-varying headache frequency | 263.5 | 14 | <0.0001 | 0 headache (reference) | - |
| 1 headache | 0.96 (0.65, 1.42) | ||||
| 2 headaches | 1.37 (0.91, 2.07) | ||||
| 3 headaches | 1.26 (0.75, 2.12) | ||||
| 4 headaches | 1.78 (1.10, 2.90) | ||||
| Time-varying headache frequency | 1.53 (1.46, 1.60)† | ||||
| 4. Headache frequency at baseline + time-varying lagged headache frequency | 137.6 | 14 | <0.0001 | 0 headache (reference) | - |
| 1 headache | 1.04 (0.71, 1.54) | ||||
| 2 headaches | 1.42 (0.94, 2.14) | ||||
| 3 headaches | 1.47 (0.88, 2.48) | ||||
| 4 headaches | 2.13 (1.31, 3.48) | ||||
| Time-varying lagged headache frequency | 1.36 (1.28, 1.45)† |
Abbreviations: LR = Likelihood Ratio, df = degrees of freedom, 95% CI = 95% confidence interval.
All models are adjusted for study site (4 categories), gender (2 categories), race/ethnicity (5 categories), and age (continuous measure).
This hazard ratio expresses the hazard associated with an increase by one standard deviation (3 headaches per month) in time-varying headache frequency.
3.3. Headache contribution to developing first-onset TMD in the nested case-control study of incident TMD cases and TMD-free controls
The temporal patterns in headache prevalence during follow-up in the nested case-control study differed between incident TMD cases and controls (Figure 1A). The mixed model used to evaluate those temporal patterns revealed significant main effects of case status (P<0.0001) and time (P=0.007), and their interaction (P<0.0001). Relative to TMD-free controls, incident TMD cases were more likely to report a headache at all four time points prior to the TMD onset (Figure 1A). When contrasts were performed to compare headache prevalence at each time point within TMD and control groups, the prevalence of headache was significantly greater among TMD cases at the last quarter (94%) relative to the first quarter (79%), while the prevalence among controls did not differ across all four time points (50–60%).
In the mixed model evaluating the temporal patterns in number of headaches per month, there were likewise significant main effects of case status (P<0.0001) and time (P<0.0001), and their interaction (P<0.0001). Relative to controls, incident TMD cases reported significantly (P≤0.0002) higher frequency of headache at all four time points prior to the TMD onset (Figure 1B). When contrasts were performed to compare headache frequency at each time point within TMD and control groups, headache frequency was greater among TMD cases at the penultimate and last quarters relative to the first quarter, while it did not differ across all four time points for controls. For TMD cases, headache frequency increased from 3 headaches per month at the first quarter to 7 headaches per months at the last quarter, while for controls headache frequency stayed around 1 headache per month.
3.4. Dynamic patterns of headache type change during follow-up in the nested case-control study
Results of baseline and follow-up headache classification for incident TMD cases and TMD-free controls participating in the nested case-control study (n=439) are presented in Table 3. In controls, the percentage reporting no headache decreased by one third, from 28.8% to 19.9%, whereas in TMD cases, it reduced by two thirds, from 20.2% to 6.5%. Meanwhile, the prevalence of any migraine in controls increased marginally, from 8.4% to 12.0%, whereas in TMD cases, it nearly doubled, from 16.1% to 28%. The most dramatic escalation was noted in prevalence of definite migraine, which increased nearly ten-fold from 1.2% to 9.7% among TMD cases.
Table 3.
Headache classification at baseline and follow-up in the OPPERA nested case-control study (n = 439)
| Headache classification | TMD cases: number of subjects (column %) | TMD-free controls: number of subjects (column %) | ||
|---|---|---|---|---|
|
| ||||
| Baseline | Follow-up | Baseline | Follow-up | |
| No headache | 50 (20.2) | 16 (6.5) | 55 (28.8) | 38 (19.9) |
| Unclassified headache | 25 (10.1) | 32 (12.9) | 7 (3.7) | 13 (6.8) |
| Any TTH | 129 (52.0) | 124 (50.0) | 109 (57.1) | 114 (59.7) |
| Probable TTH | 55 (22.2) | 51 (20.6) | 48 (25.1) | 42 (22.0) |
| Definite TTH | 74 (29.8) | 73 (29.4) | 61 (31.9) | 72 (37.7) |
| Any migraine | 40 (16.1) | 71 (28.6) | 16 (8.4) | 23 (12.0) |
| Probable migraine | 37 (14.9) | 47 (19.0) | 15 (7.9) | 20 (10.5) |
| Definite migraine | 3 (1.2) | 24 (9.7) | 1 (0.5) | 3 (1.6) |
| Mixed headache | 4 (1.6) | 5 (2.0) | 4 (2.1) | 3 (1.6) |
Abbreviations: TTH = tension-type headache, TMD = temporomandibular disorder.
