Abstract
Synthetic cathinones are beta-ketone amphetamine analogs that have emerged as a heterogeneous class of abused compounds that function as either monoamine transporter substrates or inhibitors. Preclinical drug discrimination procedures are useful for interrogating structure-activity relationships (SAR) of abuse-related drug effects; however, in vivo SAR comparisons between synthetic cathinones with different mechanisms of action are lacking. The aim of the present study was to determine whether the cocaine-like discriminative stimulus effects of the monoamine transporter inhibitor alpha-pyrrolidinovalerophenone (alpha-PVP) and the monoamine transporter substrate methcathinone were differentially sensitive to 3,4-methylenedioxy and 4-methyl substitutions. Male rhesus monkeys (n=4) were trained to discriminate intramuscular cocaine (0.32 mg/kg) from saline in a two-key food-reinforced discrimination procedure. Potency and timecourse of cocaine-like discriminative stimulus effects were determined for (±)-alpha-PVP, (±)-methcathinone, and their 3,4-methylenedioxy or 4-methyl analogs. Alpha-PVP and methcathinone produced dose- and time-dependent cocaine-like effects. A 3,4-methylenedioxy addition to either alpha-PVP (MDPV) or methcathinone (MDMC; methylone) did not alter the potency or efficacy to produce cocaine-like effects, but did prolong the time course. A 4-methyl addition to alpha-PVP (4MPVP; pyrovalerone) did not alter the potency or efficacy to produce cocaine-like effects, but did prolong the time course. In contrast, addition of a 4-methyl moiety to methcathinone (4MMC; mephedrone) significantly attenuated efficacy to produce cocaine-like effects. Overall, these results suggest different structural requirements for cocaine-like discriminative stimulus effects of monoamine transporter inhibitor and substrate synthetic cathinone analogs. Given that 4MMC is more hydrophobic than MDMC, these results suggest hydrophobicity may be an important determinant for limiting monoamine transporter substrate abuse-related behavioral effects.
Introduction
Synthetic Cathinones have emerged worldwide as a heterogeneous class of abused compounds (Davies et al., 2010). The compounds are termed synthetic cathinones because they are synthetic derivatives of the naturally occurring beta-ketone amphetamine analog cathinone. Furthermore, the pharmacological properties of synthetic cathinone analogs that have been identified in packages labeled as “bath salts” can be broadly classified as either monoamine transporter inhibitors or substrates (Baumann et al., 2012; Cozzi et al., 2013; Schneir et al., 2014). Two synthetic cathinones that appear particularly problematic are 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxymethcathinone (MDMC; “methylone”). Both MDPV and MDMC were classified as schedule I controlled substances by the United States Drug Enforcement Administration (DEA) in 2011 (DEA, 2011). However, despite this schedule I status, both MDPV and MDMC continue to be two of the most frequently reported synthetic cathinones by the National Forensic Laboratory Information System and MDMC use has increased 12-fold since DEA scheduling (DEA, 2014). Furthermore, the persistent MDPV and MDMC use contrasts with a trend for declining use of another synthetic cathinone, 4-methylmethcathinone (4MMC; “mephedrone”), which was classified schedule I by the DEA at the same time as MDPV and MDMC; reports of mephedrone prevalence have decreased 200-fold since DEA scheduling (DEA, 2014). Moreover, these DEA data are supported by data from other countries noting an overall decline in 4MMC abuse compared to MDMC since controlled substance scheduling (Caudevilla-Gálligo et al., 2013; Thai et al., 2016; Wood et al., 2013). Overall, these epidemiological results suggest that chemical structure modifications impact abuse potential.
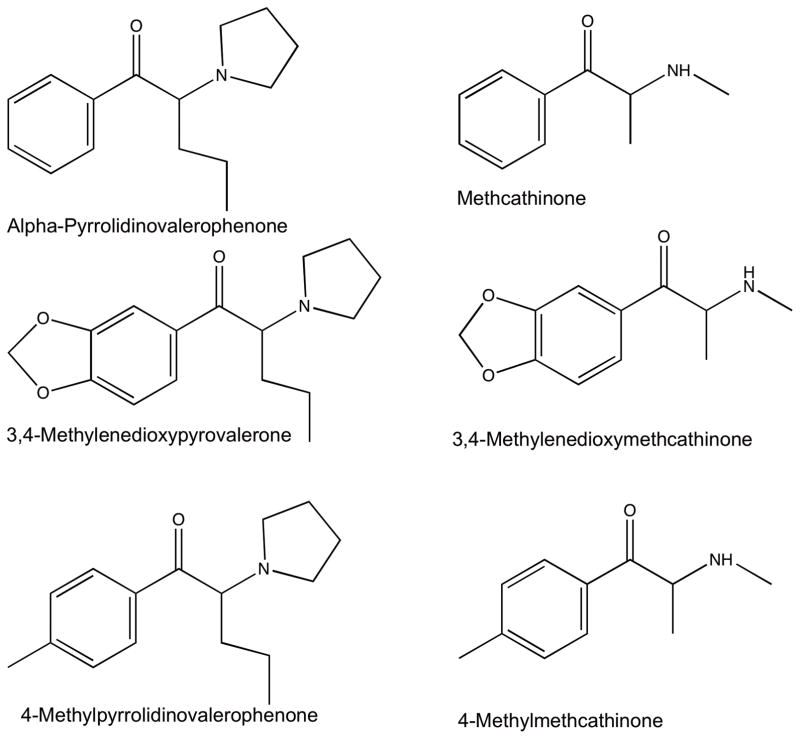
Chemical structure modifications alter the pharmacodynamics of both monoamine transporter inhibitors and substrates that may influence expression of abuse potential. For example, alpha-pyrrolidinovalerophenone (alpha-PVP) and methcathinone function as monoamine transport inhibitors and substrates, respectively, and their chemical structures and corresponding analogs of interest for this study are shown in Figure 1. A 3,4-methylenedioxy addition to alpha-PVP (to create MDPV) had little effect on in vitro selectivity to inhibit the dopamine (DAT) versus serotonin (SERT) transporter (Marusich et al., 2014); however, addition of this same moiety to methcathinone (to produce MDMC) resulted in a 100-fold decrease in the in vitro selectivity to function as a substrate at DAT versus SERT (Baumann et al., 2012; Cozzi et al., 2013; Simmler et al., 2013). Similarly, a 4-methyl addition to alpha-PVP (to create 4MPVP; “pyrovalerone”) did not alter selectivity of DAT versus SERT inhibition (Meltzer et al., 2006; Simmler et al., 2013), but addition of the same moiety to methcathinone (to create 4MMC) again produced a 100-fold decrease in selectivity to function as a DAT versus SERT substrate (Baumann et al., 2012; Simmler et al., 2013). These differences in DAT versus SERT selectivity have been hypothesized to be an important determinant of monoamine transport inhibitor and substrate abuse-related effects (Negus and Miller, 2014; Simmler et al., 2013; Wee et al., 2005). However, direct chemical structure modification comparisons between monoamine transport inhibitors and substrates are lacking. Furthermore, the degree to which these chemical structure modifications alter the pharmacokinetics, i.e. time course of drug action, of monoamine transport inhibitor and substrate abuse-related effects have not been as well characterized.
