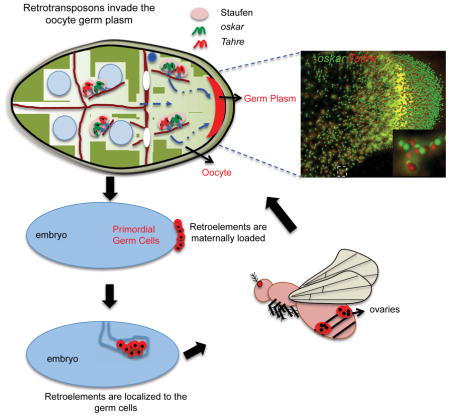
SUMMARY
Retrotransposons are a pervasive class of mobile elements present in the genomes of virtually all forms of life [1, 2]. In metazoans these are preferentially active in the germline, which, in turn, mounts defenses that restrain their activity [3, 4]. Here we report that certain classes of retrotransposons insure transgenerational inheritance by invading presumptive germ cells before they are formed. Using sensitized Drosophila and zebrafish models, we found that diverse classes of retrotransposons migrate to the germ plasm, a specialized region of the oocyte that prefigures germ cells and specifies the germline of descendants in the fertilized egg. In Drosophila, we found evidence for a ‘stowaway’ model, whereby Tahre retroelements traffic to the germ plasm by mimicking oskar RNAs and engaging the Staufen-dependent active transport machinery. Consistent with this germ plasm determinants attracted retroelement RNAs, even when these components were ectopically positioned in bipolar oocytes. Likewise, vertebrate retrotransposons similarly migrated to the germ plasm in zebrafish oocytes. Together, these results suggest that germ plasm targeting represents a fitness strategy adopted by some retrotransposons to insure transgenerational propagation.
Graphical Abstract
RESULTS AND DISCUSSION
Tahre retroelements migrate to the oocyte germ plasm
In the Drosophila ovary, retrotransoposon RNAs are often transcribed in neighboring nuclei and subsequently migrate to the oocyte. For example, Factor I retrotransposon RNAs produced in nurse cells migrate to the oocyte nucleus [5–7] and, likewise, Gypsy I RNAs produced in follicle cells traverse the perivitelline membrane to access the oocyte [6, 8, 9].
Recently, we discovered that p53 restrains mobile elements, raising the possibility that p53−/− genotypes could expose properties of transposons that are otherwise below detection limits [10–12]. Using this sensitized genetic background, we observed localization of Tahre retroelements to a posterior cortical region of the fly oocyte, consistent with possible migration to the so-called germ plasm [10]. To confirm this interpretation, we compared Tahre RNA localization patterns with well studied oskar and nanos RNAs. As a pioneer factor that initiates germ plasm formation, oskar transcripts migrate to the posterior region of the oocyte in mid oogenesis using an active and uniquely dedicated pathway [13]. Subsequently, nanos transcripts are recruited to this structure during the later stages of oogenesis [13, 14]. In situ hybridization experiments revealed early and efficient posterior localization of Tahre transcripts that mirrored oskar RNAs in oocytes of stage 9–10 egg chambers from p53−/− females. As shown in Figures 1A, Tahre localization patterns were virtually identical to oskar patterns and preceded the gradient of nanos transcripts seen in later-staged oocytes (Figure S1C). These studies demonstrated accumulation of Tahre retroelement in the p53−/− germ plasm (Figure 1A) (see also Figures S1B and 1C) and affirmed that little or no Tahre was detected in WT oocytes (see Figure S1A).
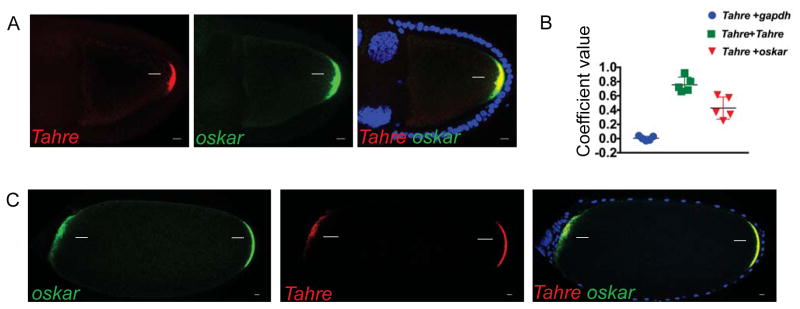
Figure 1. Tahre transposons mimic a germ plasm determinant.
A, confocal images of the simultaneous in situ hybridization of Tahre fluorescently labeled with Quasar 570, oskar 670 and their merge in Drosophila egg chambers of p53−/− ovaries with maximum Z-series projections spanning 5 μm in oocytes at stages 9–10. Scale bars: 10 μm. (Blue) DAPI counterstain. Arrows indicate germ plasm associated signal. (B) Co-localization of Tahre RNAs with oskar RNAs was quantified using automated image analyses. Controls included probes for gapdh and differentially labeled Tahre probes (see STAR Methods). n=5 egg chambers. C, confocal images of in situ co-hybridizations using oskar and Tahre probes (as in A) on stage 14 egg chambers from animals expressing the transgene oskar-bcd3′UTR ovaries in the p53−/− background. Note that Tahre RNAs mirror oskar RNAs, localizing to both the native (rightward arrows) and ecoptic germ plasms (leftward arrows) in these bipolar oocytes. See also Figure S1 and STAR Methods.
oskar RNAs migrate from nurse cells to the oocyte posterior in ribonucleoparticles (RNPs) [15]. To determine if Tahre RNAs were co-packaged in these granules, we quantified pairwise co-localization of Tahre with either oskar or nanos RNAs (See STAR Methods). Co-localization of Tahre and oskar (0.43) was significantly higher than Tahre and the gapdh control (.004) (Figure 1B) or Tahre and nanos (see Figure S1C) indicating Tahre transcripts often (but not always) co-localized with oskar RNAs. Furthermore, our co-localization analyses of oskar and Tahre RNAs in early (Figure S1G) and later-staged egg chambers (Figure S1H) suggested that co-packaging of Tahre RNAs in oskar RNPs initiates when these particles exit the nurse cell nuclei. As these cargoes traversed the oocyte, coincident signals steadily increased and peaked at the posterior cortical region (Figure S1I). Hence, as Tahre transcripts migrate to the posterior pole they were often - but not always - transported with the pioneer factor that initiates germ plasm assembly [16, 17].