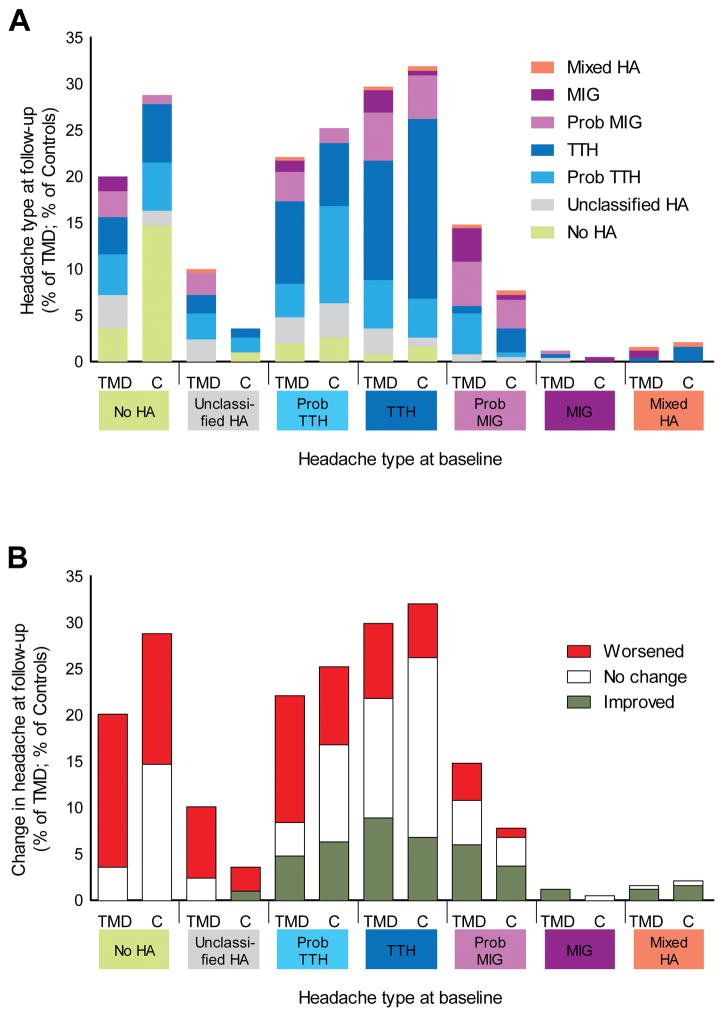
The more detailed investigation of headache types across the follow-up periods revealed markedly different patterns of headache change in TMD cases compared to controls (Figure 2A). In comparison to TMD cases, a much higher percentage of controls with no headache at baseline remained headache-free at follow-up (14.7% vs. 3.6% for the cases). Progression to any migraine was observed from all hierarchically-lower types of headache and was greater for TMD cases relative to controls: from no headache, 4.4% vs.1%; from unclassified headache, 2.4% vs. 0%; from probable TTH, 4.4% vs. 1.6%; and from definite TTH, 7.6% vs. 5.2%.
Fig. 2.
Temporal dynamics of headache for incident TMD cases and TMD-free controls in the OPPERA nested case-control study (n=439). (2A) Distribution of headache types at follow-up visit in TMD cases and controls. Denominator for percentages of TMD cases is n=248 subjects who developed TMD between baseline and follow-up. Denominator for percentages of controls is n=191 subjects who remained TMD-free at baseline and follow-up. (2B) The same data from 2A with patterns of change between baseline and follow-up collapsed to signify worsening, no change, or improvement in headache types. Abbreviations: TMD = temporomandibular disorder, C = TMD-free controls, HA = headache, MIG = migraine, TTH = tension-type headache, Prob. = probable.