Figure 1.
Chemical structure of alpha-pyrrolidinovalerophenone (alpha-PVP), methcathinone, and their 3,4-methylenedioxy or 4-methyl analogs examined.
Preclinical drug discrimination procedures have been utilized for over 40 years to improve our understanding of the pharmacological mechanisms of central nervous system active compounds (Overton, 1971; Schuster and Balster, 1977). The present study aim was to directly compare effects of alpha-PVP, methcathinone, and their 3,4-methylenedioxy and 4-methyl analogs in rhesus monkeys trained to discriminate intramuscular cocaine from saline in a two-key food-reinforced discrimination procedure. Cocaine was chosen as the training drug because it is a prototype monoaminergic abused drug, and its discriminative stimulus effects have been extensively characterized in rhesus monkeys (Garza and Johanson, 1983; Kleven et al., 1990). Moreover, cocaine has also been used as the training stimulus in rodent drug discrimination studies examining some of the synthetic cathinones examined in the present study (Gatch et al., 2015a; Gatch et al., 2013), thus allowing for a translational comparison between rodents and nonhuman primates. We hypothesized the cocaine-like effects of alpha-PVP would be more resistant than effects of methcathinone to addition of a 3,4-methylenedioxy or 4-methyl moiety. Moreover, we also hypothesized that addition of the 3,4-methylenedioxy moiety to alpha-PVP and methcathinone would prolong the duration of cocaine-like effects of both compounds..
Methods
Subjects
Drug discrimination studies were conducted in 4 adult male rhesus monkeys (Macaca mulatta) of Indian and Chinese origin and weighing between 9–13 kg. All monkeys had an experimental history of primarily monoaminergic compound exposure and were maintained on a diet of biscuits (Lab Diet High Protein Monkey Biscuits, PMI Feeds, Inc., St. Louis, MO) and fresh fruit provided after the behavioral session. Water was continuously available in the home chamber, which also served as the experimental chamber. Additionally, monkeys could earn 1-gm banana-flavored pellets (5TUR grain-based precision primate tablets, Test Diets, Richmond, IN) during daily experimental sessions (described below). A 12 h light-dark cycle was in effect (lights on from 6AM to 6PM), and temperature and humidity levels were monitored daily. Monkeys had visual, auditory, and olfactory contact with other monkeys throughout the experiments. Environmental enrichment consisting of various food puzzles, TV, or radio was provided daily after behavioral session conclusion. Facilities were licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Experiments and animal maintenance were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (Council, 2011) and reported according to the ARRIVE guidelines (Kilkenny et al., 2010). The Institutional Animal Care and Use Committee approved the research and environmental enrichment protocols.
Cocaine Discrimination Procedure
Experimental sessions were conducted in each monkey’s home chamber as described previously (Banks et al., 2013). On the front wall of each chamber was a custom operant response panel with three horizontally arranged square response keys, and only the left and right keys were used in the present studies. Attached to each panel was a pellet dispenser (Med Associates, ENV-203-1000, St. Albans, VT). Equipment operation and data collection were accomplished with a Windows-based computer and MED-PC software (Med Associates).
Monkeys were trained to discriminate 0.32 mg/kg cocaine intramuscularly (IM) from saline in a two-key, food-reinforced drug discrimination procedure as previously described (Banks et al., 2013). Discrimination training was conducted 5 days per week during daily sessions composed of multiple components. Each component consisted of a 5-minute response period, during which the right and left response keys were transilluminated red and green, respectively, and monkeys could earn up to 10 food pellets by responding under a fixed-ratio (FR) 30 schedule of food presentation. Training sessions were composed of three components presented at 2-h intervals, and either saline or (0.32 mg/kg cocaine) was administered IM approximately 15 min prior to the start of each component. Thus, on training days, monkeys would receive a sequence of saline (S) and cocaine (C) injections in the order SSS, SSC, SCS, CSS, SCC, CSC, CCS, or CCC. These training sequences were randomly presented to engender daily experience with randomized sequences of saline- and cocaine-appropriate components. The 2h duration of inter-component intervals was selected to exceed the time course of discriminative stimulus effects produced by the training dose of cocaine in rhesus monkeys (Lamas et al., 1995) and to thereby minimize effects of cocaine administered in earlier trials on performance during later trials on the same day. Following administration of saline, only responding on the green key (the saline-appropriate key) produced food, whereas following administration of 0.32 mg/kg cocaine, only responding on the red key (the cocaine-appropriate key) produced food. Responses on the inappropriate key before completing the response requirement on the appropriate key reset the FR requirement. The criterion for accurate discrimination was ≥85% injection-appropriate responding before delivery of the first reinforcer, ≥90% injection-appropriate responding for the entire component, and response rates ≥0.1 responses/s (sufficient to earn at least one pellet) for all components during 7 of 8 consecutive sessions.