To ask whether the germ plasm is itself sufficient to attract Tahre transcripts, we examined Tahre localization in samples expressing engineered oskar RNAs, (oskar-bcd3′UTR) that are misdirected to the anterior pole of developing oocytes by virtue of a bicoid (bcd) localization signal [18]. In these egg chambers, ectopic localization and translation of oskar-bcd3′UTR transcripts at the anterior pole triggers germ plasm formation, resulting in so-called ‘bipolar oocytes’ containing germ plasms at both the anterior (ectopic) and posterior (normal) pole [19]. As seen in Figures 1C, Tahre retroelements localized to both poles of these oocytes, exhibiting characteristic bipolar patterns that continued through the later stages of oogenesis and persisted in early embryos as well (Figure S1E, F). Together, these studies established that the germ plasm, rather than the posterior cortex, is sufficient to attract retrotransposons.
The oskar transport machinery conveys Tahre to the germ plasm
Staufen is an RNA binding protein required for transport of oskar RNAs to the posterior pole of the oocyte [20, 21]. Tahre contains very long 3′UTRs, which is an important feature of Staufen bound transcripts [22]. Therefore, we hypothesized that Staufen might also be required to transport Tahre RNAs to the germ plasm. To test this possibility, we inspected localization of Tahre and oskar patterns in Staufen−/−p53−/− [staufenHL/staufenry9; p53−/−] ovaries. Upon loss of Staufen [20, 21], oskar RNAs failed to localize to the germ plasm region as expected (Figures 2A and 2B). Likewise, when staufen was defective, Tahre retroelements similarly failed to localize (Figures 2C, 2D, S2A and S2B) and equivalent results were observed in RNAi strains depleted for staufen (see Figures S2 E–H). Importantly, like oskar RNAs (see Figure S2C), mislocalized Tahre transcripts (Figure 2D) accumulated at the anterior boundary of staufen-depleted oocytes. Furthermore, these retroelement RNAs were often - but not always - colocalized with oskar containing RNPs that were similarly mislocalized (Figure S2D).
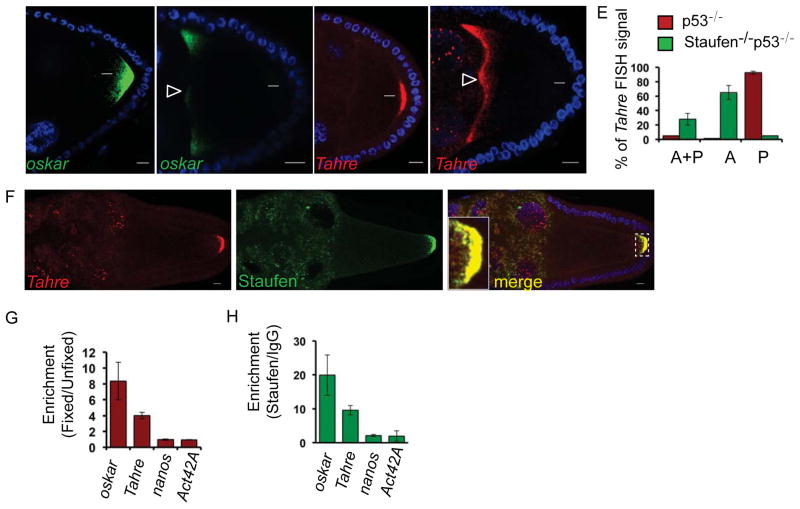
Figure 2. Tahre migration to the germ plasm requires oskar localization machinery.
In situ hybridizations for oskar and Tahre on p53−/− (A and C respectively) and Staufen−/− p53−/− (B and D respectively) egg chambers that were DAPI counterstained (Blue). Arrows indicate germ plasm region. The percentage of egg chambers exhibiting Tahre FISH signal at the anterior and posterior (A+P), anterior only (A) and posterior only (P) poles of stages 9 egg chambers of p53−/− and Staufen−/− p53−/−, were quantified from three biological replicates and are represented in bar graphs (E), n=50. Error bars represent ±SD. Note that in Staufen mutant oocytes, neither oskar nor Tahre localize at the posterior pole and, instead, these RNAs are stalled at the anterior pole (indicated by open arrow heads). Scale bar: 10 μm. Note that Tahre retroelements were similarly delocalized in egg chambers depleted for kinesin or Tm1C, see Supplemental Figures S3D and E. F show that Tahre transcripts co-associate with Staufen in the germ plasm (inset). Tahre in situ hybridization on GFP-Staufen; p53−/− ovaries depicting localization of Tahre, GFP-Staufen and merged. DAPI counterstain (Blue). Scale bar: 10 μm. (G, H) Tahre transcripts physically associate with Staufen. RIP assays were performed in formaldehyde fixed and unfixed p53−/− ovaries using IgG controls and Staufen antibodies. Fold enrichment for binding of the indicated RNAs were calculated relative to unfixed samples (G) or samples precipitated with IgG (H). Note that like the positive control oskar, Tahre transcripts were significantly enriched by precipitation of Staufen. For negative controls, nanos and act42A RNAs were used. Bar graphs are averages of three biological replicates. Error bars represent ±SD. See also Figures S2 and S3.
Staufen contains five RNA binding domain (RBD) motifs [23]. One domain, RBD2 is known to be crucial for the posterior localization of oskar transcripts but another domain, RBD5 is dispensable [21]. To investigate whether RBD2 is similarly required for localization of retroelement RNAs, we visualized Tahre transcripts using staufen alleles mutated for either the RBD2 or RBD5 and quantified Tahre RNAs using our FISH assays. As seen in Figures S2, the RBD2 of Staufen was critical for migration of Tahre to the germ plasm region while the RBD5 was dispensable (Figures S2I–K). Therefore, not only is Staufen required to transport Tahre to the germ plasm, but Tahre localization also requires the same RNA binding domain needed to convey oskar transcripts. Using the same approach, we also examined other factors that transport oskar RNAs, including kinesin (Figures S3D and S3E), and its interacting partner Tropomyosin1C (Tm1C) (Figures S3F and S3G), [24–26]. Here we observed that Tahre and oskar were similarly mislocalized, providing further support for the idea that these RNAs target the germ plasm region using shared molecular machinery.