Differences between TMD cases and controls became more notable when the same data were reduced to three possible patterns of change that assumed a gradient of severity across headache types (Figure 2B). In general, controls showed greater stability in headache types relative to TMD cases. Specifically, classification of probable TTH was stable in 10.5% of controls vs. 3.6% of TMD cases, classification of definite TTH was stable in 19.4% of controls vs. 12.9% of TMD cases, and classification of probable migraine was similarly stable in both groups (3.1% of controls vs. 4.6% of TMD cases). Due to the small number of cases meeting criteria for definite migraine and for mixed headache at baseline, their degree of stability or change could not be precisely quantified. Another general trend was a greater tendency for headache “worsening” (i.e., progression) among TMD cases compared to controls, regardless of the type of headache at baseline. Specifically, baseline unclassified headache worsened in 7.7% of TMD cases vs. 2.6% of controls, probable TTH worsened in 13.7% of TMDs vs. 8.4% of controls, definite TTH worsened in 8.1% of TMDs vs. 5.8% of controls, and probable migraine worsened in 4% of TMDs vs. 1% of controls. The overall statistical significance of these temporal changes was confirmed using Bowker’s test of symmetry for matched contingency tables which revealed a significant degree of change among incident TMD cases (chi-square=99.7, P<0.0001) but not among TMD-free controls (chi-square=19.2, P>0.05).
When change was investigated for binary categories of headache using the three different thresholds as cut-points, as described under data analysis, incident TMD cases had statistically significant increases for all three thresholds of dichotomizing the headache hierarchy (P≤0.002, Table 4), with progression/remission odds ratios ranging from 1.9 (95% CI: 1.2, 2.8) for definite TTH threshold to 2.8 (95% CI: 1.6, 4.8) for probable migraine threshold. In contrast, among TMD-free controls, there was significant change only at a threshold of definite TTH, with a progression/remission odds ratio of 2.1 (95% CI: 1.2, 3.9; Table 4).
Table 4.
Variation among TMD cases and TMD-free controls in headache prognosis measured at dichotomized headache categories in the OPPERA nested case-control study (n = 439)
| Dichotomized headache categories | TMD cases | TMD-free controls | ||
|---|---|---|---|---|
|
| ||||
| McNemar’s test chi-square (p-value) | Progression/remission OR (95% CI) | McNemar’s test chi-square (p-value) | Progression/remission OR (95% CI) | |
| Any classified headache vs. the rest | 9.7 (0.002) | 2.1 (1.3, 3.5) | 2.6 (0.109) | 1.6 (0.9, 2.9) |
| Definite TTH and worse vs. the rest | 9.3 (0.002) | 1.9 (1.2, 2.8) | 6.1 (0.013) | 2.1 (1.2, 3.9) |
| Probable migraine and worse vs. the rest | 15.1 (0.0001) | 2.8 (1.6, 4.8) | 1.4 (0.239) | 1.6 (0.7, 3.5) |
Abbreviations: TTH = tension-type headache, TMD = temporomandibular disorder, OR = odds ratio, 95% CI = 95% confidence interval.
4. Discussion
In this 5-year prospective study, we found that baseline reports of migraine, mixed headache, or headache frequency predicted increased risk of developing TMD. Both headache prevalence and frequency increased across the observation period among those who developed TMD but not among matched controls. Although patterns of change were complex when multiple types of headache were considered, incident cases of TMD were more likely to experience worsening in headache type and less likely to experience improvement compared to matched controls. For TMD cases, the most striking change was observed in prevalence of definite migraine which increased ten-fold. Among all headache types experienced by TMD cases, migraine had the highest progression/remission odds ratio, while increased likelihood of progression for controls emerged only for TTH.
The 1-year prevalence of 10.3% for migraine and 54.4 % for TTH reported in this study is similar to the previously published estimates [26,57]. For example, a recent population-based American Migraine Prevalence and Prevention study found 11.7% prevalence for migraine (17.1% in women and 5.6% in men) among individuals aged 12 years and older [34]. While assessments of migraine prevalence are remarkably stable across different populations, the estimates of TTH prevalence vary widely between studies, depending of case definition, sampling procedures, and data collection methodology [46]. The largest American study reporting data on TTH prevalence observed the 1-year prevalence of 38.3%, peaking in the fourth decade of life in both women (46.9%) and men (42.3%) [53]. In contrast, the largest European study conducted in Denmark demonstrated the 1-year prevalence of 86.0% (92.5% in women and 78.9% in men) [47].
