Abstract
Human factors/ergonomics recognizes work as embedded in and shaped by levels of social, physical, and organizational context. This study investigates the contextual or macroergonomic factors present in the health-related work performed by patients. We performed a secondary content analysis of findings from three studies of the work of chronically ill patients and their informal caregivers. Our resulting consolidated macroergonomic patient work system model identifies seventeen factors across physical, social, and organizational domains and household and community levels. These factors are illustrated with examples from the three studies and discussed as having positive, negative, or varying effects on health and health behavior. We present three brief case studies to illustrate how macroergonomic factors combine across domains and levels to shape performance in expected and unexpected ways. Findings demonstrate not only the importance of context for patients’ health-related activities but also specific factors to consider in future research, design, and policy efforts.
Keywords: Healthcare ergonomics, Macroergonomics, self-care, sociotechnical systems, qualitative research
1. INTRODUCTION
“If work is becoming flexible, socially determined, and dependent on context … then where does it leave us?” (Moray, 1994, p. 529)
The notion of work being embedded in and shaped by context is a fundamental principle of human factors/ergonomics (HFE) (Wilson, 2014). The elements of context shaping work performance can be called macroergonomic factors, referring to higher-level factors such as those related to the organization, society, culture, politics, regulations, or the economy (Carayon, 2006).
There are depictions of macroergonomic factors in “classic” HFE models. The hierarchical depiction considers macroergonomic factors as nested layers of context enveloping workers, with each layer representing increasing levels of abstraction, and different factors becoming important as the scope of analysis expands (Karsh et al., 2006; Moray, 2000; Rasmussen, 1997). The domain-based depiction views macroergonomic factors as elements of any sociotechnical system, large or small (Carayon, 2006; Kleiner, 2006; Smith & Sainfort-Carayon, 1989). The 2012 international statement on HFE also represents a domain-based depiction, specifying three macroergonomic domains: “the physical environment (‘things’), the organizational environment (how activities are organized and controlled), and the social environment (other people, culture)” (Dul et al., 2012, p. 378). Some models combine levels and domains (Henriksen et al., 2009; Holden & Karsh, 2009; Karsh et al., 2006; Moray, 2000).
In HFE research, macroergonomic factors are increasingly measured and shown to influence performance of all types (Holden et al., 2015b). A classic example is that organizational climate and the psychosocial work environment influence the biomechanics of physical work and work-related musculoskeletal disorders (Carayon et al., 1999; Moon & Sauter, 1996). Organizational policies and priorities are known to affect human error, safety violations, and resilience (Alper & Karsh, 2009; Woods et al., 2010). The acceptance and use of new technology is influenced by social forces from peers, clients, organizations, and professional societies (Holden, 2012; Rogers, 1995). In the growing area of healthcare HFE, empirical studies have investigated how macroergonomic factors contribute to specific healthcare phenomena, for example, violations of safety protocols and the performance of nurses, physicians, and pharmacists (Alper et al., 2012; Chui et al., 2012; Gurses et al., 2010; Wiegmann et al., 2010).
Work on macroergonomic factors appears to follow the cycle in Figure 1 (Karsh et al., 2014). The theorizing phase is mainly about establishing the importance of macroergonomic factors as a whole (Hendrick, 2002). What follows is the specification of particular macroergonomic factors and their roles for a given domain of work or HFE phenomenon. This means decomposing broad categories such as “physical environment” into relevant subcategories, for example, “lighting” and “layout,” and sub-subcategories, e.g., “open vs. closed layout.” Notably, apart from human error taxonomies (van Vuuren et al., 1997; Wiegmann & Shappell, 2003), there are few examples of this kind of macroergonomic factor decomposition. At the Specify stage, testable hypotheses can also be posited, such as task interruptions are more likely in open physical layouts (Werner & Holden, 2015). Once particular factors are defined and illustrated, they can be studied and hypotheses can be tested, resulting in an evidence base for formulating related design concepts or interventions. In the last stage, designs are created and tested, followed by revisiting earlier phases. Writing about the science and practice of macroergonomics, Karsh et al. (2014) urged HFE professionals to pay special consideration to the “Specify” phase, because the specification of macroergonomic factors drives subsequent scientific inquiry and design projects. Doing so is especially important for work scenarios or settings receiving relatively little HFE research attention, for example, the personal health management work performed by patients and informal caregivers in community settings, or what may be called “patient work” (Holden et al., 2013; Valdez et al., 2015). Before presenting the results of three studies specifying macroergonomic factors involved in patient work, we briefly describe the concepts of patient work and patient work systems.
Figure 1.
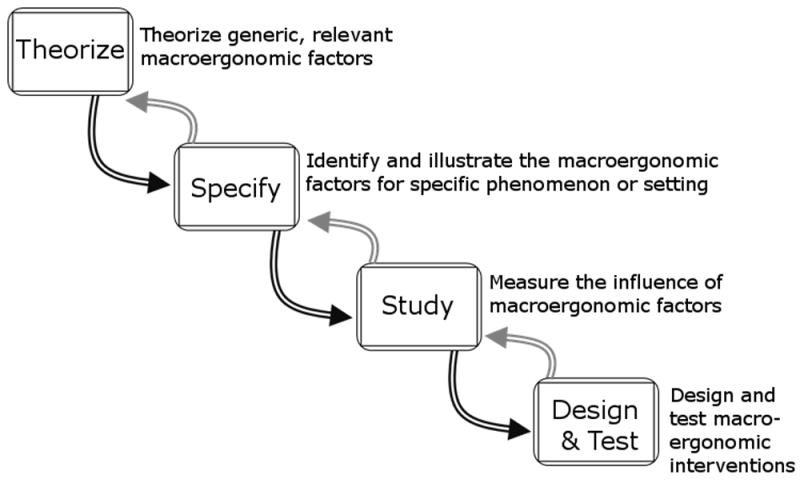
Cycle of macroergonomic research and design.
1.1. Patient work and patient work systems
Patient work is defined as the “exertion of effort and investment of time on the part of patients or family members to produce or accomplish something” (Strauss, 1993, pp. 64-65). Although this form of work is almost always unpaid and may go unrecognized (Ancker et al., 2015; Senteio & Veinot, 2014), its performance is often critical to successful health maintenance or recovery. One example is the case of chronically ill patients who self-manage their condition by taking medications, managing symptoms, dieting, and exercising (Bodenheimer et al., 2002; Mitzner et al., 2013). Informal caregiving for people is another form of health-related work that, while not performed by healthcare professionals, has profound health implications for both care recipient and provider (Schulz et al., 2007; Walker et al., 1995). Patient work often occurs alongside work performed by healthcare professionals, and patients, informal caregivers, and professionals usually play some role in one another’s activities (Holden et al., 2013).
Recognizing the importance of patient work, Holden and colleagues (2013) called for additional “application of human factors theories and principles, methods and tools, analyses, and interventions to study and improve work done by patients and families, alone or in concert with healthcare professionals” (p.1679). They and others have noted that applying HFE to patient work can raise the value of HFE in an emerging era of patient-centered care and patient engagement (Carayon et al., 2014; Hignett et al., 2013; Holden & Mickelson, 2013; Unruh & Pratt, 2007; Vincent & Coulter, 2002).
Patient work occurs in and is shaped by what can be called a patient work system (Holden et al., 2015d; Valdez & Holden, 2014; Valdez et al., 2015). Several groups of HFE researchers have conceptualized the elements of a patient work system, for example, the person, task, technology, environmental, organizational, and community factors that affect patient and informal caregiver performance (Carayon et al., 2013; Holden et al., 2013; National Research Council, 2011; Zayas Cabán & Valdez, 2012). A recent empirical study of the sociotechnical work systems of patients with heart failure and their informal caregivers described in detail person-, task-, and technology-related barriers to the performance of self-care work (Holden et al., 2015d). That study also indicated but did not fully explicate various macroergonomic barriers such as social isolation, obstacles in the physical environment, and inadequate health insurance (see also Holden et al., 2015c).
In sum, what patients and informal caregivers do can be viewed as a type of work that appears to be shaped by macroergonomic factors. Early attempts to explicate those factors have identified high-level categories such as “social environment” but have not specified a taxonomy of macroergonomic factors relevant across multiple scenarios (e.g., patient groups differing in age, disease, and location), definitions and examples of those factors, and explanations of how those factors may shape patient work performance. The present study addresses this gap.