Test sessions were identical to training sessions except that (a) response requirement completion (FR30) on either key produced food, (b) monkeys received only one vehicle or test drug dose injection at the start of the session, and (c) 5-min response components began 10, 30, 56, 100, 180, 300, and 560 min after the injection to assess the time course of drug effects. As in training sessions, switching response keys reset the response requirement. The drugs and dose ranges tested were: (±)-alpha-PVP (0.032–0.32 mg/kg), (±)-MDPV (0.01–0.32 mg/kg), (±)-4MPVP (0.01–0.32 mg/kg), (±)-methcathinone (0.032–0.32 mg/kg), (±)-MDMC (0.1–3.2 mg/kg), and (±)-4MMC (0.32–3.2 mg/kg). Test sessions were generally conducted on Tuesdays and Fridays with training sessions conducted on Mondays, Wednesdays, and Thursdays. Test sessions were conducted only if performance during the previous two training sessions met the criteria for accurate discrimination (described above). All test drug doses were evaluated once in each monkey, and all test drug doses were evaluated in a given monkey before testing the next drug. Vehicle (saline) test sessions were conducted before and after evaluation of each test drug. The order of drug doses and drugs was counterbalanced across monkeys.
Data Analysis
The primary dependent measures were (1) percent cocaine-appropriate responding {defined as (number of responses on the cocaine-associated key divided by the total number of responses on both the cocaine-and saline-associated keys)*100}, and (2) response rates during each component. These dependent measures were then plotted as a function of time after drug or saline administration. Percent cocaine-appropriate responding and response rates were analyzed using linear mixed effect analysis with drug dose and time as the main fixed effects and subjects as the random effect (JMP Pro 11.1.1, SAS, Cary, NC). A significant drug×time interaction was followed by the Dunnett’s post-hoc test for comparison to vehicle (saline) conditions within a given time point.
In addition, data analysis was supplemented with two other analytic approaches to provide summary measures of drug effects. First, drugs that produced ≥ 90% cocaine-appropriate responding were considered to produce full substitution. Second, an ED50 value for each drug in each monkey was calculated and defined as the test drug dose that produced 50% cocaine-appropriate responding at the 30 min time point. Log ED50 values were calculated by log-linear interpolation from individual subject dose-effect functions when only two data points were used (one above and below the 50% level) or by linear regression when three or more data points were used. Log ED50 values where then averaged to yield mean values and 95% confidence limits, and these log values were converted to linear values for presentation.
Drugs
(−)-Cocaine HCl, (±)-MDPV HCl, (±)-4MPVP HCl, (±)-methcathinone HCl, (±)-MDMC HCl, and (±)-4MMC HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Bruce Blough (RTI) synthesized (±)-alpha-PVP HCl. All drugs were dissolved in sterile saline and stock solutions were used for approximately 7–14 days. Results regarding 4MMC stock solution stability are reported in supplemental materials. Drug doses were calculated and expressed using the salt forms listed above.
Results
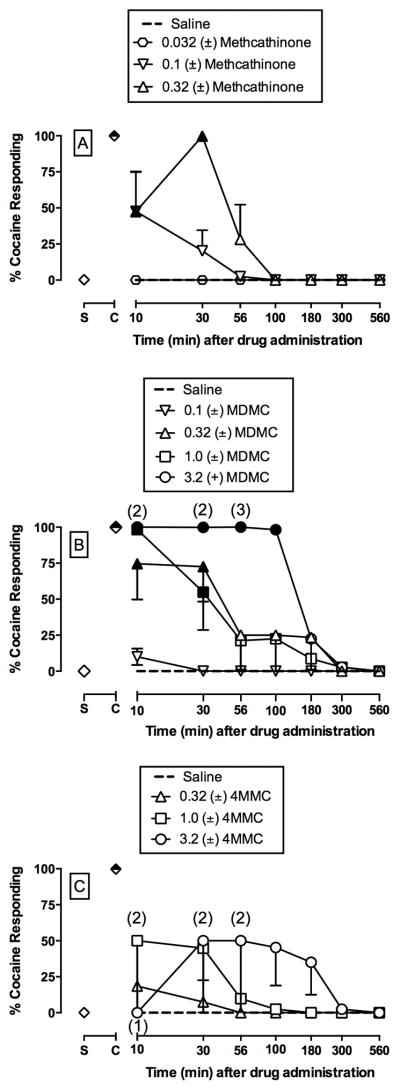
On all training days preceding test days, mean ± SEM percentages of injection-appropriate responding were 99.9 ± 0.1 and 99.8 ± 0.2%, and rates of responding were 3.0 ± 0.1 and 2.7 ± 0.2 responses/s for cocaine and saline components, respectively. For reference, the cocaine-training dose (0.32 mg/kg) produced a time course of discriminative stimulus effects no longer than 30 min (Banks et al., 2013). Saline administration produced ≤ 10% cocaine-appropriate responding at all time points (Figures 2–3) and had no significant effect on rates of responding in all time course test sessions (Supplemental Figures 1–2). Alpha-PVP, MDPV, 4MPVP, methcathinone, and MDMC all produced dose-dependent and full substitution, ≥90% cocaine-appropriate responding, at the 30 min time point in all four monkeys (Table 1; Supplemental Figure 3). In contrast, 4MMC produced full substitution in 1 out of 4 monkeys and supplemental Figure 4 shows the 4MMC substitution profile of individual monkeys. Table 1 also shows the ED50 values to produce cocaine-like discriminative stimulus effects. Alpha-PVP, MDPV, and 4MPVP were equally potent. Methcathinone and MDMC were also equally potent.