Although many studies demonstrate an association between TMD and headache, the underlying reasons for this association are poorly understood. It might be that people with TMD have another pathophysiologic condition or predisposition leading to both TMD and headache; that TMD causes headache; or that headache causes TMD. Obstructive sleep apnea might serve as an example of the first scenario, because it was associated both with TMD onset [48] and morning headache [15]. The second scenario can be illustrated by headache attributed to TMD, a secondary headache newly redefined by recent research [50] and recognized by the ICHD-3 beta [1]. While all three scenarios are plausible, the current study – because of its prospective design – provides evidence for the last option: headache as a cause of TMD.
Our finding that migraine but not TTH was a risk factor for incident TMD is especially intriguing because TMD traditionally is thought to be associated with TTH [59]. Supporting our results, higher migraine disability assessment (MIDAS) scores were positively associated with masticatory and cervical myalgia in a study of orofacial pain patients [10]. In addition, a few case-control studies reported association between migraine and TMD in adults [17,18] and children [32]. However, others found no difference in TMD prevalence between headache groups [7,52], although none of the previous studies of adults assessed the impact of various headache types on TMD onset in a prospective design. The association between migraine and TMD may be due to multiple biopsychosocial factors, such as shared physiology, genetics, psychological traits, and environmental influences. Both migraine and TMD pain are mediated by the trigeminocervical complex [16,19] and migraine-specific sensitization of trigeminal nociception [28] is likely to facilitate the onset of TMD. Dysregulation of pain modulatory mechanisms in the central and peripheral nervous systems has been reported in both conditions [3,33,38,43]. Disrupted central modulation in migraine is supported by neuroimaging studies which demonstrated that activation of specific brainstem areas occurred not only during the migraine attacks but persisted after successful treatment [61]. This long-lasting alteration in central pain processing may be another mechanism contributing to incidence of TMD. On the other hand, the extent to which migraine and TMD share common genetic risks appears only modest: a recent twin study revealed that only 12% of the genetic component of TMD pain is shared with migraine [45]. In contrast, among psychological factors, depression and anxiety are consistently reported as risk factors for both migraine and TMD [2,13,20,30]. In addition, behavioral factors, such as stress, can also contribute to the pathogenesis of both conditions [13,23].
This study for the first time identified headache frequency as a significant predictor of TMD onset. This is consistent with previous case-control studies reporting an association between headache frequency and severity of TMD pain in adults [18] and higher TMD scores in children [32]. In addition, headache frequency was substantially correlated with reduced physical and emotional functioning and aggravation of TMD symptoms in TMD patients with temple headache [5,35].
The observed temporal fluidity of headache types underscores the importance of using prospective studies when investigating patterns and consequences of headache. The existing evidence suggests substantial change, not temporal stability, in predominant headache types. In a 12-year prospective Danish population-based study, 42% of participants with migraine and 45% of participants with previous frequent episodic TTH experienced remission at follow-up, while 20% of participants with migraine and 16% of TTH sufferers developed chronic severe headaches [36]. In a 30-year prospective Swiss cohort study, only 21% of people with migraine and 7% of those with TTH continued to have the same headache for more than half of the follow-up period [39]. A bi-directional crossover of 20–25% between migraine and TTH was reported in a 7-year prospective study of a clinical sample of children and adolescents [29]. Our results showed similar complex headache patterns and demonstrated that, among those patterns, progression to migraine was the most essential factor distinguishing between Incident TMD cases and controls. The important implication of this finding for clinical care is that screening, thorough monitoring, and adequate treatment of migraine should be implemented as a preventive strategy for reducing the risk of development of TMD.
The present study had several strengths. To our knowledge, it is the first large prospective cohort study examining the role of headache in first-onset TMD in adults. Second, the prospective design of the study established the temporal relationship between headache and the first onset of TMD, thereby satisfying a prominent criterion for causal inference and supporting our conclusion that headache is a cause of TMD. Specifically, headache type was determined at baseline, when all study participants were free from TMD. Third, the use of a standardized RDC/TMD clinical examination and a reliable headache questionnaire permitted consistent classification of both conditions. Fourth, we employed very frequent (quarterly) follow-up surveys which allowed recurrent assessment of headache frequency as well as very precise ascertainment of the time of TMD onset, while eliminating the possibility of reverse causation. Only one previous study investigating TMD onset in early adolescence used such short follow-up intervals [31]. Fifth, our nested case-control study permitted a direct comparison of dynamic headache patterns among incident TMD cases and TMD-free controls.