1.2. Consolidating three studies of patient work
Given the apparent importance of macroergonomic factors in the patient work system, we conducted a secondary analysis of data from three studies of patients living with chronic disease. The purpose of the analysis was to specify, i.e., identify, illustrate, and hypothesize the role of macroergonomic factors in the patient work system. The studies collected data on and from patients and informal caregivers (often family members) differing in age, disease condition, and geographic region. They were performed by three separate research teams and were guided by three distinct but related sociotechnical systems models. All three studies focused on health management in the home and community. Information technology was of particular interest in two of the studies and a case management intervention was the focus of the third. Across these studies, we were able to identify areas of overlap, permitting us to produce and specify a consolidated model of macroergonomic factors influencing the patient work performance of chronically ill patients and informal caregivers.
2. METHODS
2.1. Overview of the three studies
Between 2012 and 2015, three separate research teams performed three studies: the Asthma and Technology Study (Study A), Keystone Beacon Project (Study B), and Caring Hearts Study (Study C). Each study’s principal investigator was not involved in the other studies. Patient age ranges differed between Studies A (19-50), B (46-80+), and C (65-86) (Appendix A).
The Asthma and Technology Study was a qualitative exploration of the positive and negative experiences faced by ten individuals using the beta version of a novel commercial asthma management technology. This technology consisted of a sensor device that attached to participants’ inhalers, enabling capture of the time and place of inhaler use. This device was also paired with a mobile health application that facilitated tracking of multiple parameters of asthma management, including medication use, symptom occurrence, and symptom triggers. Study participants were located across the United States, and data were collected through Skype-based interviews. The research was guided by the critical incident technique (Flanagan, 1954; Gurses & Carayon, 2009) and the systems model in Figure 2a. This systems model was introduced in the National Research Council (2011) report, “Health Care Comes Home: The Human Factors,” and was based on an earlier HFE systems model (Fisk et al., 2009). Study participants were asked to consider how elements of the system in Figure 2a contributed to their positive and negative experiences with the technology. Findings were provided to the vendor to be used in redesign efforts.
Figure 2.
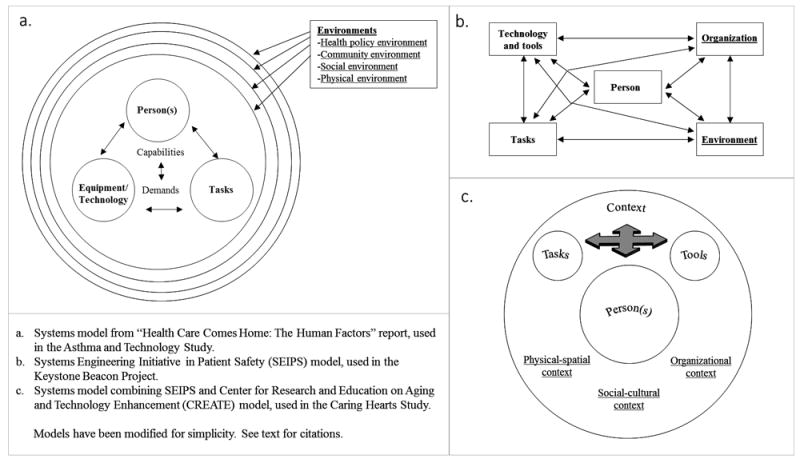
Sociotechnical systems models used in each original research study. Macroergonomic factors in each model are underlined.
The Keystone Beacon Project assessed the impact of health information technology-mediated inpatient and outpatient care management on outcomes (i.e., (re)admissions and emergency department visits) for patients with heart failure and/or chronic obstructive pulmonary disease in five counties in the US northeast. Although a significant focus of the project addressed the work of the care managers, researchers captured patient satisfaction with and assessment of the quality of the standard care management activities they received. These activities included: reconciling medication lists, providing education upon discharge, and scheduling follow-up care with primary care providers and community health services. Data were captured through in-home and telephonic interviews (n=10), four focus groups conducted in healthcare settings (clinic and hospital meetings rooms) (n=9), and patient surveys distributed to intervention and control groups (n=160). Data collection and analysis were guided by the Systems Engineering Initiative for Patient Safety (SEIPS) model of work system and patient safety (Carayon et al., 2006), depicted in Figure 2b. Results of the analyses were used to make improvements to care management work systems.
The Caring Hearts Study was a mixed method, field-based investigation of older people with heart failure and their informal caregivers, tools and technologies, tasks, and contexts. Data were collected from 61 patients aged ≥65 and 35 caregivers, living in the Southeast US, within a 300 mile radius of an academic medical center. Data collection included in-clinic observations and interviews, in-home observations and interviews, standardized surveys, and medical record review. Findings from the study were used to design a mobile health application to promote self-care behavior, knowledge, and motivation. Data collection and analyses were guided by the systems model in Figure 2c, which merged the two models in Figures 2a and 2b. Findings served as input into the design and testing of a mobile health application for heart failure self-care.
2.2. Secondary analysis procedures
Each study team initially analyzed its own data using that study’s conceptual model (Figure 2) and a variant of qualitative content analysis (Graneheim & Lundman, 2004; Saldaña, 2013). The Asthma and Technology Study analysis primarily used Hsieh and Shannon’s (2005) procedure for conventional content analysis. Inductive coding was used to generate work system sub-elements and sub-sub-elements in each of the four environments of the systems model: physical, social, community, and health policy. Two researchers collaboratively coded all transcripts. Disagreements were resolved through consensus building.
In the Keystone Beacon Project, one researcher analyzed all transcripts, first grouping patients according to the type of case manager involved in the patient’s care (inpatient, outpatient or telephonic follow-up). Participants’ responses were then categorized using the SEIPS model. Significant patient quotations were also identified and noted for later reporting. Survey results were used to confirm and clarify findings from the interviews and focus groups.
In the Caring Hearts Study, an interdisciplinary team of three analysts performed a stepwise, iterative content analysis on observation and interview transcriptions (Holden et al., 2015d). Macroergonomic factors were initially coded in one or more broad categories of ‘physical,’ ‘social,’ or ‘organizational’ context and then iteratively assigned to subcategories such as ‘physical-distances-proximity of resources and facilities’ or ‘social-social influence-compliance.’ The coding team followed a convergence-seeking process of multiple group coding discussions, with ad-hoc discussion and coding conflict resolution in between. Quantitative data from surveys were used to support or clarify qualitative analyses.
After the above individual analyses, two of the principal investigators and then members of all three study teams participated in group meetings and e-mail discussions to create a generic patient work system model consolidating the three frameworks in Figure 2 and each study’s initial findings. Each team then performed a secondary analysis of their data to specify and illustrate the macroergonomic factors in the consolidated model. In the results, we present the agreed-upon generic model, followed by specific illustrations of identified macroergonomic factors in the model, and finally the fully specified model.
3. RESULTS
3.1. Consolidated model of the patient work system
Figure 3 depicts the initial, generic model agreed upon by the three research teams. It consolidates the three original models in Figure 2. The model has three nested levels of abstraction: the triad of person-task-tools; the household; and the community. The inner triad consists of three important interacting microergonomic components:
Figure 3.
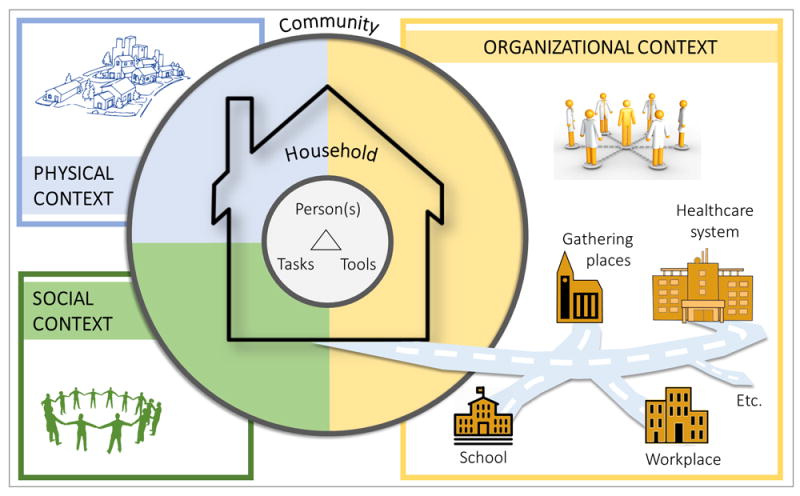
Consolidated model of the macroergonomic patient work system, with three levels—the microergonomic triad of person(s)-tasks-tools, household, and community—and three macroergonomic domains of physical, social, and organizational context.