Trafficking of oskar RNAs that form the germ plasm is mediated by physical association with Staufen protein. Therefore, to test whether Tahre retroelements might similarly complex with Staufen we used complementary methods. First, using a tagged allele of Staufen [27] we found extensive co-localization with Tahre RNAs, at the germ plasm (Figures 2F). Second, we performed RNA immunoprecipitation (RIP) assays and detected significant binding of Tahre retroelements to Staufen. As seen in Figures 2G and 2H, these interactions were comparable to binding of Staufen to oskar RNAs [22], which served as our benchmark for these studies. Furthermore, these interactions were specific, since neither nanos (posteriorly localizing) nor act42A mRNAs were enriched (Figures 2G and 2H). Together, these observations suggest that Tahre, like oskar, physically associates with Staufen to reach the presumptive germ plasm.
Tahre retroelements persist in germ cells of the next generation
Tahre retroelements are co-transported with oskar RNPs in a Staufen-dependent manner, suggesting that these selfish elements may target the germ plasm as a fitness strategy that insures transmission to the F2 generation. To evaluate this possibility, we determined whether Tahre RNAs persisted in the germline of the F1 generation by inspecting recently fertilized embryos prior to the onset of zygotic expression. We observed Tahre transcripts localized to the pole plasm of precellularized embryos (see Figure S4E–G) as well as at the pole cells of the cellularized embryos (Figures 3A and S4A). We used nanos transcripts to mark embryonic pole cells (Figure S4B) in these studies and, as seen in Figure S4C, Tahre and nanos RNAs were extensively co-localized. Hence, these retroelement RNAs are transmitted from the oocyte germ plasm to the embryo and, furthermore, these maternally derived transcripts persist in the presumptive germ cells of the F1 blastula. To genetically verify this maternal effect, we inspected early embryos produced from either p53+ or p53− mothers. In these reciprocal out crosses, maternally loaded Tahre RNAs appeared only in samples produced from p53− mothers, as expected (Figure S4D). Hence, Tahre retroelements can invade the germline of subsequent generations by targeting of the oocyte germ plasm. Furthermore, as shown in Figures 3B and 3C, we also detected Tahre transcripts in the primordial gonads of later staged embryos (stage 8), suggesting that these maternally loaded mobile elements persist in the differentiated germline of descendants.
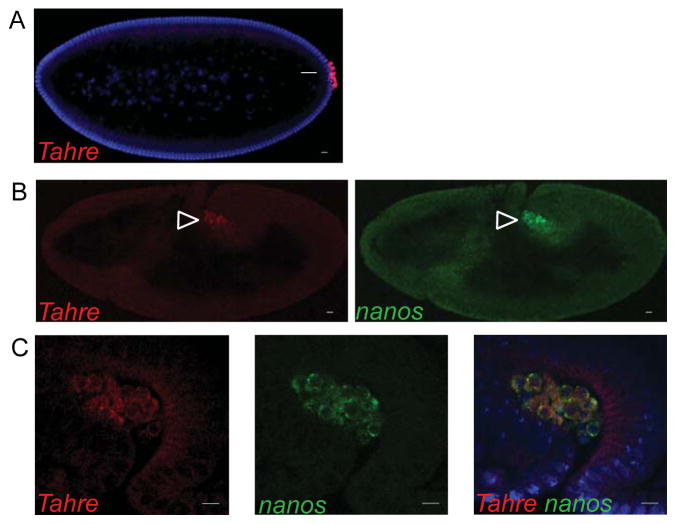
Figure 3. Tahre elements from the oocyte germ plasm persist in the embryonic germline.
In (A), Tahre RNAs localize to the germline progenitors, the pole cells (arrow) of 0–3 hr p53−/− embryos. (B) in situ hybridizations reveal that Tahre localizes to the gonads (arrowheads) of later staged p53−/− embryos (stage 8), similar to the nanos marker. (C) Magnified images of Tahre, nanos and their merge. DAPI counterstain (Blue). Scale bars: 10 μm. See also Figure S4.
Migration of retrotransposons to the germ plasm is a conserved phenomenon
To ask whether germ plasm targeting by retroelements is a broadly conserved phenomenon we surveyed other classes of Drosophila retroelements. Like Tahre retroelements, Burdock and HeT-A also localized to the oocyte germ plasm (Figures 4A–F and see also Table S1) and, similarly, Burdock RNAs (see Figures S4H–I) persisted in the pole plasm of precellularized embryos. In contrast, other retroelements such as Idefix were not localized to germ plasm. Hence, some but not all classes of Drosophila retrotransposons target the germ plasm and persist in pole cells.