However, this study had several limitations. First, as in any prospective cohort study, there was loss to follow-up although, as reported elsewhere [6]; this loss was non-differential, i.e., the degree of the loss generally did not differ in groups classified by a range of TMD risk factors. Second, because participants with 5 or more headaches per month were excluded from enrolling in the study, this criterion could have led to overly-conservative estimates of impact of headache frequency on TMD onset. While inclusion of individuals with higher headache frequency might have revealed a greater influence of headache on risk of TMD onset, we feared it would mask signs and symptoms of underlying TMD, with the consequence of inadvertent enrollment of TMD cases. Third, headache types were classified only by questionnaire whilst a clinical assessment by a specialist and use of daily diaries would be more valid. Unfortunately, this approach is difficult to employ in a large-scale epidemiologic study. Fourth, we couldn’t classify medication-overuse headache as detailed data on analgesic use were not collected in the study. Finally, the external validity of study estimates could be limited because study participants were not selected at random from the community. However, the participants were recruited from 4 geographically distant study sites using multiple recruitment strategies, thereby providing good representation of major US demographic groups [54].
In summary, these data support the hypothesis that migraine and frequent headaches contribute to risk of developing TMD. Future studies should address the question if timely and optimal migraine therapy could reduce the risk of TMD onset.
Supplementary Material
Acknowledgments
The authors would like to thank the OPPERA research staff for their invaluable contribution to this work. Finding for this study was provided by the National Institutes of Health (NIH)/National Institute of Dental and Cranial Research (NIDCR) U01-DE017018 and K12-DE022793 grants.
Footnotes
Conflict of Interest Statement
Drs. Slade, Fillingim, and Maixner have equity ownership in Algynomics Inc., a company providing research services in personalized pain treatment and diagnostics. Dr. Maixner is President of the company and has equity holdings in Proove Biosciences Inc. and Orthogen. All other authors declare no financial relations that might represent a possible conflict of interest.
References
- 1.The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia : an international journal of headache. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. Risk factors for onset of chronic oro-facial pain--results of the North Cheshire oro-facial pain prospective population study. Pain. 2010;149(2):354–359. doi: 10.1016/j.pain.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akerman S, Holland PR, Goadsby PJ. Diencephalic and brainstem mechanisms in migraine. Nature reviews Neuroscience. 2011;12(10):570–584. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- 4.Allison PD SAS Institute. Survival analysis using SAS : a practical guide. Cary, NC: SAS Institute; 1995. [Google Scholar]
- 5.Anderson GC, John MT, Ohrbach R, Nixdorf DR, Schiffman EL, Truelove ES, List T. Influence of headache frequency on clinical signs and symptoms of TMD in subjects with temple headache and TMD pain. Pain. 2011;152(4):765–771. doi: 10.1016/j.pain.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, Maixner W, Smith SB, Diatchenko L, Gonzalez Y, Gordon SM, Lim PF, Ribeiro-Dasilva M, Dampier D, Knott C, Slade GD. Study Protocol, Sample Characteristics, and Loss to Follow-Up: The OPPERA Prospective Cohort Study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T2–T19. doi: 10.1016/j.jpain.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballegaard V, Thede-Schmidt-Hansen P, Svensson P, Jensen R. Are headache and temporomandibular disorders related? A blinded study. Cephalalgia : an international journal of headache. 2008;28(8):832–841. doi: 10.1111/j.1468-2982.2008.01597.x. [DOI] [PubMed] [Google Scholar]
- 8.Ciancaglini R, Radaelli G. The relationship between headache and symptoms of temporomandibular disorder in the general population. Journal of dentistry. 2001;29(2):93–98. doi: 10.1016/s0300-5712(00)00042-7. [DOI] [PubMed] [Google Scholar]