Person(s), who may be patients, informal caregivers, or healthcare professionals, and their attributes such as individual knowledge, motivation, functional ability, personality and attitude, and demographic factors such as race and gender.
Tasks, which include illness-related activities such as taking medications or monitoring symptoms, personal health-related tasks such as bathing and grocery shopping, health-related logistical tasks such as managing medical insurance and expenditures and obtaining medical supplies, and secondary tasks such as caring for others that are not health-related but may influence health. Tasks have properties such as difficulty, timing, and complexity.
Tools or technologies, including record-keeping systems, communication technologies, organizing equipment, and other hardware and software used for health-related entertainment, education, or engagement. Tool and technology properties include accessibility, availability, usability, and effectiveness (Mickelson, Willis, & Holden, 2015).
This person(s)-task-tools triad has been discussed elsewhere in the context of patient work and a full description is not in the scope of this macroergonomic paper. The triad is embedded in both the household and community. At those levels, the model proposes three domains of macroergonomic factors: social; physical; and organizational. In the community, we theorize multiple places or settings outside the home where patient work occurs and that potentially shape patient work performance. These may be places such as one’s primary care clinic, church, place of work or volunteering, or community food market. The following sections identify and illustrate specific macroergonomic factors within the three domains found to influence patient work across the three studies. While we discuss aspects of the three macroergonomic domains separately, this is only a simplifying strategy, as various household and community settings or phenomena are often a combination of physical, social, and organizational aspects.
In the results below, factors and themes are presented if the phenomenon was seen across multiple participants in one of the three studies and in participants across at least two studies. References are given to 69 representative quotations or field notes (R1-R69, Tables 1 - 3), for illustrative purposes. Because of differences in methods and content units across the three studies, and in light of the overall study objective—to specify, not compare, macroergonomic factors—we do not quantify the findings.
Table 1.
Physical context factors, their definitions, and examples from three studies.
| Factor and its definition | Examples from data* |
|---|---|
Weather and environment
|
|
Distance, layout, and surface
|
|
Workspace
|
|
(A) refers to the Asthma and Technology Study; (B) refers to the Keystone Beacon Project; (C) refers to the Caring Hearts Study.
Table 3.
Organizational context factors, their definitions, and examples from three studies.
| Factor and its definition | Examples from data* |
|---|---|
Structural properties
|
|
Rules and roles
|
|
Routines
|
|
Disruptions
|
|
Workload
|
|
Health-management resources and facilities
|
|
Healthcare delivery services and facilities
|
|
Communication
|
|
Financial factors
|
|
Legal and policy factors
|
|
Health system values
|
|
(A) refers to the Asthma and Technology Study; (B) refers to the Keystone Beacon Project; (C) refers to the Caring Hearts Study.
3.2. Physical context factors (Table 1)
Weather and environment are known to directly affect people’s health, for example, through the biological effects of air quality on people with lung disease (Frumkin et al., 2008). The studies demonstrated that internal and external environmental conditions also shaped patients’ health-related behaviors. Indoor smoking, outdoor air quality (e.g., pollen, sawdust, smells) and insects aggravated breathing problems and limited mobility and exercise (R1). Among asthma patients, changes in pollen also influenced patient-perceived value of self-care behaviors such as using a portable asthma technology (R2).
Distance, layout, and surface factors affecting primarily older patients included stairs and distances within households, in residential areas such as driveways, and at places such as stores or clinics (R3). Rural-dwelling adults (R4) and patients relying on others for transportation (R5) also reported inconvenient distances to places such as hospitals and clinics, exercise facilities, friends and family, grocery stores, and pharmacies. Among more mobile patients, traffic congestion and drive times affected movement (R6). We found patients weighed travel costs (e.g., of returning for something) against the burden of carrying objects such as health equipment or medications (R7).
Workspaces were dedicated or ad-hoc areas for performing health-related tasks. For some, these were places in the home containing equipment and supplies, medications, records and logs, pens and paper, and sometimes a computer (Figure 4). This helped organize health-related activities (R8). For many others, health-related artifacts and tasks were spread across the home, portable locations (e.g., purse, pocket, car), and even different homes (R9). Informal assessments of homes revealed inadequate lighting, clutter and disorganization, and problems with electrical or Internet access (R10). In the community, patients often encountered places ill-designed for health-related tasks, i.e., lacking privacy, surface area, or resources (e.g., Internet connectivity, electricity) (R11).
Figure 4.

Photos from the Caring Hearts Study show the “health workspaces” of patients. The top line illustrates that people used different rooms for these workspaces (left: living room, right: bathroom). The middle line illustrates a disorganized (left) versus organized (right) workspace. The bottom line illustrates that within the same household, objects for managing health could be distributed across multiple rooms (left: computer room, right: bedroom).
3.3. Social context factors (Table 2)
Table 2.
Social context factors, their definitions, and examples from three studies.
| Factor and its definition | Examples from data* |
|---|---|
Interpersonal influence
|
|
Cultural influence
|
|
Social support and engagement
|
|
(A) refers to the Asthma and Technology Study; (B) refers to the Keystone Beacon Project; (C) refers to the Caring Hearts Study.
Patient work was decidedly a social activity, supported and influenced by informal caregivers, family members, healthcare professionals, and various social forces. Patients’ social networks were often a source of interpersonal influence. In the home, patients’ family members encouraged (R12) or directly manipulated their behavior, for better (R13) or worse (R14). Outside the home, physicians and nurses were largely reported as positively influencing and supporting patients’ behaviors (R15). Patients were also affected by observing and modeling others’ healthy or unhealthy behaviors, e.g., of a health-conscious daughter vs. those of visiting relatives during the holidays (R16). Patients were influenced by the expectations of others. In some cases, a health-related topic became the focal point of personal or public social interaction (R17) but in other cases one’s disease or self-care routine invoked feelings of being judged (R18).
A related aspect of the social environment was the cultural influence exerted by living or growing up in a particular region or group. At the household-level, some reported a positive family or household culture promoting positive attitude, knowledge, and education. Younger patients in the asthma study described how growing up in a “hypochondriac family” (R19) or currently living in an “electronic family” (R20) influenced behavior, while older cardiac patients mentioned family values such as hard work, which could undermine one’s health (R21). At the community-level, older patients described how growing up in the South, in rural areas, or outside the US influenced dietary habits they now had to overcome (R22). Other community-level cultural influences were one’s profession (R23) or a life of partying and drug use. Cultural influences were complex because they could promote healthy behaviors or serve as barriers. In our studies, cultural beliefs and practices were often deep-seated and occasionally reinforced during rituals such as Christmas or Thanksgiving holiday meals. They were also transient, such as being part of “teen culture” (R24).
A final aspect of the social environment was social support and engagement, reflecting the instrumental value of a social network and the health consequences. The social network included informal caregivers in the household and people outside the home such as formal healthcare professionals, friends, and community members. Informal caregivers and other companions provided general assistance and emotional support. They also helped with specific tasks such as transportation, grocery shopping, or medication preparation. Live-in companions, often family members, were particularly helpful for older, chronically ill adults. Examples included when a patient had physical needs (R25), memory impairment (R26), or the need to move in with someone else (R27). Some younger asthma patients also benefited from family reminders (R28). Patients in all studies received support from family members living outside the home, physicians, nurses, pharmacists, therapists, or friends from church. Interestingly, patients often viewed clinicians as part of their social network, as opposed to merely medical consultants (R29). Members of the social network provided both support and opportunities for social engagement. However, among older adults, social isolation and inactivity were recurring problems (R30). Even among cohabitating older couples, it was common to hear, “we really don’t do much.” More active older adults experienced social engagement through part-time work, volunteer efforts, festivals, visits with friends and family, and meals outside the home.