Figure 2.
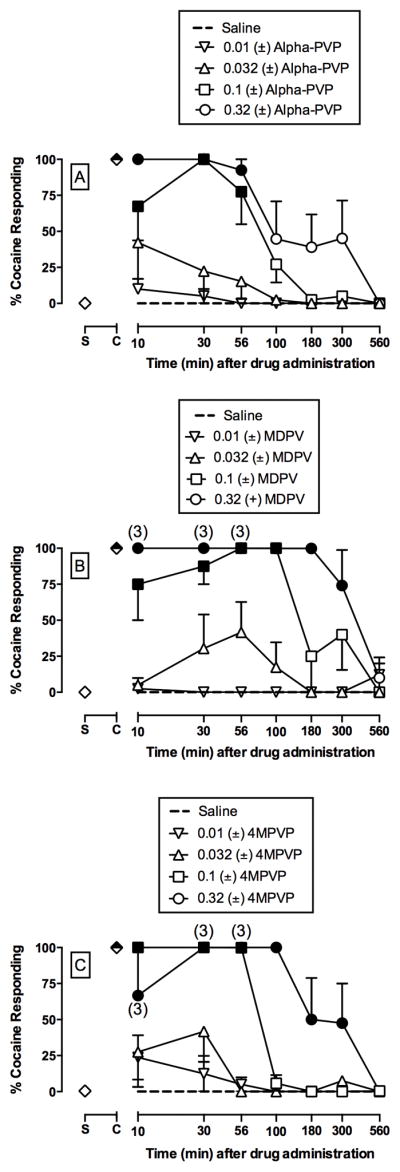
Time course of the discriminative stimulus effects of (±)-alpha-PVP (A; 0.01 – 0.32 mg/kg, IM), (±)-3,4-methylendioxypyrovalerone (MDPV) (B; 0.01 – 0.32 mg/kg, IM), and (±)-4-methylpyrrolidinovalerophenone (4MPVP) (C; 0.01 – 0.32 mg/kg, IM) in male rhesus monkeys (n=4). Abscissae: time in min after injection. Ordinates: percent cocaine-appropriate responding. Symbols above “S” and “C” represent the group averages for all saline- and cocaine-training sessions preceding test sessions, respectively. Filled symbols indicate statistical significance compared to saline at a given time point (p < 0.05). Number in parentheses indicate the number of subjects contributing to that data point if fewer than the total number (4) of subjects tested (i.e. indicative of a time point where one or more monkeys failed to complete at least one ratio requirement).
Figure 3.
Time course of the discriminative stimulus effects of (±)-methcathinone (A; 0.032 – 0.32 mg/kg, IM), (±)-3,4-methylenedioxymethcathinone (MDMC) (B; 0.1 – 3.2 mg/kg, IM), and (±)-4-methylmethcathione (4MMC) (C; 0.32 – 201 mg/kg, IM) in male rhesus monkeys (n=4). Ordinates: percent cocaine-appropriate responding. Symbols above “S” and “C” represent the group averages for all saline- and cocaine-training sessions preceding test sessions, respectively. Filled symbols indicate statistical significance compared to saline at a given time point (p < 0.05). Number in parentheses indicate the number of subjects contributing to that data point if fewer than the total number (4) of subjects tested (i.e. indicative of a time point where one or more monkeys failed to complete at least one ratio requirement).
Table 1.
Mean (95% confidence limits) ED50 values for alpha-PVP, methcathinone, and their analogs in rhesus monkeys trained to discriminate 0.32 mg/kg intramuscular cocaine from saline. The fraction of monkeys in which an ED50 value could be determined is also shown under “# Full Substitution.” Values were determined from dose-effect curves shown in Figures 2 and 3 at the 30 min time point.
| Compound | # Full Substitution | ED50 (95% CL) in mg/kg |
|---|---|---|
| (±)-Alpha-Pyrrolidinovalerophenone | 4/4 | 0.04 (0.03 – 0.07) |
| (±)-3,4-Methylenedioxypyrovalerone (MDPV) | 4/4 | 0.04 (0.02 – 0.08) |
| (±)-4-Methylpyrrolidinovalerophenone (4MPVP) | 4/4 | 0.02 (0.01 – 0.05) |
| (±)-Methcathinone | 4/4 | 0.15 (0.11 – 0.20) |
| (±)-3,4-Methylendioxymethcathinone (MDMC) | 4/4 | 0.31 (0.11 – 0.89) |
| (±)-4-Methylmethcathinone (4MMC) | 1/4 | 0.57 |
Figure 2 shows the time courses of saline and individual (A) alpha-PVP, (B), MDVP, and (C) 4MPVP doses. All three compounds produced dose- and time-dependent cocaine-like stimulus effects (alpha-PVP dose × time: F24,102 = 3.5, p<0.0001; MDPV dose × time: F24,99 = 6.3, p<0.0001; 4MPVP dose × time: F24,99 = 6.3, p<0.0001). Although the potency to produce cocaine-like effects was similar between alpha-PVP, MDPV, and 4MPVP, both MDPV and 4MPVP produced significantly longer cocaine-like effects compared to alpha-PVP. Supplemental Figure 1 shows drug effects on rates of operant responding. Alpha-PVP and 4MPVP had no effect on rates of responding, whereas 0.032 mg/kg MDPV significantly increased rates of responding at one time point (MDPV dose × time: F24,102 = 2.1, p=0.0069).