- 9.Dahlstrom L, Carlsson GE. Temporomandibular disorders and oral health-related quality of life. A systematic review. Acta odontologica Scandinavica. 2010;68(2):80–85. doi: 10.3109/00016350903431118. [DOI] [PubMed] [Google Scholar]
- 10.Dando WE, Branch MA, Maye JP. Headache disability in orofacial pain patients. Headache. 2006;46(2):322–326. doi: 10.1111/j.1526-4610.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 11.Dao TT, Lund JP, Lavigne GJ. Comparison of pain and quality of life in bruxers and patients with myofascial pain of the masticatory muscles. Journal of orofacial pain. 1994;8(4):350–356. [PubMed] [Google Scholar]
- 12.Dworkin S, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. Journal of craniomandibular disorders : facial & oral pain. 1992;6(4):301–355. [PubMed] [Google Scholar]
- 13.Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, Maixner W. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T75–90. doi: 10.1016/j.jpain.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiss JL. Statistical methods for rates and proportions. New York: Wiley; 1981. [Google Scholar]
- 15.Freedom T. Headaches and sleep disorders. Disease-a-month : DM. 2015;61(6):240–248. doi: 10.1016/j.disamonth.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Goadsby PJ, Charbit AR, Andreou AP, Akerman S, Holland PR. Neurobiology of migraine. Neuroscience. 2009;161(2):327–341. doi: 10.1016/j.neuroscience.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Goncalves DA, Bigal ME, Jales LC, Camparis CM, Speciali JG. Headache and symptoms of temporomandibular disorder: an epidemiological study. Headache. 2010;50(2):231–241. doi: 10.1111/j.1526-4610.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 18.Goncalves DA, Camparis CM, Speciali JG, Franco AL, Castanharo SM, Bigal ME. Temporomandibular disorders are differentially associated with headache diagnoses: a controlled study. The Clinical journal of pain. 2011;27(7):611–615. doi: 10.1097/AJP.0b013e31820e12f5. [DOI] [PubMed] [Google Scholar]
- 19.Graff-Radford SB. Temporomandibular disorders and headache. Dental clinics of North America. 2007;51(1):129–144. vi–vii. doi: 10.1016/j.cden.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Hamelsky SW, Lipton RB. Psychiatric comorbidity of migraine. Headache. 2006;46(9):1327–1333. doi: 10.1111/j.1526-4610.2006.00576.x. [DOI] [PubMed] [Google Scholar]
- 21.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia : an international journal of headache. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 22.Hill AB. The Environment and Disease: Association or Causation? Proceedings of the Royal Society of Medicine. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- 23.Houle T, Nash JM. Stress and headache chronification. Headache. 2008;48(1):40–44. doi: 10.1111/j.1526-4610.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 24.Isong U, Gansky SA, Plesh O. Temporomandibular joint and muscle disorder-type pain in U.S. adults: the National Health Interview Survey. Journal of orofacial pain. 2008;22(4):317–322. [PMC free article] [PubMed] [Google Scholar]
- 25.Janal MN, Raphael KG, Nayak S, Klausner J. Prevalence of myofascial temporomandibular disorder in US community women. Journal of oral rehabilitation. 2008;35(11):801–809. doi: 10.1111/j.1365-2842.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 26.Jensen R, Stovner LJ. Epidemiology and comorbidity of headache. The Lancet Neurology. 2008;7(4):354–361. doi: 10.1016/S1474-4422(08)70062-0. [DOI] [PubMed] [Google Scholar]
- 27.Kang JK, Ryu JW, Choi JH, Merrill RL, Kim ST. Application of ICHD-II criteria for headaches in a TMJ and orofacial pain clinic. Cephalalgia : an international journal of headache. 2010;30(1):37–41. doi: 10.1111/j.1468-2982.2009.01866.x. [DOI] [PubMed] [Google Scholar]
- 28.Katsarava Z, Lehnerdt G, Duda B, Ellrich J, Diener HC, Kaube H. Sensitization of trigeminal nociception specific for migraine but not pain of sinusitis. Neurology. 2002;59(9):1450–1453. doi: 10.1212/wnl.59.9.1450. [DOI] [PubMed] [Google Scholar]
- 29.Kienbacher C, Wober C, Zesch HE, Hafferl-Gattermayer A, Posch M, Karwautz A, Zormann A, Berger G, Zebenholzer K, Konrad A, Wober-Bingol C. Clinical features, classification and prognosis of migraine and tension-type headache in children and adolescents: a long-term follow-up study. Cephalalgia : an international journal of headache. 2006;26(7):820–830. doi: 10.1111/j.1468-2982.2006.01108.x. [DOI] [PubMed] [Google Scholar]