Notably, while people’s social networks were largely beneficial, in some cases, patients were surrounded by individuals who were unavailable, stressed, underinformed, overwhelmed with other responsibilities, or engaged in behavior negatively affecting their health (R31). Patients’ social networks could create risks such as neighbors taking advantage of an older person (e.g., “I live in the government projects, and everybody’s always trying to play a little game with you”) or patients becoming too dependent on help from others (e.g., “Sometimes I ask [my children to help with medication] when I really shouldn’t”).
3.4. Organizational context factors (Table 3)
We defined organizational context factors as structures organizing time, space, resources, and activity in patients’ homes and communities.
Structural properties were often people’s in- and out-of-home social network arrangements. At home, a typical example was living alone vs. living with family members. Living with others imposed health-related constraints such as eating communal meals or needing to secure medications when there were young children in the home (R32). As an example of community-level structural properties, patients living apart from friends and family described health-management consequences of being away from social networks (R33).
Analogous to formal workplaces, patients and their informal caregivers encountered rules and roles, imposed by self or others. In addition to family “rules” or norms (e.g., for diet, exercise, or self-care), patients followed personal rules such as slowing down, indulging (e.g., in foods), or adjusting medications as the situation warranted (R34). Patients often described their health-related activity as simply following clinicians’ rules and orders (R35). Other sources of rules were community entities such as schools (R36) and seasonal cycles such as needing to be healthy for the holidays. Patients’ roles in part related to the household’s division of labor—who would do the cleaning, shopping, cooking, finances, and repairs—and power, in terms of who directed whom (R37). Patients also varied in how active vs. passive they were in managing their health, with some taking full control, others sharing self-care with an informal caregiver, and some placing their health in the hands of informal caregivers, healthcare professionals, or God (R38).
In most cases, patients observed specific routines, which structured health-related activities. These were personal routines such as chores, staying up late, or eating out—all of which affected people’s health—and medical routines such as taking medications or attending clinic visits. Of note, personal and medical routines were often organized around one another. When older adults took diuretic medications, the entanglement of personal and medical routines was obvious: diuretics cause urination, meaning they sometimes disrupted sleep, travel, and social activities, leading some to adjust either their medication regimen or their personal lives (R39). We observed both the creation and use of helpful routines (R41) to support or simplify health-related activity and the occasional absence of such routines (R42).
Many reported disruptions which could be minor or major, temporary or permanent, and medical or non-medical. Major, permanent life disruptions included losing the ability to travel, drive, hike, work, eat as before, or perform a hobby; moving into a retirement home; and coping with the death of a loved one. More temporary disruptions were power outages, periods of bad weather or natural disaster, temporary relocation, and holidays (R42). Health and treatment-related disruptions included inadvertent lapses in medication taking or other self-care behaviors (at times described as “falling-off-the-wagon”), infections, hospitalizations, surgeries, new medical devices (e.g., pacemaker), and acute events such as stroke or heart attack (R43). Patients experiencing disruptions not only had to recover from the event itself but often reported rethinking and re-structuring their lives (R44).
Workload was another organizational concept clearly applicable to patients and informal caregivers. Workload is a function of demands (or load) and resources (or capacity) (Holden et al., 2011; Vidulich & Tsang, 2012). For chronically ill people, mornings were often a time of multiple, intertwined, and sometimes laborious health-related tasks done at home (R45). Health-management tasks such as logging health information were also performed outside the home, in the community (R46). Other household demands included housework and caregiving for young or sick family members (R47). Of note for some patients, caregiving compromised their own care and health, but in some cases a history of caregiving provided a foundation of knowledge about how to take care of oneself. Outside the home, patients worked or volunteered and performed what sociologists call articulation work (Suchman, 1994), or logistical work that enables health-related activities: e.g., arranging transportation and assistance, scheduling and attending appointments, gathering and interpreting medical information, or obtaining and managing medications and medical supplies (R48).
Two types of organizational resources were fundamental to self-care performance. First, the availability of and access to health-management resources played a major role in patients’ health-related performance. Educational-informational resources were available at home via TV, Internet, or print materials and in the community through the public library or clinic (R49). Other resource factors included food delivery, proximity to grocery stores, and restaurants with healthy choices; personal or public transportation; in-house exercise equipment, community gyms, and neighborhood pools; and mail-order or community shops (R50).
A second type of resource was healthcare delivery services and facilities. These were largely community-based, but also included home-based services such as home health nursing or physical therapy (R51). Patients described the importance of accessing primary and specialty care for information, treatment, diagnosis, and as a way to have time to be engaged with one’s health (R52). However, participants also reported access barriers, such as not being able to reach the doctor (R53). Variable quality of information, the loss of records, and lack of integration between different clinicians or entities (clinics, pharmacies, hospitals) (R54) were also noted as problematic. Participants described undesirable disparities in healthcare services (R55) and personal encounters (R56), showing that access to healthcare resources did not ensure quality care.
Communication factors within the household and between patients, informal caregivers, and professionals affected performance. Reliable communication was essential for patients and informal caregivers to work together toward common goals; we observed both breakdowns in interpersonal communication (R57) and successful use of technology to support communication (R58). Much communication between patients and healthcare professionals occurred face-to-face and was separated by several months, even when prompt interactions were needed in emergencies. Again, there were both communication gaps and evidence that technology could bridge those gaps for both younger (R59) and older patients (R60) (Mickelson et al., 2015).
Financial factors affected the performance of younger and older patients, particularly due to the cost of care, medication expenses, and software or physical equipment costs. Some patients avoided filling high-cost medications (R61). A patient with asthma used hand-me-down inhalers rather than purchasing new ones (R62). Even patients with “good insurance” experienced treatment limitations and out-of-pocket costs: for example, one patient could not afford the co-payment for a diagnostic test needed to determine treatment for a suspected medical condition (R63). Patients described needing to place medical expenses on credit cards or receiving financial help from their family.
Legal and policy factors included variations in healthcare policies and insurance coverage, which sometimes created difficulties keeping the same doctor; dictated whether, when, and where a patient received treatment (R64); and necessitated out-of-pocket payments for medications, medical equipment, testing supplies, and services. Changes in patients’ medications were also dictated by insurance policies (R65). As one caregiver for an older patient stated, “You live and die by your insurance company.” On a broader scale, patients also described health-relevant community-level policies such as clean air initiatives (R66).
Lastly, the factor health system values reflected the practical realities—norms, priorities, and practices—of health systems and individual organizations. Patients were often aware of these and valued technology for its ability to present seemingly objective information to healthcare providers (R67). Because patients could have mistaken views of how the health system worked (R68), we observed multiple cases of healthcare professionals explaining the system to patients and informal caregivers (R69).
3.5. Interactions between context domains and levels
As described, our consolidated model of macroergonomic factors shaping health-related performance of patients and caregivers, distinguishes between three context domains (physical, social, organization) and two levels of analysis (household and community), but recognizes the interactions among domains and among levels. We illustrate these interactions with three case examples from the Caring Hearts Project.
Case #1
A 74 year-old Black male with heart failure described not having a computer at home (household-level organizational resource) for health-related tasks. Asked if he would ever visit the library to use their public computers (community-level resource), he replied, “I know. I want to go up there so bad, but there’s a lot of women hang out there.” He clarified that the library is located near a women’s shelter (community-level physical proximity) and the women from the shelter occupy all the computer stations—“their computers be full.” However, this patient has another reason to avoid the library: he wants to minimize the temptation to socialize with the women (social influence and self-imposed rules): “I just leave that alone.”
Case #2
An 86 year-old Black wheelchair-bound female with heart failure is observed to be unable to access her medications from a tall kitchen cabinet (household-level physical layout). Asked if the patient can reach the medications, her daughter replies “If she’s standing up, but she can’t stand up.” The daughter also explains the placement of the medication, explaining there are young children in the house (household-level organizational arrangement) and “We don’t want them to get ahold [of it].” The daughter continues to describe a rotating informal caregiving system the family set up (household-level social support), before “routines simply changed and now mama’s alone during the day.” In the absence of helpers, the patient reveals “I didn’t take it [medicine] on time.” This prompted another change to the informal caregiving schedule, and lately a second daughter has been coming to help with medications: “One sister … comes in the morning before she go to work, and puts on her clothes, and see that the medicine is administrated. Then, she also attends when she gets off of work, because one pill is a nighttime pill [a diuretic]. She makes sure that when she gets off of work, she comes by and see that she [patient] gets that.” Ultimately, this process (household-level organizational routine) is still suboptimal, because when the daughter administers the diuretic medication in the evening after work, the diuretic ends up keeping the patient up all night, affecting her sleep: “it is a problem cause I be sleepy, you know, and, uh, then, I have to wake myself up.”