Figure 3 shows the time courses of saline and individual (A) methcathinone, (B) MDMC, and (C) 4MMC doses. Methcathinone and MDMC produced dose- and time-dependent cocaine-like effects (methcathinone dose × time: F18,81 = 4.5, p<0.0001; MDMC dose × time: F24,96 = 4.0, p<0.0001). In contrast, 4MMC did not produce a significant cocaine-like stimulus effect. Supplemental Figure 2 shows drug effects on rates of operant responding. Methcathinone had no effect on rates of responding whereas both MDMC (MDMC dose × time: F24,102 = 2.2, p=0.0028) and 4MMC (4MMC dose × time: F18,81 = 3.5, p<0.0001) decreased rates of operant responding.
Discussion
The aim of the present study was to determine within the same monkeys how similar chemical structure modifications to the monoamine transporter inhibitor alpha-PVP and the monoamine transporter substrate methcathinone impacted abuse-related discriminative stimulus effects in a cocaine discrimination procedure. There were three main findings. First, consistent with the known abuse liability of alpha-PVP and methcathinone, both compounds produced dose- and time-dependent cocaine-like stimulus effects. Second, a 3,4-methylenedioxy addition did not alter the potency or efficacy of alpha-PVP or methcathinone to produce cocaine-like effects, although this addition did prolong the time course of MDPV and increase the expression of rate-decreasing effects for both compounds. Lastly, a 4-methyl addition attenuated the cocaine-like effects and increased the expression of rate-decreasing effects of methcathinone. In contrast, a 4-methyl addition prolonged the time course of alpha-PVP without altering the potency or efficacy to produce cocaine-like effects. Overall, these results demonstrate different structural requirements for the cocaine-like discriminative stimulus effects of synthetic cathinone analogs that function as monoamine transporter inhibitors or substrates and highlight the importance of determining the behavioral pharmacology of centrally active compounds in multiple species. Additionally, the hydrophobicity of structural additions may be an important determinant for monoamine transporter substrate abuse-related behavioral effects.
Effects of alpha-PVP and methcathinone
The complete substitution of alpha-PVP and methcathinone for cocaine in the present study is consistent with previous results in rats trained to discriminate cocaine (Gatch et al., 2015a; Gatch et al., 2015b). Moreover, although this is the first report of alpha-PVP effects in nonhuman primates, methcathinone was also reported previously to substitute completely for cocaine in rhesus monkeys (Kohut et al., 2013). These studies differed in cocaine training doses and routes of administration (10 mg/kg IP in rats; 0.32–0.4 mg/kg IM in rhesus monkeys) as well as in species of experimental subjects; however, these differences in training doses did not result in equivalent potency differences for alpha-PVP and methcathinone substitution. Specifically, comparison of ED50 values indicates that alpha-PVP was approximately 100-fold more potent in monkeys than in rats, whereas methcathinone potency was similar in the two monkeys studies and only approximately 3-fold more potent than in rats. The larger species difference in potency of alpha-PVP than methcathinone suggests the potential for larger species differences in pharmacokinetics of alpha-PVP. The present study extended on these earlier studies by evaluating the time courses of alpha-PVP and methcathinone. At equivalent doses, both alpha-PVP and methcathinone produced similar time courses compared to cocaine (Banks et al., 2013). In summary, the consistency of the present results, albeit with potential species differences in regards to potency, with the existing literature provide an empirical foundation for the determining the impact of two different chemical structure modifications on the abuse-related subjective effects of these two cathinone analogs possessing different mechanisms of action.
Effects of a 3,4-methylenedioxy substituent
Consistent with previous results in rats trained to discriminate cocaine (Gatch et al., 2015a; Gatch et al., 2013), both MDPV and MDMC produced dose-dependent and full substitution for the training drug. Compared to rats (Gatch et al., 2013), MDPV was approximately 20-fold and MDMC was approximately 8-fold more potent in monkeys to produce cocaine-like stimulus effects. Another noted difference between the present results and previous rat results was that alpha-PVP and MDPV were equally potent to produce cocaine-like effects in monkeys, whereas MDPV was 5-fold more potent than alpha-PVP to produced cocaine-like effects in the rat (Gatch et al., 2015a; Gatch et al., 2013). Furthermore, methcathinone was 2-fold more potent than MDMC in monkeys compared to a 3-fold potency difference in rats (Gatch et al., 2015b; Gatch et al., 2013). These potency differences again provide evidence for species differences in the pharmacokinetics of synthetic cathinone analogs. The present results also extend on previous findings in rats by characterizing the time courses of the cocaine-like discriminative stimulus effects of MDPV and MDMC in comparison to their unsubstituted parent compounds (alpha-PVP and methcathinone, respectively). In general, MDPV produced longer lasting discriminative stimulus effects compared to alpha-PVP, whereas the time courses of MDMC and methcathinone discriminative stimulus effects were similar. Overall, the present results suggest that a 3,4-methylenedioxy addition does not attenuate the abuse-related cocaine-like discriminative stimulus effects of either alpha-PVP or methcathinone.
Effects of a 4-methyl substituent
Addition of the 4-methyl substituent to alpha-PVP did not alter the efficacy, potency, or time course to produce cocaine-like stimulus effects. To the best of our knowledge, this is the first preclinical study to report on the abuse-related behavioral effects of 4MPVP. There is a single published French report of 4MPVP (pyrovalerone) abuse (Deniker et al., 1975). Previous 4MPVP studies have mainly focused on in vitro pharmacology (Meltzer et al., 2006) or metabolism (Michaelis et al., 1970). Given the similar efficacy and potency of alpha-PVP, MDPV, and 4MPVP to produce cocaine-like discriminative stimulus effects, the present in vivo behavioral results are consistent with previous in vitro neurochemical studies demonstrating that similar DAT potency and DAT versus SERT selectivity (Meltzer et al., 2006; Rickli et al., 2015). Moreover, our behavioral results are inconsistent with the DEA schedule V controlled substance classification of 4MPVP as a compound that has low abuse potential relative to compounds scheduled I–IV.