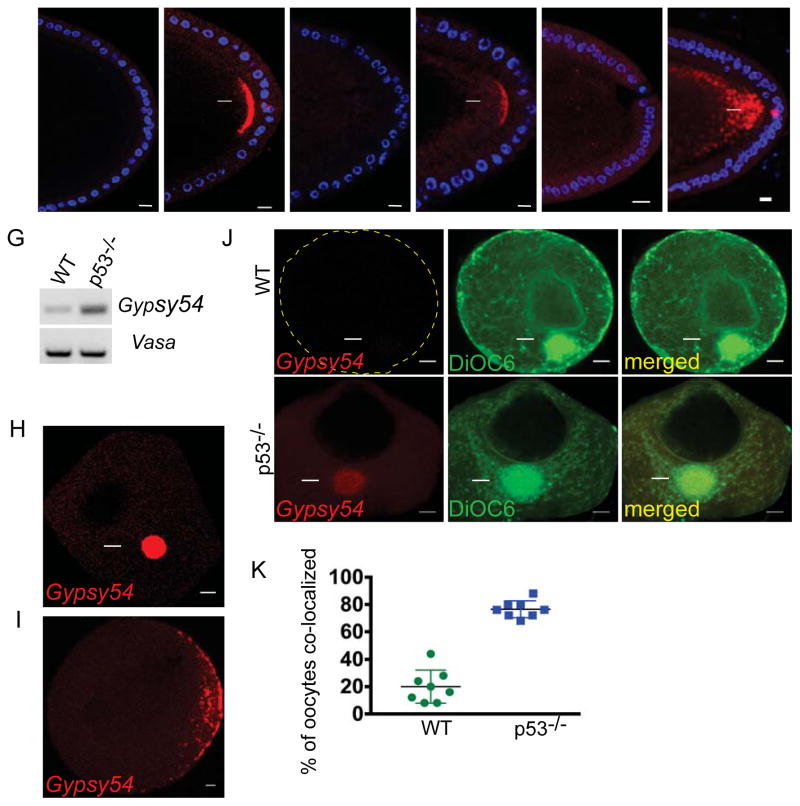
Figure 4. Germ plasm localization is conserved in invertebrates and vertebrates.
Patterns of other retrotransposons in the fly oocyte were profiled by in situ hybridization. Examples of Tahre (A and B), Burdock (C and D), HeT-A (E and F) on WT and p53−/− oocytes are shown. DAPI counterstain (Blue). Like Tahre, Burdock and HeT-A RNAs also localized to the germ plasm region (arrows). Scale bars: 10 μm. G–K illustrate that Gypsy54 localizes to the germ plasm of zebrafish oocytes. (G) RT-PCR analysis of Gypsy54 and vasa (loading control) from WT and p53−/− zebrafish ovaries. (J–I) shows in situ hybridizations for Gypsy54 RNA (red) on WT and p53−/− oocytes. Arrows point to the Balbiani body, the presumptive germ plasm of stage I oocytes (H). The arrowhead in (I) indicates the germ plasm at the cortical region of stage II oocytes. DiOC6 (green) was used to label the Balbiani body (J, arrow) in p53−/− and WT oocytes. Images are single optical sections. The scatter plot in (K) shows percentages of DiOC6-stained Balbiani bodies that were also positive for Gypsy54 RNA. See also Figure S4 and See STAR Method.
To determine whether germ plasm targeting might also occur in vertebrate systems, we examined developing oocytes in zebrafish and, as above, we used p53−/− mutants as a sensitized background. In preliminary studies, we profiled native retroelements that erupt in early p53−/− zebrafish embryos (A.W. and A.J., unpublished observations) and, among these, we selected Gypsy54 for FISH analyses, since this retroelement was highly derepressed in p53−/− ovaries (Figure 4G). Using Gypsy54-specific probes, we observed that these RNAs localized to the Balbiani body, an aggregate of mitochondria, Golgi and endoplasmic reticulum [28, 29] that marks the germ plasm of zebrafish oocytes at this stage (Figure 4H). The Balbiani body is present in oocytes of all animals including mammals [30, 31] and can be routinely detected by staining with DiOC6 [32]. Therefore, to confirm our interpretation, we conducted FISH experiments followed by DiOC6 staining and, as seen in Figures 4H–J, Gypsy54 transcripts accumulated in the germ plasm of these early oocytes (Figure 4J), co-localizing with DiOC6-stained Balbiani bodies at high penetrance (Figure 4K). In later staged oocytes, the germ plasm migrates to the cortical region [33] and, as seen in Figure 4I, Gypsy54 transcripts similarly localized to these positions as well. Hence, retroelements present in vertebrate genomes can target the oocyte germ plasm. We also followed Gypsy54 RNAs after fertilization prior to zygotic transcription and, in these studies, we detected localization to the cleavage planes of 4 cell stage embryos (Figure S4N). Since gametes form at these positions [34], our results suggest that, as in flies, retroelement RNAs can persist in the presumptive gametes of the F1 generation.
Conclusion
We found that diverse classes of retrotransposons migrate to - and accumulate in - the germ plasm, a structure within the oocyte that prefigures germ cells and ultimately specifies the germline of F1 descendants. In this way, certain mobile elements appear to invade rudimentary components of germ cells that eventually form ‘grandchildren’ through the maternal lineage. Hence, germ plasm targeting could represent a fitness strategy that promotes transmission in subsequent generations and, at the same time, may evade un-necessary costs associated with transposition in somatic cells [35]. We found compelling support for this in our case study of the Drosophila Tahre retroelement. First, RNA coded by this transposon mirrored the migratory behaviors of both native and ectopic oskar RNAs, which specify germ plasm formation. Second, germ plasm targeting by Tahre required at least three factors (Staufen, kinesin heavy chain and Tm1C) that normally enable oskar RNAs to construct the germ plasm. Third, Tahre RNAs physically associated with Staufen, recruiting the same machinery (Figures S2 and S3) and RNA binding domain (Figure S2I and S2J) needed to transport oskar RNAs.
An attractive explanation for these observations proposes that Tahre transcripts mimic oskar RNAs, acting as molecular stowaways to gain passage from their site of production in nurse cells to the oocyte germ plasm. Consistent with this, we found that Tahre RNAs were indeed co-packaged within oskar granules (Figures S1G–I) that are transported by the Staufen complex. Since oskar RNAs multimerize within RNPs [36], it is plausible that Tahre retroelements indirectly associate with Stafuen by tethering to oskar RNAs en route to the posterior pole. However, we favor direct physical binding to Staufen for two reasons. First, in both normal and perturbed settings we infrequently, but consistently, observed RNPs that contained Tahre RNAs but lacked detectable oskar RNAs (e.g. Figure S1H). Second, Tahre retroelements were decoupled from germ plasm accumulation of oskar RNAs in stage 10 osk84 oocytes that lacked Oskar protein (see Figure S3C, where oskar RNAs accumulated in the germ plasm region but Tahre retroelements did not). While Tahre mislocalization in these mutants could reflect a role for Oskar protein, more likely explanations for this phenotype trace to the absence of a germ plasm in osk84 oocytes [37, 38]. Nevertheless, taken together, these observations suggest that co-packaging with oskar transcripts is often incidental but is not required to transport Tahre to the germ plasm.