- 30.Kindler S, Samietz S, Houshmand M, Grabe HJ, Bernhardt O, Biffar R, Kocher T, Meyer G, Volzke H, Metelmann HR, Schwahn C. Depressive and anxiety symptoms as risk factors for temporomandibular joint pain: a prospective cohort study in the general population. The journal of pain : official journal of the American Pain Society. 2012;13(12):1188–1197. doi: 10.1016/j.jpain.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 31.LeResche L, Mancl LA, Drangsholt MT, Huang G, Von Korff M. Predictors of onset of facial pain and temporomandibular disorders in early adolescence. Pain. 2007;129(3):269–278. doi: 10.1016/j.pain.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liljestrom MR, Le Bell Y, Anttila P, Aromaa M, Jamsa T, Metsahonkala L, Helenius H, Viander S, Jappila E, Alanen P, Sillanpaa M. Headache children with temporomandibular disorders have several types of pain and other symptoms. Cephalalgia : an international journal of headache. 2005;25(11):1054–1060. doi: 10.1111/j.1468-2982.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 33.Lin CS. Brain signature of chronic orofacial pain: a systematic review and meta-analysis on neuroimaging research of trigeminal neuropathic pain and temporomandibular joint disorders. PloS one. 2014;9(4):e94300. doi: 10.1371/journal.pone.0094300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 35.List T, John MT, Ohrbach R, Schiffman EL, Truelove EL, Anderson GC. Influence of temple headache frequency on physical functioning and emotional functioning in subjects with temporomandibular disorder pain. Journal of orofacial pain. 2012;26(2):83–90. [PMC free article] [PubMed] [Google Scholar]
- 36.Lyngberg AC, Rasmussen BK, Jorgensen T, Jensen R. Prognosis of migraine and tension-type headache: a population-based follow-up study. Neurology. 2005;65(4):580–585. doi: 10.1212/01.wnl.0000172918.74999.8a. [DOI] [PubMed] [Google Scholar]
- 37.Macfarlane TV, Blinkhorn AS, Davies RM, Kincey J, Worthington HV. Oro-facial pain in the community: prevalence and associated impact. Community dentistry and oral epidemiology. 2002;30(1):52–60. doi: 10.1034/j.1600-0528.2002.300108.x. [DOI] [PubMed] [Google Scholar]
- 38.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63(3):341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 39.Merikangas KR, Cui L, Richardson AK, Isler H, Khoromi S, Nakamura E, Lamers F, Rossler W, Ajdacic-Gross V, Gamma A, Angst J. Magnitude, impact, and stability of primary headache subtypes: 30 year prospective Swiss cohort study. BMJ. 2011;343:d5076. doi: 10.1136/bmj.d5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitrirattanakul S, Merrill RL. Headache impact in patients with orofacial pain. J Am Dent Assoc. 2006;137(9):1267–1274. doi: 10.14219/jada.archive.2006.0385. [DOI] [PubMed] [Google Scholar]
- 41.Ohrbach R, Fillingim RB, Mulkey F, Gonzalez Y, Gordon S, Gremillion H, Lim PF, Ribeiro-Dasilva M, Greenspan JD, Knott C, Maixner W, Slade G. Clinical findings and pain symptoms as potential risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case-control study. The journal of pain : official journal of the American Pain Society. 2011;12(11 Suppl):T27–45. doi: 10.1016/j.jpain.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okeson JP, de Leeuw R. Differential diagnosis of temporomandibular disorders and other orofacial pain disorders. Dental clinics of North America. 2011;55(1):105–120. doi: 10.1016/j.cden.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. The Lancet Neurology. 2009;8(7):679–690. doi: 10.1016/S1474-4422(09)70090-0. [DOI] [PubMed] [Google Scholar]
- 44.Plesh O, Adams SH, Gansky SA. Self-reported comorbid pains in severe headaches or migraines in a US national sample. Headache. 2012;52(6):946–956. doi: 10.1111/j.1526-4610.2012.02155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plesh O, Noonan C, Buchwald DS, Goldberg J, Afari N. Temporomandibular disorder-type pain and migraine headache in women: a preliminary twin study. Journal of orofacial pain. 2012;26(2):91–98. [PubMed] [Google Scholar]
- 46.Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Seminars in neurology. 2010;30(2):107–119. doi: 10.1055/s-0030-1249220. [DOI] [PubMed] [Google Scholar]