In contrast to results with 4MPVP, addition of a 4-methyl group to methcathinone produced a decrease in both efficacy and potency to produce cocaine-like discriminative stimulus effects to the extent that a group mean ED50 value could not be determined. The failure of 4MMC to substitute fully for cocaine here contrasts with a previous report that 4MMC substituted completely for cocaine in rats and was equipotent to MDMC based on ED50 values (Gatch et al., 2013). However, cocaine produced only partial substitution in rats trained to discriminate 4MMC (Varner et al., 2013), whereas cocaine completely substituted in rats trained to discriminate methcathinone (Young and Glennon, 1998). Thus, the asymmetrical generalization between 4MMC and cocaine and the symmetrical generalization between methcathinone and cocaine in rat discrimination studies is consistent with the present nonhuman primate results. Overall, these results suggest that discriminative stimulus effects of cocaine overlap less with those of 4MMC than with those of the other synthetic cathinone analogs evaluated here.
Recent human studies also provide support for non-overlapping subjective effects between 4MMC and cocaine. For example, only 28.9% of 4MMC users reported that subjective effects of 4MMC and cocaine were “very similar” or “nearly the same,” whereas 36% reported that 4MMC and cocaine effects were only “somewhat similar” or “not similar at all” (Kapitány-Fövény et al., 2013). Furthermore, of 4MMC users who also previously used 3,4-methylenedioxymethamphetamine (MDMA) and cocaine, twice as many users responded that 4MMC effects were more comparable to MDMA than cocaine (Carhart-Harris et al., 2011). Lastly, approximately 56% of dual 4MMC and cocaine users reported that 4MMC was less addictive than cocaine (Winstock et al., 2011). In summary, the present nonhuman primate results are consistent with these human studies to suggest modest and incomplete overlap in subjective effects of 4MMC and cocaine in humans.
Implications for predicting novel monoamine transport inhibitor and substrate abuse
Insofar as cocaine-like discriminative stimulus effects are indicative of abuse potential, the present results support two main implications for predicting the abuse of future synthetic cathinone analogs that function as either monoamine transport inhibitors or substrates. First, the abuse-related subjective effects of synthetic cathinone analogs that function as monoamine transporter inhibitors and possess an N-pyrrolidine substituent were resistant to the phenyl ring modifications studied here. The present behavioral results in nonhuman primates and previous results in rats (Aarde et al., 2015; Bonano et al., 2014; Gatch et al., 2015a) are consistent with the broader literature demonstrating that synthetic cathinone analogs containing an N-pyrrolidine are potent and selective DAT inhibitors in vitro (Kolanos et al., 2015; Meltzer et al., 2006).
Second, the present results suggest that a 4-methyl substitution may attenuate the abuse-related effects of a monoamine transporter substrate more than a 3,4-methylenedioxy substitution. These results extend previous intracranial self-stimulation (ICSS) results in rats demonstrating that 4MMC produced weaker abuse-related facilitation than MDMC (Bonano et al., 2014). However, inconsistent with the present monkey discrimination and previous rat ICSS results are drug self-administration data suggesting that 4MMC has higher reinforcing efficacy than MDMC in rats (Creehan et al., 2015; Vandewater et al., 2015) and other behavioral data suggesting 4MMC produced similar effects to methamphetamine (Gatch et al., 2013; Wright et al., 2012) and cocaine (Winstock et al., 2011). These apparent discrepancies warrant further study; however, the broader scientific literature suggests that a 4-methyl addition more consistently attenuates the abuse-related effects of monoamine transporter substrates compared to a 3,4-methylenedioxy addition. For example, a 4-methyl addition to amphetamine (4-methylamphetamine, aka PAL-313) attenuated both abuse-related neurochemical and behavioral endpoints in both nonhuman primates (Kimmel et al., 2009; Wee et al., 2005) and rats (Bauer et al., 2013; Baumann et al., 2011). In contrast, a 3,4-methylenedioxy addition to either amphetamine or cathinone has produced equivocal results with some studies demonstrating no effect (Griffiths et al., 1976; Kamien et al., 1986; Markert and Roberts, 1991) and other studies demonstrating an attenuated abuse-related effect (Dal Cason et al., 1997). Moreover, the broader human literature suggests that 4MMC use has declined more than MDMC use since controlled substance classification (DEA, 2014; Caudevilla-Gálligo et al., 2013; Lai et al., 2013; Thai et al., 2016). Overall, the present results suggest differential abuse-related impact of 4-methyl and 3,4-methylenedioxy additions to monoamine transporter substrates.
One potential explanation for the differential abuse-related behavioral results between MDMC and 4MMC may be related to the hydrophobicity of monoamine transporter substrate. For monoamine transport inhibitors such as alpha-PVP (Pubchem CID: 11148955), MDPV (Pubchem CID: 20111961), and 4MPVP (Pubchem CID: 14373), lipophilicity has been reported to improve potency to inhibit DAT in vitro (Kolanos et al., 2015). Lipophilicity also improves blood-brain barrier penetration and could thus contribute to the potency of these three compounds to produce abuse-related behavioral and neurochemical effects. However, hydrophobicity may also alter drug-monoamine transporter interactions that may have implications for expression of abuse-related behavioral effects (Kolanos et al., 2015). For example, the rank order of the monoamine transporter substrates examined from most hydrophobic to least hydrophobic based on Log P values was 4MMC (Pubchem CID: 45266826) > MDMC (Pubchem CID: 45789647) > methcathinone (Pubchem CID: 1576). Moreover, hydrophobicity has been recently reported to be an important determinant of potency to release 5HT, but not DA, in vitro (Sakloth et al., 2015). Consistent with this hypothesis, 4MMC was approximately 2-fold more potent to release 5HT than MDMC (Baumann et al., 2012). Thus, the degree to which hydrophobicity enhances or attenuates the abuse-related effects of novel synthetic cathinone analogs may depend upon its function at the different monoamine transporters.
Supplementary Material
Acknowledgments
We acknowledge the technical assistance of Crystal Reyns. We also acknowledge Kevin Costa for writing the original version of the behavioral programs.