Together, our observations provide compelling evidence that Tahre retrotransposons use molecular mimicry to traffic to the germ plasm by co-opting a pathway dedicated to forming this structure. Consistent with this, germ plasm targeting is a property shared by other retroelements in Drosophila and in zebrafish (Figures 4 and S4E–M). Furthermore, transposon RNAs in the oocyte germ plasm persisted in the germline progenitors of the next generation (Figures S4E–N) and, in the case of Tahre, these likely perdured in gametes of these descendants. Since other retrotransposons migrate to the oocyte nucleus [5–7], this work illustrates how diverse retrotransposons can adopt different propagation strategies by targeting distinct subcellular components within the oocyte.
Retrotransposons and their host genomes exert reciprocal selective pressures that are often exposed in reproductive contexts, including oogenesis. Since Tahre elements support telomere formation [39], the need to maintain telomeres could potentially drive germ plasm targeting. However, this explanation is not generally satisfying since other retrotransposons with no role in telomere function also migrate to the germ plasm (e.g., Burdock in flies, Gypsy54 in zebrafish). In closing, it is tempting to speculate that some mobile elements may have contributed to the evolution of germline specification by targeting the germ plasm in some lineages. Consistent with this, evolutionary models teach that transposons “tend to create, and then to sharpen, distinctions between the germline and soma” [40]. Considering the evidence presented here we believe this is a plausible scenario.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for protocols and datasets should be directed to and will be fulfilled by the Lead Contact, John Abrams (john. abrams@utsouthwestern.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fly stocks were maintained at 22°C–25°C on standard food medium. Two p53−/− null alleles, p53−/−, 238H (ns) and 5A-1-4 (k1), were used in transcombination. For comparison, yw and w1118 were used in transcombination. For knockdown studies, staufen UAS-RNAi (BL#43187) were obtained from the Bloomington Stock Center (Indiana University, Bloomington, IN) and crossed with 238H (ns). The driver line gal4-nanos (BL#4442) was crossed with 5A-1-4 (k1) to generate p53 mutations. staufenHL (BL#1507) and staufenry9 (BL#10742) [20], were crossed with 5A-1-4 (k1) to generate p53 mutations, and used in (transcombinations). Mutant stocks stauD3, P{w+, staufenΔRBD5}/CyO; stauD3, P{w+, staufenΔloop2}, sp/CyO [21] were crossed with staufenHL (BL#1507) to visualize Tahre localization. w;pUASp-oskar (CDNA, w+)-bcd3′UTR;PrDr/Tm3,Ser [19] was a kind gift from Dr. Ruth Lehmann, The Howard Hughes Medical Institute, The Skirlball Institute of Biomolecular Medicine, Newyork University School of Medicine, were crossed with p53 null strains to generate p53 mutation.
METHOD DETAILS
In situ hybridization on Drosophila ovaries
Stellaris probes (Biosearch Technologies) were designed, to detect the Tahre, Gypsy, Burdock, HeT-A, oskar and nanos mRNAs, using the Biosearch design tool (Table S2). Quasar 570-conjugated Stellaris oligonucleotide probes against gapdh were obtained from LGC Biosearch Technologies (Petaluma, CA). Ovaries were hybridized with the Stellaris RNA FISH probe sets following the manufacturer’s instructions. Briefly, ovaries were dissected into PBS and fixed for 35 min at room temperature with 4% formaldehyde solution in PBS. After fixation, ovaries were placed in 70% ethyl alcohol overnight at 4°C. The following day, the ethyl alcohol was aspirated, and wash buffer (2x SSC, 10% deionized formamide in nuclease-free water) was added for 5 min. The probe was diluted at a concentration of 50 nM in hybridization buffer (2x SSC, 10% dextran sulfate [Sigma, D8906], 1 mg/mL tRNA [Sigma, R8759], 2 mM vanadyl robonucleoside complex [New England Biolabs], 10% deionized formamide (in nuclease free water). Hybridization solution with probes was added to each sample which were then placed at 37°C for 24 h. The samples were then washed with wash buffer twice for 15 min each at 37°C. Vecta Shield (Vector Laboratories) with DAPI was added before mounting and imaging. For Figure S3, RNA in situ hybridization was followed as described in [25].
Immunostaining of fly ovary
Well-fed females were dissected in PBS and fixed in 4% formaldehyde (Thermo scientific, 28908) diluted in PBS–0.1% Triton X-100 (PBST) and three volumes of heptane. After washing, tissues were blocked in 1.5% BSA and then incubated with 1:250 GFP antibody (rabbit, Invitrogen Molecular Probes, A11122) overnight at 4°C and probed with Alexa-488 (Invitrogen) secondary antibody. After washing, ovaries were mounted in Vecta Shield (Vector Laboratories) for imaging.
Drosophila embryo collections and in situ hybridization
Embryos (0–2 h) were collected from WT (yw/w1118) and p53−/− (k1/ns) strains on standard grapes juice agar plates with yeast paste. RNA in situ hybridizations were performed using manufacturers (Stellaris) protocol. Embryos were dechorionated in ~50% bleach for 2 minutes and washed thoroughly with double distilled H2O. Embryos were transferred to a scintillation vial-containing fixative (1ml 4% PBS and 4ml heptane) and vortexed for 25 min. The embryos at the bottom were collected and rinsed with methanol 3 times and stored in methanol overnight prior to in situ hybridization. The same protocol was used on 0–3 h and 2–6 h embryos to analyze pole cells and primordial gonad cells respectively.