- 47.Russell MB, Levi N, Saltyte-Benth J, Fenger K. Tension-type headache in adolescents and adults: a population based study of 33,764 twins. European journal of epidemiology. 2006;21(2):153–160. doi: 10.1007/s10654-005-6031-3. [DOI] [PubMed] [Google Scholar]
- 48.Sanders AE, Essick GK, Fillingim R, Knott C, Ohrbach R, Greenspan JD, Diatchenko L, Maixner W, Dubner R, Bair E, Miller VE, Slade GD. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. Journal of dental research. 2013;92(7 Suppl):70S–77S. doi: 10.1177/0022034513488140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanders AE, Slade GD, Bair E, Fillingim RB, Knott C, Dubner R, Greenspan JD, Maixner W, Ohrbach R. General health status and incidence of first-onset temporomandibular disorder: the OPPERA prospective cohort study. The journal of pain : official journal of the American Pain Society. 2013;14(12 Suppl):T51–62. doi: 10.1016/j.jpain.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schiffman E, Ohrbach R, List T, Anderson G, Jensen R, John MT, Nixdorf D, Goulet JP, Kang W, Truelove E, Clavel A, Fricton J, Look J. Diagnostic criteria for headache attributed to temporomandibular disorders. Cephalalgia : an international journal of headache. 2012;32(9):683–692. doi: 10.1177/0333102412446312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, List T, Svensson P, Gonzalez Y, Lobbezoo F, Michelotti A, Brooks SL, Ceusters W, Drangsholt M, Ettlin D, Gaul C, Goldberg LJ, Haythornthwaite JA, Hollender L, Jensen R, John MT, De Laat A, de Leeuw R, Maixner W, van der Meulen M, Murray GM, Nixdorf DR, Palla S, Petersson A, Pionchon P, Smith B, Visscher CM, Zakrzewska J, Dworkin SF. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. Journal of oral & facial pain and headache. 2014;28(1):6–27. doi: 10.11607/jop.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schokker RP, Hansson TL, Ansink BJ. Craniomandibular disorders in patients with different types of headache. Journal of craniomandibular disorders : facial & oral pain. 1990;4(1):47–51. [PubMed] [Google Scholar]
- 53.Schwartz BS, Stewart WF, Simon D, Lipton RB. Epidemiology of tension-type headache. Jama. 1998;279(5):381–383. doi: 10.1001/jama.279.5.381. [DOI] [PubMed] [Google Scholar]
- 54.Slade GD, Bair E, By K, Mulkey F, Baraian C, Rothwell R, Reynolds M, Miller V, Gonzalez Y, Gordon S, Ribeiro-Dasilva M, Lim PF, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Dampier D, Knott C, Ohrbach R. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. The journal of pain : official journal of the American Pain Society. 2011;12(11 Suppl):T12–26. doi: 10.1016/j.jpain.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slade GD, Sanders AE, Bair E, Brownstein N, Dampier D, Knott C, Fillingim R, Maixner WO, Smith S, Greenspan J, Dubner R, Ohrbach R. Preclinical episodes of orofacial pain symptoms and their association with health care behaviors in the OPPERA prospective cohort study. Pain. 2013;154(5):750–760. doi: 10.1016/j.pain.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slade GD, Sanders AE, Ohrbach R, Fillingim RB, Dubner R, Gracely RH, Bair E, Maixner W, Greenspan JD. Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain. 2014;155(10):2134–2143. doi: 10.1016/j.pain.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53(3):427–436. doi: 10.1111/head.12074. [DOI] [PubMed] [Google Scholar]
- 58.Stovner LJ, Al Jumah M, Birbeck GL, Gururaj G, Jensen R, Katsarava Z, Queiroz LP, Scher AI, Tekle-Haimanot R, Wang SJ, Steiner TJ. The methodology of population surveys of headache prevalence, burden and cost: principles and recommendations from the Global Campaign against Headache. The journal of headache and pain. 2014;15:5. doi: 10.1186/1129-2377-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Svensson P. Muscle pain in the head: overlap between temporomandibular disorders and tension-type headaches. Current opinion in neurology. 2007;20(3):320–325. doi: 10.1097/WCO.0b013e328136c1f9. [DOI] [PubMed] [Google Scholar]
- 60.Von Korff M, Dworkin SF, Le Resche L, Kruger A. An epidemiologic comparison of pain complaints. Pain. 1988;32(2):173–183. doi: 10.1016/0304-3959(88)90066-8. [DOI] [PubMed] [Google Scholar]
- 61.Weiller C, May A, Limmroth V, Juptner M, Kaube H, Schayck RV, Coenen HH, Diener HC. Brain stem activation in spontaneous human migraine attacks. Nature medicine. 1995;1(7):658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.