Footnotes
Author Contributions:
MLB was responsible for the study concept and design. DAS contributed to animal data acquisition. JLP designed the methodology and performed the 4MMC stock solution analysis. BEB synthesized alpha-PVP. DAS and MLB drafted the manuscript. SSN and BEB assisted with data analysis and interpretation of findings, and provided critical revision of the manuscript for intellectual content. All authors critically reviewed content and approved the final manuscript version for publication.
Funding and Disclosures:
Research reported in this publication was supported by National Institutes of Health grants R01-DA026946, R01-DA012970, R01-DA033930, P30-DA033934, and institutional professional development funds. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Mr. Smith and Mr. Poklis declare no conflicts.
Dr. Negus and Dr. Blough declare that NIH has funded their research.
Dr. Banks declares that NIH has funded his research. During the past 3 years, he has received compensation as a collaborator with the pharmaceutical companies Abbott and Purdue for projects related to opioid pharmacology and analgesic drug development. Dr. Banks declares that the present study was not related to this professional relationship and should not be perceived as constituting a potential conflict of interest.
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology. 2015;232:3045–3055. doi: 10.1007/s00213-015-3944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Role of phenmetrazine as an active metabolite of phendimetrazine: Evidence from studies of drug discrimination and pharmacokinetics in rhesus monkeys. Drug Alcohol Depend. 2013;130:158–166. doi: 10.1016/j.drugalcdep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 2013;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In Vivo Effects of Amphetamine Analogs Reveal Evidence for Serotonergic Inhibition of Mesolimbic Dopamine Transmission in the Rat. J Pharmacol Exp Ther. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, Felice LJ, Banks ML, Negus SS. Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology. 2014;231:199–207. doi: 10.1007/s00213-013-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, King LA, Nutt DJ. A web-based survey on mephedrone. Drug Alcohol Depend. 2011;118:19–22. doi: 10.1016/j.drugalcdep.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Caudevilla-Gálligo F, Ventura M, Indave Ruiz BI, Fornís I. Presence and composition of cathinone derivatives in drug samples taken from a Drug Test Service in Spain (2010–2012) Human Psychopharmacol. 2013;28:341–344. doi: 10.1002/hup.2296. [DOI] [PubMed] [Google Scholar]
- Council National Research. Guide for the care and use of laboratory animals. 8. National Academies Press; Washington DC: 2011. [Google Scholar]
- Cozzi NV, Brandt SD, Daley PF, Partilla JS, Rothman RB, Tulzer A, Sitte HH, Baumann MH. Pharmacological examination of trifluoromethyl ring-substituted methcathinone analogs. Eur J Pharmacol. 2013;699:180–187. doi: 10.1016/j.ejphar.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creehan KM, Vandewater SA, Taffe MA. Intravenous self-administration of mephedrone, methylone and MDMA in female rats. Neuropharmacology. 2015;92:90–97. doi: 10.1016/j.neuropharm.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cason TA, Young R, Glennon RA. Cathinone: An Investigation of Several N-Alkyl and Methylenedioxy-Substituted Analogs. Pharmacol Biochem Behav. 1997;58:1109–1116. doi: 10.1016/s0091-3057(97)00323-7. [DOI] [PubMed] [Google Scholar]
- Davies S, Wood DM, Smith G, Button J, Ramsey J, Archer R, Holt DW, Dargan PI. Purchasing ‘legal highs’ on the Internet—is there consistency in what you get? QJM. 2010;103:489–493. doi: 10.1093/qjmed/hcq056. [DOI] [PubMed] [Google Scholar]
- DEA. Schedules of controlled substances: temporary placement of three synthetic cathinones in Schedule I. Final Order. Federal register. 2011;76:65371–65375. [PubMed] [Google Scholar]
- DEA. National Forensic Laboratory Information System Special Report: Synthetic cannabinoids and synthetic cathinones reported in NFLIS, 2010–2013. Office of Diversion Control DoJ (ed). Drug Enforcement Administration; Springfiled, VA: 2014. [Google Scholar]
- Deniker P, Loo H, Cuche H, Roux JM. Abuse of pyrovalerone by drug addicts. Ann Med Psychol (Paris) 1975;2:745–748. [PubMed] [Google Scholar]
- Garza RD, Johanson CE. The discriminative stimulus properties of cocaine in the rhesus monkey. Pharmacol Biochem Behav. 1983;19:145–148. doi: 10.1016/0091-3057(83)90323-4. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Dolan SB, Forster MJ. Comparative Behavioral Pharmacology of Three Pyrrolidine-Containing Synthetic Cathinone Derivatives. J Pharmacol Exp Ther. 2015a;354:103–110. doi: 10.1124/jpet.115.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Rutledge MA, Forster MJ. Discriminative and locomotor effects of five synthetic cathinones in rats and mice. Psychopharmacology. 2015b;232:1197–1205. doi: 10.1007/s00213-014-3755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Winger G, Brady JV, Snell JD. Comparison of behavior maintained by infusions of eight phenylethylamines in baboons. Psychopharmacology. 1976;50:251–258. doi: 10.1007/BF00426841. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Johanson CE, Schuster CR, Woolverton WL. The effects of (±)-methylenedioxymethamphetamine and (±)-methylenedioxyamphetamine in monkeys trained to discriminate (+)-amphetamine from saline. Drug Alcohol Depend. 1986;18:139–147. doi: 10.1016/0376-8716(86)90046-3. [DOI] [PubMed] [Google Scholar]