In situ hybridization on Zebrafish embryos and ovaries
Zebrafish of the AB and tp53M214K/M214K [41] strains were used for these experiments. Stellaris probes (Biosearch Technologies) were designed to detect the Gypsy54 mRNAs using the Biosearch design tool. Sequence of zebrafish Gypsy54 was obtained from Repbase [42]. Probes were labeled with Quasar 570; sequences are listed in Table S2. 2–4 cell-staged embryos were washed in 1X PBS and fixed in 4% EM-grade paraformaldehyde (Electron Microscopy Sciences) overnight at 4°C. Fixed embryos were wash ed in 1X PBS and hand-dechorionated. After washing in 1X PBS, embryos were stored in 100% methanol at least one day, and rehydration of embryos were carried out as described in [43]. Hybridization protocols, hybridization buffer and washing buffer were used as described for Drosophila ovary and embryos.
For in situ hybridization on ovary, WT and p53−/− fish were euthanized with an overdose of Tricaine. A small portion of the ovary was removed and stored in TRIzol for RNA isolation. The remainder of the ovary was fixed in situ in the fish overnight in 4% PFA/1X PBS at 4°C. Fixed ovaries were dissected out and and stored in 100% methanol at –20°C for at least 24 hours. On the third day, ovaries were rehydrated as described before for the Zebrafish embryos. Ovaries were digested with prewarmed proteinase K solution at 37°C for 30 min and digestion was stopped by incubating in 4% PFA in 1X PBST 5 min, followed by 4 washes with PBST. Ovaries were prehybridized in hybridization buffer for 2–5 hours at 37°C. Next, Hybridization buffer was removed and hybridization buffer containing probes and then incubated at 37°C for 24 h. The samples were then washed with wash buffer twice for 15 min each at 37°C. DiOC6 staining was performed with 5ng/ml DiOC6 (Invitrogen, D273) in 1X PBS for 10 minutes in rotating condition at room temperature. The ovaries were washed with 1X PBS 3 times; Vecta Shield (Vector Laboratories) without DAPI was added before mounting and imaging.
RNA Immunoprecipitation assay
A modified version of the protocol described in [44] was used with some modifications. Dissected ovaries were cross-linked using 0.08% formaldehyde in 1X PBS for 10 min and fixation was quenched with glycine. The ovaries were then placed on ice and rinsed with 1X PBS and followed by radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, 200 mM NaCl, 0.4% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA, 200 mM NaCl). The samples were lysed in RIPA plus (RIPA, 2.5 mg/ml yeast tRNA, 1 mM PMSF, 50 U/ml Roche RNAse inhibitor) and clarified by centrifugation. Supernatant was collected and mixed with either mixed with either anti-Staufen or anti-goat IgG antibodies and incubated at 4°C for 12 h followed by addition of protein A/G agarose beads (Santa Cruz). Samples were then incubated for 4 h at 4°C under constant rotation. The beads were then washed with 500 μl of RIPA plus U (RIPA plus supplemented with 1 M urea) and incubated in 1 mM Tris-HCl (pH 6.8), 5 mM EDTA, 10 mM DTT, 1.0% SDS at 70 °C for 45 min. Proteinase K was added to a final concentration of 0.1 mg/ml and samples were incubated for 25 min at 37°C. RNA was isolated using TRIzol, cDNA synthesis was performed using an iScript kit. Quantification was performed with digital droplet PCR (BIORAD) using Eva Green supermix on a QX200 droplet reader. All concentration values for RIP assay targets were normalized either to IgG or unfixed samples. Primers used in this study were adapted from [10, 45]
Microscopy
Images were captured using 20X and 40X oil objectives with a Zeiss LSM 780 and LSM 880 inverted confocal microscopes and were prepared for presentation using the Fiji software package.
QUANTIFICATION AND STATISTICAL ANALYSIS
Co-localization analyses
For each experiment, image stacks were acquired from 3–5 different egg chambers/oocytes at each developmental stage shown. Images were taken at 0.3 μm intervals throughout the egg chamber using Zeiss LSM 880 inverted confocal microscope. For comparative analyses, acquisition settings were identical for all datasets. Images were deconvolved using the blind deconvolution algorithm of Autoquant X (Media Cybernetics). Co-localization was evaluated quantitatively using the coloc module of Imaris (Bitplane). Intensity thresholds of 500 was set and applied to all datasets. Co-localization statistics for each two-channel pair were obtained as csv files and analyzed with prism7 software. For upper limits of co-localization Quasar 570 and 670-labelled probes targeting overlapping regions on Tahre mRNAs were generated and cohybridized. For negative control, Tahre probes labeled-670 was co-hybridized with gapdh probe labeled with Quasar 570. As controls for the upper limits of co-localization, we co-hybridized Tahre probes, labeled with Quasar 570 and 670 (see Figure S1B). In addition, as a negative control, we also hybridized Tahre probes with a probe for gapdh (a non posterior localizing mRNA) (see Figure S1D). Pairwise co-localization of the two transcripts was measured by calculating the Pearson’s coefficient in colocalized volume [46] using Imaris 8.0 (Biplane) software from and plotted using Prism7 software.
Supplementary Material
Table S2. Oligonucleotide sequences for RNA in situ hybridization experiments. Related to STAR Methods and Figures 1, 2, 3, and 4.
Acknowledgments
B.T. was supported by the CPRIT training grant to UT Southwestern. This work was supported by grants to PK (1F31GM108472-04), AW (NRSA 1F31CA189691-01), AEJ (ACS 128847-PF-15-160-01-DDC), GBG (R01GM100088), JFA (NIH R01CA135731 and CPRIT RP110394) and JMA (NIH R01GM072124, R01GM115682, and the Welch Foundation I-1865). GFP-Staufen, staufenΔRBD2 and staufenΔRBD5 strains were a kind gift from Andrea H. Brand Ph.D., from Wellcome/CRC Institute and Department of Genetics; Cambridge. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. We thank the University of Texas Southwestern Live Cell Imaging Core, especially Kate Luby-Phelps Ph.D. and Abhijit Bugde, for assisting in microscopy and co-localization analyses. We would like to acknowledge Angelica Sanchez Ph.D. (J. Amatruda Lab at UTSW) for help in Zebrafish RNA in situ hybridization experiments. We thank Varsha Bhargava (M. Buszczak lab at UTSW) for advice on the RIP assay. We also thank Dr. Nicholas Conrad Department of Microbiology at UTSW for his helpful discussions.