Case #3
A 66 year-old White male with heart failure described having broken his sodium dietary restriction as a result of eating at a movie theater (community-level health-management resources), prompted by his girlfriend (household-level social influence): “We went to the movies probably Saturday night, and I didn’t have any dinner and she said well if you get popcorn I’ll eat some. And I said I really don’t want the popcorn from here ‘cause it’s dang salty. I said I’d rather get the caramel corn, she said well it’s got a lot of sugar. I said the sugar I can control a lot easier than the salt. I can take a little extra insulin but I can’t take the salt out of my system without taking Lasix [diuretic]. So I had the regular popcorn cause you know we [men] never win a battle so (laughs) so she ate the popcorn, I ate the popcorn, I tasted the salt and, and everything else and I knew the next morning my reading would be [high] and it was (I1: Hmm hmm) you know because of the salt.”
The cases illustrate intersections of physical, social, and organizational domains and interactions between household- and community-level factors. As above, in many cases, the various factors must be considered together to understand the ultimate performance outcomes.
4. DISCUSSION
Recognizing the importance of context was a necessary but not sufficient step in progress towards HFE science and practice of value to performance in complex domains such as aviation, education, and healthcare (Moray, 1994, 2000; Wilson, 2014). Today’s canonical HFE texts acknowledge the importance of context; for instance, in their introductory textbook, Wickens et al. (2003) write:
“Most notably, individual behavior is a function of the social context, referring to the attitudes and behaviors of coworkers and others in the work environment, and a function of the organizational context, which includes variables such as management structure, reward or incentive systems, and so forth.” (p.492, emphasis theirs)
We note two aspects of this quote. First, contextual factors are clearly portrayed as de facto HFE topics. Second, “coworkers,” “management,” and reward or incentive systems” are appropriate contextual factors in many work settings but may not be applicable to settings and domains of work such as the health-related work activity of patients and informal caregivers such as family members and friends. Some contextual factors, such as “the attitudes and behaviors … of others,” do apply, and were identified in our three studies of patient work. Of course, some of the contextual factors are unique to specific settings, for instance, the life and medical disruptions described in the results, above. Indeed, each setting or work domain is expected to have a combination of common and unique contextual or macroergonomic factors, which is why the specify step in the cycle in Figure 1 is so important, and why the present analysis was conducted.
In terms of specific macroergonomic factors, we found a large variety of physical, social, and organizational factors shaping the health-related work performance and outcomes of patients with chronic illness. Figure 5 consolidates the findings into a fully specified model of macroergonomic factors, which we propose can be used to guide future research, design, and policy initiatives related to the work of patients with chronic illness and their informal caregivers. It may also be applicable to other forms of unpaid work taking place in home and community settings. While some of the factors had direct influence on general health, adherence to one’s self-care regimen, and quality of life (e.g., the effect of ambient air quality on asthma symptoms or social isolation on mood in older adults), the current study was unique in identifying and illustrating specific macroergonomic factors affecting the way patients and informal caregivers performed their work. These factors included how physical layouts and distances made it either harder or easier to attend appointments and obtain health-related supplies; how family and other social actors encouraged positive health behavior and helped control unhealthy behaviors; and how the workload of caring for others made it harder to take care of oneself but sometimes served as an opportunity to learn more about health and self-care. Of course, many of these and other individual factors have been identified and studied in other literatures such as health geography, ecological approaches to health behavior, medical sociology, and public health.
Figure 5.
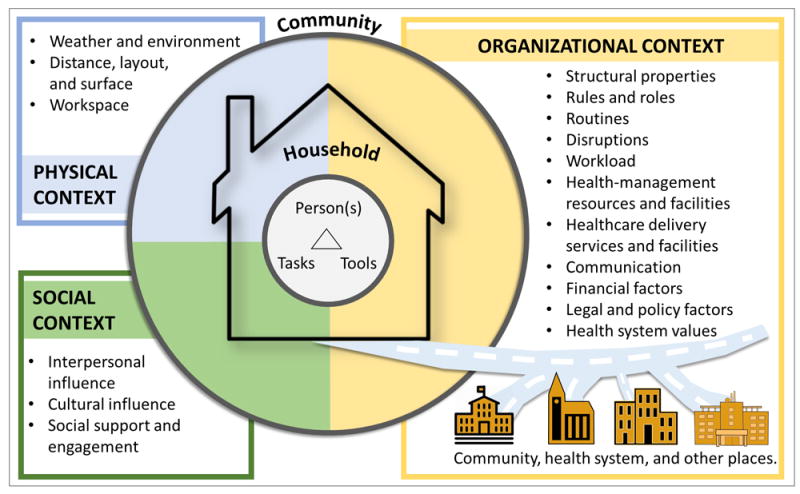
Empirically-specified macroergonomic patient work system model, depicting seventeen physical, social, and organizational macroergonomic factors in the household and community.
A number of noteworthy macroergonomic factors were found across the three studies. In the physical environment, important geospatial phenomena were observed, including distance from resources such as food—i.e., being located in so called ‘food deserts’ (Walker et al., 2010)—or being relatively close to a location but not being able to get there due to mobility and transportation barriers. Both homes and community settings could have layouts, surfaces, and other design characteristics enabling better patient and caregiver performance but these were not always intentionally built in (National Research Council, 2011).
Social factors were found across levels of analysis, from close social partners (e.g., spouse, child, friend) to others in the household, healthcare professionals, community members, and cultural groups. This accords with the notion of “networks” of people who assist, influence, inform, or otherwise influence patients (e.g., Penrod et al., 1995; Valdez & Brennan, 2015). Of interest, patients do not always benefit from the presence of others. Consistent with Mayberry and Osborn’s (2012) focus group study of diabetic adults, we found patients in the three studies perceived, experienced, and on occasion suffered from “nonsupportive behaviors” of family members and others. However, like much of the chronic illness literature, we found social support and engagement to be a largely positive presence in people’s lives (e.g., Graven & Grant, 2014)—and in our case, in people’s self-care performance. Our studies also found nonsupportive behaviors from healthcare professionals, although these too were exceptions to the norm.
Our findings on the organizational factors shaping patient work performance were perhaps the most intriguing, because they are the most varied and numerous. Not surprisingly, the structures of households and communities were important performance-shaping factors, as were resources and financial, legal, and policy factors. However, routines, disruptions, and workload factors are hallmarks of organizational sciences and HFE rarely formally considered in chronic illness research. Emerging conceptualizations of patient workload describe illness and treatment burdens for patients (Gallacher et al., 2011; Shippee et al., 2012), whereas the concept of “caregiver burden” has a longer history in areas such as aging and dementia care (Etters et al., 2008; Novak & Guest, 1989). Important follow-up questions relate to the origin, management, variability, and dynamics of patient workload, especially around key events such as transitions of care or changes in health (Nathan-Roberts et al., 2015; Shippee et al., 2012). Generally speaking, understanding the dynamics of macroergonomic factors’ impact on patient and caregiver performance will help determine how routines are built and rebuilt, and what happens during planned or unplanned disruptions such as medical or life events. In line with newer areas of HFE such as cultural ergonomics (Smith-Jackson et al., 2014) and community ergonomics (Smith et al., 2002), we observed the presence and influence of factors at the community and policy levels: from insurance regulations to access to healthcare services to the costs of medications. These high-level factors may not be considered by microergonomists and may not be perceived as modifiable by macroergonomists. As such, one strategy is to align intervention to the present reality while others strive for change. A second strategy is that HFE professionals partner with experts in management, public policy, sociology, anthropology, economics, and other fields concerned with high-level change (Koningsveld et al., 2005; Moray, 2000; Zink, 2000). This external partnership strategy coincides with Howell’s (2002) Presidential address, “The Human Factors-Ergonomics Parade: A Tale of Two Models” and the 2014 vision statement of the US Human Factors & Ergonomics Society (HFES):
We envision a future in which the reach, relevance, and quality of human factors/ergonomics are greatly expanded by enriching the science and enhancing its impact on solving societal problems by embracing outward-facing collaborations. (Imada, 2014, emphasis ours)
The present study revealed not only individual macroergonomic factors but combinations or interactions of macroergonomic factors across domains and levels, summarized in three typical cases. Interactions are the sine qua non of contemporary HFE (Wilson, 2000) and are unmistakably present in all complex sociotechnical systems (Carayon, 2006). They must be studied in all domains, including that of patient work, to understand the multiple related causes of performance. Three recent articles present additional cases featuring system interactions shaping patient work (Holden et al., 2015c; Holden et al., 2015d; Valdez & Holden, 2014) and social scientists have described scenarios in which interactions are evident (e.g., Hinder & Greenhalgh, 2012).