- Kapitány-Fövény M, Kertész M, Winstock A, Deluca P, Corazza O, Farkas J, Zacher G, Urbán R, Demetrovics Z. Substitutional potential of mephedrone: an analysis of the subjective effects. Human Psychopharmacol. 2013;28:308–316. doi: 10.1002/hup.2297. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Manvich DF, Blough BE, Negus SS, Howell LL. Behavioral and neurochemical effects of amphetamine analogs that release monoamines in the squirrel monkey. Pharmacol Biochem Behav. 2009;94:278–284. doi: 10.1016/j.pbb.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1990;254:312–317. [PubMed] [Google Scholar]
- Kohut SJ, Fivel PA, Blough BE, Rothman RB, Mello NK. Effects of methcathinone and 3-Cl-methcathinone (PAL-434) in cocaine discrimination or self-administration in rhesus monkeys. Int J Neuropsychopharmacol. 2013:1–14. doi: 10.1017/S146114571300059X. [DOI] [PubMed]
- Kolanos R, Sakloth F, Jain AD, Partilla JS, Baumann MH, Glennon RA. Structural Modification of the Designer Stimulant α-Pyrrolidinovalerophenone (α-PVP) Influences Potency at Dopamine Transporters. ACS Chem Neurosci. 2015;6:1726–1731. doi: 10.1021/acschemneuro.5b00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai FY, Thai PK, O’Brien J, Gartner C, Bruno R, Kele B, Ort C, Prichard J, Kirkbride P, Hall W, Carter S, Mueller JF. Using quantitative wastewater analysis to measure daily usage of conventional and emerging illicit drugs at an annual music festival. Drug Alcohol Rev. 2013;32:594–602. doi: 10.1111/dar.12061. [DOI] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Hall E, Mello NK. Relationship between the discriminative stimulus effects and plasma concentrations of intramuscular cocaine in rhesus monkeys. Psychopharmacology. 1995;121:331–338. doi: 10.1007/BF02246072. [DOI] [PubMed] [Google Scholar]
- Markert LE, Roberts DCS. 3,4-methylenedioxyamphetamine (MDA) self-administration and neurotoxicity. Pharmacol Biochem Behav. 1991;39:569–574. doi: 10.1016/0091-3057(91)90129-p. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV) Neuropharmacology. 2014;87:206–213. doi: 10.1016/j.neuropharm.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer PC, Butler D, Deschamps JR, Madras BK. 1-(4-Methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one (Pyrovalerone) Analogues: A Promising Class of Monoamine Uptake Inhibitors. J Med Chem. 2006;49:1420–1432. doi: 10.1021/jm050797a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis W, Russel JH, Schindler O. The metabolism of pyrovalerone hydrochloride. J Med Chem. 1970;13:497–503. doi: 10.1021/jm00297a036. [DOI] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial Self-Stimulation to Evaluate Abuse Potential of Drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton D. Discriminative control of behavior by drug states. In: Thompson T, Pickens R, editors. Stimulus properties of drugs. Appelton-Century-Crofts; New York: 1971. pp. 87–110. [Google Scholar]
- Rickli A, Hoener MC, Liechti ME. Monoamine transporter and receptor interaction profiles of novel psychoactive substances: Para-halogenated amphetamines and pyrovalerone cathinones. Eur Neuropsychopharmacol. 2015;25:365–376. doi: 10.1016/j.euroneuro.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Sakloth F, Kolanos R, Mosier PD, Bonano JS, Banks ML, Partilla JS, Baumann MH, Negus SS, Glennon RA. Steric parameters, molecular modeling and hydropathic interaction analysis of the pharmacology of para-substituted methcathinone analogues. Br J Pharmacol. 2015;172:2210–2218. doi: 10.1111/bph.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneir A, Ly BT, Casagrande K, Darracq M, Offerman SR, Thornton S, Smollin C, Vohra R, Rangun C, Tomaszewski C, Gerona RR. Comprehensive analysis of “bath salts” purchased from California stores and the internet. Clin Toxicol. 2014;52:651–658. doi: 10.3109/15563650.2014.933231. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Balster RL. The discriminative stimulus properties of drugs. In: Thompson T, Dews PB, editors. Advances in Behavioral Pharmacology. Academic Press; New York: 1977. pp. 86–139. [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai PK, Lai FY, Edirisinghe M, Hall W, Bruno R, O’Brien JW, Prichard J, Kirkbride KP, Mueller JF. Monitoring temporal changes in use of two cathinones in a large urban catchment in Queensland, Australia. Sci Total Environ. 2016;545–546:250–255. doi: 10.1016/j.scitotenv.2015.12.038. [DOI] [PubMed] [Google Scholar]
- Vandewater SA, Creehan KM, Taffe MA. Intravenous self-administration of entactogen-class stimulants in male rats. Neuropharmacology. 2015;99:538–545. doi: 10.1016/j.neuropharm.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner K, Daigle K, Weed P, Lewis P, Mahne S, Sankaranarayanan A, Winsauer P. Comparison of the behavioral and cardiovascular effects of mephedrone with other drugs of abuse in rats. Psychopharmacology. 2013;225:675–685. doi: 10.1007/s00213-012-2855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the Serotonergic Activity and Reinforcing Effects of a Series of Amphetamine Analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wood DM, Greene SL, Dargan PI. Emergency department presentations in determining the effectiveness of drug control in the United Kingdom: mephedrone (4-methylmethcathinone) control appears to be effective using this model. Emerg Med J. 2013;30:70–71. doi: 10.1136/emermed-2011-200747. [DOI] [PubMed] [Google Scholar]
- Wright JMJ, Vandewater SA, Angrish D, Dickerson TJ, Taffe MA. Mephedrone (4-methylmethcathinone) and d-methamphetamine improve visuospatial associative memory, but not spatial working memory, in rhesus macaques. Br J Pharmacol. 2012;167:1342–1352. doi: 10.1111/j.1476-5381.2012.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Glennon RA. Discriminative stimulus effects of S(-)-methcathinone (CAT): a potent stimulant drug of abuse. Psychopharmacology. 1998;140:250–256. doi: 10.1007/s002130050765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.