Footnotes
Author Contributions
B.T. designed experiments, carried out experiments, analyzed data and composed the manuscript. J.M.A. designed experiments, analyzed the data and contributed to manuscript composition. G.G. helped design experiments, analyze the data and gave critical suggestions. D.P.B performed in situ hybridization and image acquisition on oskar RNA and protein null strains, Tm1C and Khc deplete strains. P.K. helped conceive the study, interpret the data and conducted initial experiments. A.J. and A.W. contributed pivotal unpublished data particularly for the zebrafish studies. J.F.A. helped design experiments, interpret the data and made critical technical contributions for the zebrafish studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hua-Van A, Le Rouzic A, Maisonhaute C, Capy P. Abundance, distribution and dynamics of retrotransposable elements and transposons: similarities and differences. Cytogenet Genome Res. 2005;110:426–440. doi: 10.1159/000084975. [DOI] [PubMed] [Google Scholar]
- 2.Biemont C, Vieira C. Genetics: junk DNA as an evolutionary force. Nature. 2006;443:521–524. doi: 10.1038/443521a. [DOI] [PubMed] [Google Scholar]
- 3.van den Hurk JA, Meij IC, Seleme MC, Kano H, Nikopoulos K, Hoefsloot LH, Sistermans EA, de Wijs IJ, Mukhopadhyay A, Plomp AS, et al. L1 retrotransposition can occur early in human embryonic development. Hum Mol Genet. 2007;16:1587–1592. doi: 10.1093/hmg/ddm108. [DOI] [PubMed] [Google Scholar]
- 4.Zamudio N, Bourc’his D. Transposable elements in the mammalian germline: a comfortable niche or a deadly trap? Heredity (Edinb) 2010;105:92–104. doi: 10.1038/hdy.2010.53. [DOI] [PubMed] [Google Scholar]
- 5.Seleme MC, Disson O, Robin S, Brun C, Teninges D, Bucheton A. In vivo RNA localization of I factor, a non-LTR retrotransposon, requires a cis-acting signal in ORF2 and ORF1 protein. Nucleic Acids Res. 2005;33:776–785. doi: 10.1093/nar/gki221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van De Bor V, Hartswood E, Jones C, Finnegan D, Davis I. gurken and the I factor retrotransposon RNAs share common localization signals and machinery. Dev Cell. 2005;9:51–62. doi: 10.1016/j.devcel.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton RS, Hartswood E, Vendra G, Jones C, Van De Bor V, Finnegan D, Davis I. A bioinformatics search pipeline, RNA2DSearch, identifies RNA localization elements in Drosophila retrotransposons. RNA. 2009;15:200–207. doi: 10.1261/rna.1264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song SU, Kurkulos M, Boeke JD, Corces VG. Infection of the germ line by retroviral particles produced in the follicle cells: a possible mechanism for the mobilization of the gypsy retroelement of Drosophila. Development. 1997;124:2789–2798. doi: 10.1242/dev.124.14.2789. [DOI] [PubMed] [Google Scholar]
- 9.Touret F, Guiguen F, Terzian C. Wolbachia influences the maternal transmission of the gypsy endogenous retrovirus in Drosophila melanogaster. MBio. 2014;5:e01529–01514. doi: 10.1128/mBio.01529-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wylie A, Jones AE, D’Brot A, Lu WJ, Kurtz P, Moran JV, Rakheja D, Chen KS, Hammer RE, Comerford SA, et al. p53 genes function to restrain mobile elements. Genes Dev. 2016;30:64–77. doi: 10.1101/gad.266098.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wylie A, Jones AE, Abrams JM. p53 in the game of transposons. Bioessays. 2016;38:1111–1116. doi: 10.1002/bies.201600115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine AJ, Ting DT, Greenbaum BD. P53 and the defenses against genome instability caused by transposons and repetitive elements. Bioessays. 2016;38:508–513. doi: 10.1002/bies.201600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becalska AN, Gavis ER. Lighting up mRNA localization in Drosophila oogenesis. Development. 2009;136:2493–2503. doi: 10.1242/dev.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrest KM, Gavis ER. Live imaging of endogenous RNA reveals a diffusion and entrapment mechanism for nanos mRNA localization in Drosophila. Curr Biol. 2003;13:1159–1168. doi: 10.1016/s0960-9822(03)00451-2. [DOI] [PubMed] [Google Scholar]
- 15.Mhlanga MM, Bratu DP, Genovesio A, Rybarska A, Chenouard N, Nehrbass U, Olivo-Marin JC. In vivo colocalisation of oskar mRNA and trans-acting proteins revealed by quantitative imaging of the Drosophila oocyte. PLoS One. 2009;4:e6241. doi: 10.1371/journal.pone.0006241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehmann R. Germ Plasm Biogenesis--An Oskar-Centric Perspective. Curr Top Dev Biol. 2016;116:679–707. doi: 10.1016/bs.ctdb.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahowald AP. Assembly of the Drosophila germ plasm. Int Rev Cytol. 2001;203:187–213. doi: 10.1016/s0074-7696(01)03007-8. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald PM, Struhl G. cis-acting sequences responsible for anterior localization of bicoid mRNA in Drosophila embryos. Nature. 1988;336:595–598. doi: 10.1038/336595a0. [DOI] [PubMed] [Google Scholar]
- 19.Ephrussi A, Lehmann R. Induction of germ cell formation by oskar. Nature. 1992;358:387–392. doi: 10.1038/358387a0. [DOI] [PubMed] [Google Scholar]