Methodology discussion and limitations
A strength of the present work was the combination of three studies differing in research teams, objectives, medical conditions, geographic locations, and methods. This kind of synthesis improves generalizability but is rare because it is time consuming and difficult to coordinate. The present paper is a product of over 1.5 years of efforts involving its authors. Unlike individual studies examining a single disease from a single study’s perspective, we are more confident in suggesting that the resulting model in Figure 5 and the factors identified and defined in Tables 1-3 can be applied to a variety of conditions and patient groups. Yet, we believe further studies in different settings will find different examples or manifestations of the factors we presented. Some will find additional factors and others will find that not all factors in Figure 5 are equally relevant. For example, the impact of weather and air quality is especially salient among people with heart and lung disease, as in our studies. However, interpersonal influence and social support may have very different effects in younger groups of, for example, adolescents with Type 1 diabetes mellitus. Effects across age and other demographic groups may also be similar at one level but varying in detail, such as the source of social influence in White vs. African American communities or the role of economic factors in unemployed younger people vs. retired older individuals on fixed incomes. As such, we believe our model is only general to a limit but can easily be modified to address, for example, work in patient domains such as dementia, cancer, pediatrics, disability, acute care, or transitional care.
Although well designed to discover and specify a variety of macroergonomic factors, our three studies did not comprehensively measure each factor or seek to statistically model questions of relationships and multivariate causality. These kinds of investigations are the type needed in the “Study” stage of the research cycle we presented in Figure 1. However, we note that the “Specify” stage, which was the focus of this paper, is a crucial one because it supports testing specific hypotheses and designing specific interventions. While we do not prescribe the specific ways model relationships or the model as a whole should be tested in the Specify and Study stages, we propose some suggestions:
Survey individuals across demographic, geographic, or clinical groups on each of the macroergonomic variables to established relative frequencies and group differences;
Perform content analyses of existing datasets including online forums or social networking services (Twitter, Facebook, Youtube) to understand the specific nature of macroergonomic factors within a given community (e.g., sexual minorities at risk for communicable disease);
Test structural models for moderating effects of macroergonomic variables on health-related beliefs and behaviors or the mediation of macroergonomic influence on health outcomes through behaviors such as self-care adherence;
Collect diary or photographic data on everyday patient and informal caregiver activities to understand how macroergonomic factors interact with microergonomic factors over time and space.
We note that our synthesis of three studies was limited in not being designed to achieve the kind of research suggested above. Further, it was outside the scope of this paper to report microergonomic factors related to individuals (e.g., patient health literacy), tasks (e.g., difficulty of medication management), and tools and technologies (e.g., usability of health websites). Those factors certainly contribute to performance and interact with macroergonomic factors (for more on these factors in the context of self-care performance, see, e.g., Holden et al., 2015d; Mickelson et al., 2015). While the combined sample size of the three studies was relatively large, one study had only 10 participants. Two of three studies used multiple methods and the precise method design was not identical across studies. The three studies had different, though complementary, objectives: both a strength and a weakness. Only three individual studies contributed to the synthesis, and more would have been preferable. Our overall analysis was also limited in being descriptive as opposed to prescriptive. Future work can provide specific design implications and suggestions for using models such as those in Figures 2, 4, and 5 to study and improve patient work performance.
Lastly, we note the practical challenges of performing research on the macroergonomic factors shaping patient work, especially across household and community settings. The challenges of conducting data collection in the home and community are described in other recent work (Holden et al., 2015a). Many of them have to do with participants who are usually not healthcare professionals, not employed or otherwise governed by one entity, and vary in terms of abilities, preferences, and social situations. Data collection creates safety, ethical, and analytic implications, some of which are also found in organizational HFE research (Holden et al., 2008) but some of which are unique. Valdez and Holden (in press) propose a number of strategies to manage health-related research and practice in home and community settings.
5. CONCLUSION
HFE researchers increasingly recognize that people who are not paid employees still perform work: “the term work…refer[s] to any form of human effort or activity, including recreation and leisure pursuits” (Hendrick, 2002, p. 1). The work of patients and caregivers may be invisible to most and therefore poorly understood, and consequently “the health-care industry struggles to answer key questions… How to increase patient participation? … How to improve information provision? … How to reduce medical errors? … How to leverage information technology?” (Unruh & Pratt, 2008, pp. 34-35).
Accordingly, HFE can play an important complementary role in health and healthcare research, by supplying theories and methods for studying and improving the performance of work among patients and informal caregivers. This can be done by attending to both micro- and macroergonomic factors pertinent to patient work, as well as crossing levels, for example, by considering individual differences in performing specific health-related tasks (e.g., diet monitoring) with specific technologies (e.g., mobile applications) in multiple physical, social, and organizational contexts – for example, in a community characterized by specific geospatial conditions (e.g., food deserts), food cultures (e.g., carbohydrate-rich diets), and resource limitations (e.g., free Internet access points). Having established the importance and nature of macroergonomic factors associated with the work of patients and caregivers, we urge others to work to replicate and extend our findings as well as to take the next step in the cycle in Figure 1: studying specific relationships and mechanisms by which macroergonomic factors affect this form of work and developing and testing related interventions.
Supplementary Material
PRACTITIONER SUMMARY.
Health-related activities of patients are embedded in and shaped by levels of social, physical, and organizational context. This paper combined findings from three studies to specify seventeen contextual or macroergonomic factors in home- and community-based work systems of chronically ill patients. These factors have research, design, and policy implications.