- 20.St Johnston D, Beuchle D, Nusslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66:51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- 21.Micklem DR, Adams J, Grunert S, St Johnston D. Distinct roles of two conserved Staufen domains in oskar mRNA localization and translation. EMBO J. 2000;19:1366–1377. doi: 10.1093/emboj/19.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laver JD, Li X, Ancevicius K, Westwood JT, Smibert CA, Morris QD, Lipshitz HD. Genome-wide analysis of Staufen-associated mRNAs identifies secondary structures that confer target specificity. Nucleic Acids Res. 2013;41:9438–9460. doi: 10.1093/nar/gkt702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.St Johnston D, Brown NH, Gall JG, Jantsch M. A conserved double-stranded RNA-binding domain. Proc Natl Acad Sci U S A. 1992;89:10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaspar I, Sysoev V, Komissarov A, Ephrussi A. An RNA-binding atypical tropomyosin recruits kinesin-1 dynamically to oskar mRNPs. EMBO J. 2017;36:319–333. doi: 10.15252/embj.201696038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veeranan-Karmegam R, Boggupalli DP, Liu G, Gonsalvez GB. A new isoform of Drosophila non-muscle Tropomyosin 1 interacts with Kinesin-1 and functions in oskar mRNA localization. J Cell Sci. 2016;129:4252–4264. doi: 10.1242/jcs.194332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brendza RP, Serbus LR, Duffy JB, Saxton WM. A function for kinesin I in the posterior transport of oskar mRNA and Staufen protein. Science. 2000;289:2120–2122. doi: 10.1126/science.289.5487.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palacios IM, St Johnston D. Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development. 2002;129:5473–5485. doi: 10.1242/dev.00119. [DOI] [PubMed] [Google Scholar]
- 28.Billett FS, Adam E. The structure of the mitochondrial cloud of Xenopus laevis oocytes. J Embryol Exp Morphol. 1976;36:697–710. [PubMed] [Google Scholar]
- 29.Heasman J, Quarmby J, Wylie CC. The mitochondrial cloud of Xenopus oocytes: the source of germinal granule material. Dev Biol. 1984;105:458–469. doi: 10.1016/0012-1606(84)90303-8. [DOI] [PubMed] [Google Scholar]
- 30.Cox RT, Spradling AC. Milton controls the early acquisition of mitochondria by Drosophila oocytes. Development. 2006;133:3371–3377. doi: 10.1242/dev.02514. [DOI] [PubMed] [Google Scholar]
- 31.Pepling ME, Wilhelm JE, O’Hara AL, Gephardt GW, Spradling AC. Mouse oocytes within germ cell cysts and primordial follicles contain a Balbiani body. Proc Natl Acad Sci U S A. 2007;104:187–192. doi: 10.1073/pnas.0609923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marlow FL, Mullins MC. Bucky ball functions in Balbiani body assembly and animal-vegetal polarity in the oocyte and follicle cell layer in zebrafish. Dev Biol. 2008;321:40–50. doi: 10.1016/j.ydbio.2008.05.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosaka K, Kawakami K, Sakamoto H, Inoue K. Spatiotemporal localization of germ plasm RNAs during zebrafish oogenesis. Mech Dev. 2007;124:279–289. doi: 10.1016/j.mod.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Yoon C, Kawakami K, Hopkins N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]
- 35.Haig D. Transposable elements: Self-seekers of the germline, team-players of the soma. Bioessays. 2016;38:1158–1166. doi: 10.1002/bies.201600125. [DOI] [PubMed] [Google Scholar]
- 36.Little SC, Sinsimer KS, Lee JJ, Wieschaus EF, Gavis ER. Independent and coordinate trafficking of single Drosophila germ plasm mRNAs. Nat Cell Biol. 2015;17:558–568. doi: 10.1038/ncb3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell. 1991;66:23–35. doi: 10.1016/0092-8674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- 38.Jenny A, Hachet O, Zavorszky P, Cyrklaff A, Weston MD, Johnston DS, Erdelyi M, Ephrussi A. A translation-independent role of oskar RNA in early Drosophila oogenesis. Development. 2006;133:2827–2833. doi: 10.1242/dev.02456. [DOI] [PubMed] [Google Scholar]
- 39.Shpiz S, Kwon D, Uneva A, Kim M, Klenov M, Rozovsky Y, Georgiev P, Savitsky M, Kalmykova A. Characterization of Drosophila telomeric retroelement TAHRE: transcription, transpositions, and RNAi-based regulation of expression. Mol Biol Evol. 2007;24:2535–2545. doi: 10.1093/molbev/msm205. [DOI] [PubMed] [Google Scholar]
- 40.Johnson LJ. Selfish genetic elements favor the evolution of a distinction between soma and germline. Evolution. 2008;62:2122–2124. doi: 10.1111/j.1558-5646.2008.00433.x. [DOI] [PubMed] [Google Scholar]
- 41.Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD, Morris JP, Liu TX, Schulte-Merker S, Kanki JP, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci U S A. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao W, Kojima KK, Kohany O. Repbase Update, a database of repetitive elements in eukaryotic genomes. Mob DNA. 2015;6:11. doi: 10.1186/s13100-015-0041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 44.Carreira-Rosario A, Bhargava V, Hillebrand J, Kollipara RK, Ramaswami M, Buszczak M. Repression of Pumilio Protein Expression by Rbfox1 Promotes Germ Cell Differentiation. Dev Cell. 2016;36:562–571. doi: 10.1016/j.devcel.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeske M, Bordi M, Glatt S, Muller S, Rybin V, Muller CW, Ephrussi A. The Crystal Structure of the Drosophila Germline Inducer Oskar Identifies Two Domains with Distinct Vasa Helicase- and RNA-Binding Activities. Cell Rep. 2015;12:587–598. doi: 10.1016/j.celrep.2015.06.055. [DOI] [PubMed] [Google Scholar]
- 46.Costes SV, Daelemans D, Cho EH, Dobbin Z, Pavlakis G, Lockett S. Automatic and quantitative measurement of protein-protein colocalization in live cells. Biophys J. 2004;86:3993–4003. doi: 10.1529/biophysj.103.038422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ephrussi A, Dickinson LK, Lehmann R. Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell. 1991;66:37–50. doi: 10.1016/0092-8674(91)90137-n. [DOI] [PubMed] [Google Scholar]
- 48.Sogame N, Kim M, Abrams JM. Drosophila p53 preserves genomic stability by regulating cell death. Proc Natl Acad Sci U S A. 2003;100:4696–4701. doi: 10.1073/pnas.0736384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2. Oligonucleotide sequences for RNA in situ hybridization experiments. Related to STAR Methods and Figures 1, 2, 3, and 4.