Acknowledgments
We thank the participants in the three studies and the research personnel involved in each. Journal reviewers provided helpful feedback. The Caring Hearts Study was supported by grants from the National Institute on Aging (NIA) of the US National Institutes of Health (NIH) (K01AG044439) and grants UL1 TR000445 and KL2 TR000446 from the National Center for Advancing Translational Sciences (NCATS/NIH) through the Vanderbilt Institute of Clinical and Translational Research (VICTR). The Keystone Beacon Project was funded by the US Office of the National Coordinator for Health IT through the Beacon award program (award No. 90BC001301). The project was also supported through Grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Alper SJ, Holden RJ, Scanlon MC, Patel NR, Kaushal R, Skibinski K, Karsh B, et al. Self-reported violations during medication administration in two pediatric hospitals. BMJ Quality & Safety. 2012;21:408–415. doi: 10.1136/bmjqs-2011-000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper SJ, Karsh B. A systematic review of safety violations in industry. Accident Analysis & Prevention. 2009;41(4):739–754. doi: 10.1016/j.aap.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Ancker JS, Witteman HO, Hafeez B, Provencher T, Van de Graaf M, Wei E. The invisible work of personal health information management among people with multiple chronic conditions: Qualitative interview study among patients and providers. Journal of Medical Internet Research. 2015;17(6) doi: 10.2196/jmir.4381. http://www.jmir.org/2015/2016/e2137/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA-Journal of the American Medical Association. 2002;288:2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- Carayon P. Human factors of complex sociotechnical systems. Applied Ergonomics. 2006;37:525–535. doi: 10.1016/j.apergo.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Carayon P, Karsh B, Gurses AP, Holden RJ, Hoonakker P, Hundt AS, Wetterneck TB, et al. Macroergonomics in healthcare quality and patient safety. Reviews of Human Factors and Ergonomics. 2013;8:4–54. doi: 10.1177/1557234X13492976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayon P, Schoofs Hundt A, Karsh B, Gurses AP, Alvarado CJ, Smith M, Brennan PF. Work system design for patient safety: the SEIPS model. Quality & Safety in Health Care. 2006;15:i50–i58. doi: 10.1136/qshc.2005.015842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayon P, Smith MJ, Haims MC. Work Organization, Job Stress, and Work-Related Musculoskeletal Disorders. Human Factors: The Journal of the Human Factors and Ergonomics Society. 1999;41(4):644–663. doi: 10.1518/001872099779656743. [DOI] [PubMed] [Google Scholar]
- Carayon P, Wetterneck TB, Rivera-Rodriguez AJ, Hundt AS, Hoonakker P, Holden RJ, Gurses AP. Human factors systems approach to healthcare quality and patient safety. Applied Ergonomics. 2014;45(1):14–25. doi: 10.1016/j.apergo.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui MA, Mott DA, Maxwell L. A qualitative assessment of a community pharmacy cognitive pharmaceutical services program, using a work system approach. Research in Social & Administrative Pharmacy. 2012;8:206–216. doi: 10.1016/j.sapharm.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dul J, Bruder R, Buckle P, Carayon P, Falzon P, Marras WS, van der Doelen B, et al. A strategy for human factors/ergonomics: Developing the discipline and profession. Ergonomics. 2012;55:377–395. doi: 10.1080/00140139.2012.661087. [DOI] [PubMed] [Google Scholar]
- Etters L, Goodall D, Harrison BE. Caregiver burden among dementia patient caregivers: A review of the literature. Journal of the American Academy of Nurse Practitioners. 2008;20(8):423–428. doi: 10.1111/j.1745-7599.2008.00342.x. [DOI] [PubMed] [Google Scholar]
- Fisk AD, Rogers WA, Charness N, Czaja SJ, Sharit J. Designing for Older Adults: Principles and Creative Human Factors Approaches. 2. Boca Raton, FL: CRC Press; 2009. [Google Scholar]
- Flanagan JC. The Critical Incident Technique. Psychological Bulletin. 1954;51:327–359. doi: 10.1037/h0061470. [DOI] [PubMed] [Google Scholar]
- Frumkin H, McMichael AJ, Hess JJ. Climate Change and the Health of the Public. American Journal of Preventive Medicine. 2008;35(5):401–402. doi: 10.1016/j.amepre.2008.08.031. http://dx.doi.org/10.1016/j.amepre.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Gallacher K, May CR, Montori VM, Mair FS. Understanding patients’ experiences of treatment burden in chronic heart failure using normalization process theory. The Annals of Family Medicine. 2011;9:235–243. doi: 10.1370/afm.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graneheim UH, Lundman B. Qualitative content analysis in nursing research: Concepts, procedures and measures to achieve trustworthiness. Nurse Education Today. 2004;25:105–112. doi: 10.1016/j.nedt.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Graven LJ, Grant JS. Social support and self-care behaviors in individuals with heart failure: An integrative review. International Journal of Nursing Studies. 2014;51:320–333. doi: 10.1016/j.ijnurstu.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Gurses A, Carayon P. Exploring performance obstacles of intensive care nurses. Applied Ergonomics. 2009;40:509–518. doi: 10.1016/j.apergo.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Gurses AP, Marsteller JA, Ozok AA, Xiao Y, Owens S, Pronovost PJ. Using an interdisciplinary approach to identify factors that affect clinicians’ compliance with evidence-based guidelines. Critical Care Medicine. 2010;38:S282–S291. doi: 10.1097/CCM.0b013e3181e69e02. [DOI] [PubMed] [Google Scholar]
- Hendrick HW. An overview of macroergonomics. In: Hendrick HW, Kleiner BM, editors. Macroergonomics: Theory, methods and applications. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 1–23. [Google Scholar]
- Henriksen K, Joseph A, Zayas-Cabán T. The human factors of home health care: A conceptual model for examining safety and quality concerns. Journal of Patient Safety. 2009;5:229–236. doi: 10.1097/PTS.0b013e3181bd1c2a. [DOI] [PubMed] [Google Scholar]
- Hignett S, Griffiths P, Sands G, Wolf L, Costantinou E. Patient falls: Focusing on human factors rather than clinical conditions. Proceedings of the HFES International Symposium on Human Factors and Ergonomics in Health Care: Advancing the Cause; 2013. pp. 99–104. [Google Scholar]
- Hinder S, Greenhalgh T. “This does my head in”. Ethnographic study of self-management by people with diabetes. BMC Health Services Research. 2012;12 doi: 10.1186/1472-6963-12-83. http://www.biomedcentral.com/1472-6963/12/83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden RJ. Social and personal normative influences on healthcare professionals to use information technology: Towards a more robust social ergonomics. Theoretical Issues in Ergonomics Science. 2012;13:546–569. doi: 10.1080/1463922X.2010.549249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden RJ, Carayon P, Gurses AP, Hoonakker P, Hundt AS, Ozok AA, Rivera-Rodriguez AJ. SEIPS 2.0: A human factors framework for studying and improving the work of healthcare professionals and patients. Ergonomics. 2013;56(11):1669–1686. doi: 10.1080/00140139.2013.838643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden RJ, Karsh B. A theoretical model of health information technology usage behaviour with implications for patient safety. Behaviour & Information Technology. 2009;28:21–38. [Google Scholar]
- Holden RJ, McDougald Scott AM, Hoonakker PLT, Hundt AS, Carayon P. Data collection challenges in community settings: Insights from two field studies of patients with chronic disease. Quality of Life Research. 2015a;24(5):1043–1055. doi: 10.1007/s11136-014-0780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden RJ, Mickelson RS. Performance barriers among elderly chronic heart failure patients: An application of patient-engaged human factors and ergonomics. Proceedings of the Human Factors and Ergonomics Society. 2013;57(1):758–762. [Google Scholar]
- Holden RJ, Or CKL, Alper SJ, Rivera AJ, Karsh B. A change management framework for macroergonomic field research. Applied Ergonomics. 2008;39:459–474. doi: 10.1016/j.apergo.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Holden RJ, Rivera AJ, Carayon P. Occupational macroergonomics: Principles, scope, value, and methods. IIE Transactions on Occupational Ergonomics and Human Factors. 2015b;3:1–8. doi: 10.1080/21577323.2015.1027638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden RJ, Scanlon MC, Patel NR, Kaushal R, Escoto KH, Brown RL, Karsh B, et al. A human factors framework and study of the effect of nursing workload on patient safety and employee quality of working life. BMJ Quality & Safety. 2011;20:15–24. doi: 10.1136/bmjqs.2008.028381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden RJ, Schubert CC, Eiland EC, Storrow AB, Miller KF, Collins SP. Self-care barriers reported by emergency department patients with acute heart failure: A sociotechnical systems-based approach. Annals of Emergency Medicine. 2015c;66:1–12. doi: 10.1016/j.annemergmed.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden RJ, Schubert CC, Mickelson RS. The patient work system: An analysis of self-care performance barriers among elderly heart failure patients and their informal caregivers. Applied Ergonomics. 2015d;47:133–150. doi: 10.1016/j.apergo.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell WC. The human factors-ergonomics parade: A tale of two models. 2002 Available at: http://www.hfes.org/Web/PubPages/Howell.pdf.
- Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Imada AS. New beginnings. HFES Bulletin. 2014;57(12) http://www.hfes.org/web/HFESBulletin/dec2014NewBeginnings.html. [Google Scholar]
- Karsh B, Holden RJ, Alper SJ, Or CKL. A human factors engineering paradigm for patient safety – designing to support the performance of the health care professional. Quality & Safety in Health Care. 2006;15:i59–i65. doi: 10.1136/qshc.2005.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsh B, Waterson PE, Holden RJ. Crossing levels in systems ergonomics: A framework to support ‘mesoergonomic’ inquiry. Applied Ergonomics. 2014;45(1):45–54. doi: 10.1016/j.apergo.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner BM. Macroergonomics: Analysis and design of work systems. Applied Ergonomics. 2006;37:81–89. doi: 10.1016/j.apergo.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Koningsveld EAP, Dul J, Van Rhijn GW, Vink P. Enhancing the impact of ergonomics interventions. Ergonomics. 2005;48(5):559–580. doi: 10.1080/00140130400029136. [DOI] [PubMed] [Google Scholar]
- Mayberry LS, Osborn CY. Family support, medication adherence, and glycemic control among adults with Type 2 diabetes. Diabetes Care. 2012;35(6):1239–1245. doi: 10.2337/dc11-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelson RS, Willis M, Holden RJ. Medication-related cognitive artifacts used by older adults with heart failure. Health Policy and Technology. 2015;4(4):387–398. doi: 10.1016/j.hlpt.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzner TL, McBride SE, Barg-Walkow LH, Rogers WA. Self-management of wellness and illness in an aging population. Reviews of Human Factors and Ergonomics. 2013;8:277–333. [Google Scholar]
- Moon SD, Sauter SL, editors. Beyond Biomechanics: Psychosocial Aspects of Musculoskeletal Disorders in Office Work. Bristol, PA: Taylor & Francis; 1996. [Google Scholar]
- Moray N. “De Maximis non Curat Lex” or How context reduces science to art in the practice of human factors; Paper presented at the Human Factors and Ergonomics Society 38th Annual Meeting; Nashville, TN. 1994. [Google Scholar]
- Moray N. Culture, politics and ergonomics. Ergonomics. 2000;43:858–868. doi: 10.1080/001401300409062. [DOI] [PubMed] [Google Scholar]
- Nathan-Roberts D, Holden RJ, Yin S, Valdez RS. Examining patient work: The when and how much of self-care. Proceedings of the 19th Triennial Congress of the International Ergonomics Association; 2015. pp. 1–3. [Google Scholar]
- National Research Council. Health Care Comes Home: The Human Factors. Washington, DC: National Academies Press. Committee on the Role of Human Factors in Home Health Care, Board on Human-Systems Integration, Division of Behavioral and Social Sciences and Education; 2011. [Google Scholar]
- Novak M, Guest C. Application of a Multidimensional Caregiver Burden Inventory. The Gerontologist. 1989;29:798–803. doi: 10.1093/geront/29.6.798. [DOI] [PubMed] [Google Scholar]
- Penrod JD, Kane RA, Kane RL, Finch MD. Who cares? The size, scope, and composition of the caregiver support system. The Gerontologist. 1995;35(4):489–497. doi: 10.1093/geront/35.4.489. [DOI] [PubMed] [Google Scholar]
- Rasmussen J. Risk management in a dynamic society: A modelling problem. Safety Science. 1997;27:183–213. [Google Scholar]
- Rogers EM. Diffusion of Innovations. 4. New York: Free Press; 1995. [Google Scholar]
- Saldaña J. The Coding Manual for Qualitative Researchers. 2. Thousand Oaks, CA: SAGE; 2013. [Google Scholar]
- Schulz R, Hebert RS, Dew MA, Brown SL, Scheier MF, Beach SR, Nicols L, et al. Patient suffering and caregiver compassion: New opportunities for research, practice, and policy. The Gerontologist. 2007;47:4–13. doi: 10.1093/geront/47.1.4. [DOI] [PubMed] [Google Scholar]
- Senteio C, Veinot TC. Trying to make things right: Adherence work in high-poverty, African-American neighborhoods. Qualitative Health Research. 2014;24:1745–1756. doi: 10.1177/1049732314549027. [DOI] [PubMed] [Google Scholar]
- Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: A functional, patient-centered model of patient complexity can improve research and practice. Journal of Clinical Epidemiology. 2012;65:1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Smith-Jackson TL, Resnick ML, Johnson HT, editors. Cultural Ergonomics: Theory, Methods, and Applications. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- Smith JH, Cohen WJ, Conway FT, Carayon P, Derjani-Bayeh A, Smith MJ. Community ergonomics. In: Hendrick HW, Kleiner BM, editors. Macroergonomics: Theory, Methods, and Applications. Mahwah, NJ: Lawrence Erlbaum; 2002. pp. 289–309. [Google Scholar]
- Smith MJ, Sainfort-Carayon P. Balance theory of job design for stress reduction. International Journal of Industrial Ergonomics. 1989;4:67–79. [Google Scholar]
- Strauss A. Continual Permutations of Action. New York: Aldine de Gruyter; 1993. [Google Scholar]
- Suchman L. Supporting Articulation Work. New York: Morgan Kaufmann; 1994. [Google Scholar]
- Unruh KT, Pratt W. Patients as actors: the patient’s role in detecting, preventing, and recovering from medical errors. International Journal of Medical Informatics. 2007;76:S236–S244. doi: 10.1016/j.ijmedinf.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Unruh KT, Pratt W. The invisible work of being a patient and implications for health care: “[the doctor is] my business partner in the most important business in my life, staying alive”. Proceedings of the Ethnographic Praxis in Industry Conference (EPIC ‘08); 2008. pp. 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez RS, Brennan PF. Exploring patients’ health information communication practices with social network members as a foundation for consumer health IT design. International Journal of Medical Informatics. 2015;84:363–374. doi: 10.1016/j.ijmedinf.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Valdez RS, Holden RJ. Conceptualizing the patient work system, Part A: macroergonomic models; Paper presented at the Human Factors in Organizational Design and Management - XI.2014. [Google Scholar]
- Valdez RS, Holden RJ. Health care human factors/ergonomics, homeward bound: Practical considerations for fieldwork in home and community settings. Ergonomics in Design. doi: 10.1177/1064804615622111. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez RS, Holden RJ, Novak LL, Veinot TC. Transforming consumer health informatics through a patient work framework: Connecting patients to context. Journal of the American Medical Informatics Association. 2015;22(1):2–10. doi: 10.1136/amiajnl-2014-002826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vuuren W, Shea CE, van der Schaaf TW. The development of an incident analysis tool for the medical field. Eindhoven, Netherlands: Technische Universiteit Eindhoven; 1997. [Google Scholar]
- Vidulich MA, Tsang PS. Mental workload and situation awareness. In: Salvendy G, editor. Handbook of Human Factors and Ergonomics. 4. Wiley & Sons: Hoboken, NJ; 2012. pp. 243–273. [Google Scholar]
- Vincent C, Coulter A. Patient safety: What about the patient? Quality & Safety in Health Care. 2002;11:76–80. doi: 10.1136/qhc.11.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AJ, Pratt CC, Eddy L. Informal caregiving to aging family members: A critical review. Family Relations. 1995;44:402–411. [Google Scholar]
- Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: A review of food deserts literature. Health & Place. 2010;16(5):876–884. doi: 10.1016/j.healthplace.2010.04.013. http://dx.doi.org/10.1016/j.healthplace.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Werner NE, Holden RJ. Interruptions in the wild: Development of a sociotechnical systems model of interruptions in the emergency department through a systematic review. Applied Ergonomics. 2015;51:244–254. doi: 10.1016/j.apergo.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Wickens CD, Lee JD, Liu Y, Gordon-Becker S. An Introduction to Human Factors Engineering. 2. Englewood Cliffs, NJ: Prentice Hall; 2003. [Google Scholar]
- Wiegmann DA, Eggman AA, ElBardissi AW, Parker SH, Sundt TM. Improving cardiac surgical care: A work systems approach. Applied Ergonomics. 2010;41:701–712. doi: 10.1016/j.apergo.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegmann DA, Shappell SA. Human Error Approach to Aviation Accident Analysis: The Human Factors Analysis and Classification System. Aldershot, UK: Ashgate; 2003. [Google Scholar]
- Wilson JR. Fundamentals of ergonomics in theory and practice. Applied Ergonomics. 2000;31:557–567. doi: 10.1016/s0003-6870(00)00034-x. [DOI] [PubMed] [Google Scholar]
- Wilson JR. Fundamentals of systems ergonomics/human factors. Applied Ergonomics. 2014;45:5–13. doi: 10.1016/j.apergo.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Woods DD, Dekker SC, R I, Johannessen LK, Sarter N. Behind Human Error. 2. Aldershot, UK: Ashgate; 2010. [Google Scholar]
- ZayasCabán T, Valdez RS. Human Factors and Ergonomics in Home Care. In: Carayon P, editor. Handbook of Human Factors and Ergonomics in Health Care and Patient Safety. 2. Boca Raton, FL: CRC Press; 2012. pp. 743–762. [Google Scholar]
- Zink KJ. Ergonomics in the past and the future: From a German perspective to an international one. Ergonomics. 2000;43(7):920–930. doi: 10.1080/001401300409116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


