Abstract
Maternal over-nutrition may predispose offspring to obesity, type 2 diabetes and other adult diseases. The present study investigated long-term impact of prenatal high sucrose (HS) diets on cognitive capabilities in aged rat offspring. The fasting plasma glucose concentration did not differ between the control and HS groups. However, the fasting plasma insulin and insulin resistance index values were significantly increased in HS offspring that showed abnormal glucose tolerance test. HS offspring exhibited increased escape latency and swimming path length to the platform, and reduced time in the target quadrant and the number of crossing the platform, as compared with the control group. The expression of Grin2b/NR2B, Wnt2, Wnt3a and active form of β-catenin protein were decreased, and Dickkopf-related protein 1 was increased in the HS group. In addition, the levels of lipid peroxidation biomarker thiobarbituricacid reactive substance, nicotinamide adenine dinucleotide phosphate oxidases 2 and superoxide dismutase 1 were significantly increased, and the activity of catalase was decreased in the hippocampus in the HS group. The results demonstrate that prenatal HS-induced metabolic changes cause cognitive deficits in aged rat offspring, probably due to altered N-methyl-D-aspartate receptors/Wnt signaling and oxidative stress in the hippocampus.
Keywords: Aged offspring, Prenatal high sucrose diets, Spatial cognition, Oxidative stress, NMDARs, Wnt/β-catenin
1. Introduction
Age-related dementia such as Alzheimer’s disease (AD) is one of the most common causes of disability among older people, which is expected to reach 106 million by 2050 (Wimo et al., 2013; Ogawa, 2014). Epidemiological and experimental studies have indicated that adverse nutritional status may increase the risk of cognitive loss (Solfrizzi et al., 2011). It has been demonstrated that maternal high calorie intake during pregnancy results in various chronic diseases linked to dementia (Barker, 1990; Lakhan and Kirchgessner, 2013). Recent studies showed that prenatal high sucrose (HS) diets impaired spatial learning associated with apoptosis in the hippocampus in adolescent offspring (Kuang et al., 2014). However, whether prenatal HS diets have a long-term influence on cognitive function in aged offspring is unknown.
Excess energy intake during pregnancy results in programming of adverse metabolic outcomes in offspring such as obesity and diabetes in adulthood (Sedova et al., 2007). Both chronic hyperglycemia and insulin resistance may trigger neuronal death through oxidative stress and affect cognitive processes (Trevino et al., 2015). Oxidative stress is widely accepted as a key player in neurodegeneration, especially in age-related dementia such as AD (Smith et al., 1996; Ansari et al., 2008). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOX) are a family of enzymes in generation of reactive oxygen species (ROS) (Bedard and Krause, 2007). In the central nervous system, NOX contributes to the development of AD, Parkinson disease, anxiety, and schizophrenia (Ma et al., 2017). High fat diets enhanced hippocampal and cerebral cortex oxidative stress via the activation of NOX2 (Zhang et al., 2005; Bruce-Keller et al., 2010). However, effects of prenatal HS diets on NOX in the hippocampus remain unclear.
A recent study suggested that obesity and hypertriglyceridemia induced cognitive impairment (Farr et al., 2008). In the hippocampus, both N-methyl-D-aspartate receptors (NMDARs) and Wnt signaling play important roles in regulating cognitive functions (Chen et al., 2006; Zhao et al., 2009). NMDARs are cation-passing channels in the maintenance of synaptic plasticity, learning and memory (Park et al., 2014). NMDARs contain NR1 and NR2 subunits, and NR2A and NR2B present in high density in the hippocampus (Erreger et al., 2005). Disruption of NMDARs in the hippocampus may lead to blockade of synaptic plasticity and memory decline (Morris et al., 1990). The Wnt pathway is activated when Wnts bind to the Frizzled (FZD) and low-density lipoprotein-related protein (LRP) receptors, followed by the recruitment of cytoplasmic protein Dishevelled (DVL) and inhibition of glycogen synthase kinase-3 (GSK-3) activity. Free β-catenin is ultimately translocated to the nucleus in the control of activation of Wnt-responsive target genes (Kishida et al., 2001). The Wnt signaling pathway was altered in patients with neurodegenerative diseases (Caricasole et al., 2003; Logan and Nusse, 2004). It is unknown whether NMDARs and Wnt signaling are involved in the long-term effects on hippocampal-dependent learning and memory in aged offspring exposed to prenatal HS. Furthermore, ROS may suppress both the Wnt pathway and the function of NMDARs (Buckner, 2004; Manolopoulos et al., 2010). Thus, we determined the effect of prenatal HS on hippocampal NMDARs/Wnt signaling in aged offspring in the present study.
2. Results
2.1. The effect of prenatal HS on food and fluid intake, body and brain weight, plasma glucose, insulin and glucose tolerance in aged offspring
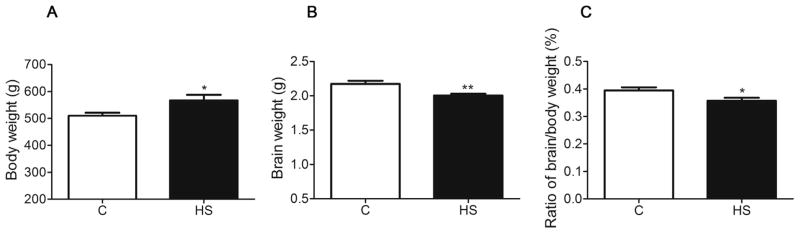
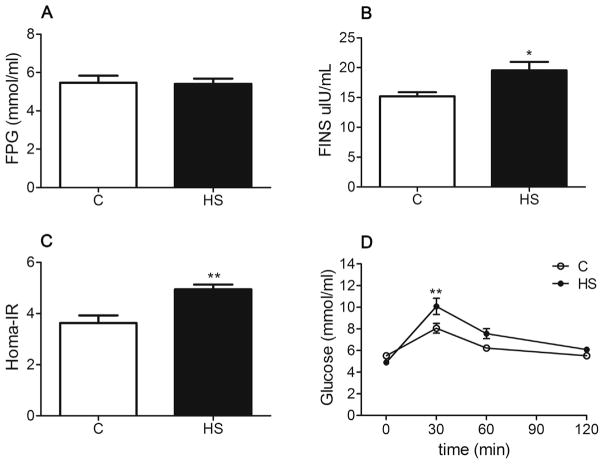
No significant differences were observed in daily food intake (22.45 ± 0.583 g/day versus 24.56 ± 0.7627 g/day, P > 0.05) and fluid intake (33.68 ± 1.764 ml/day versus 37.5 ± 1.118 ml/day, P > 0.05) between the control and HS groups. The body weight of HS offspring was greater than that of the control (P = 0.024, Fig. 1A). The brain weight (P = 0.006, Fig. 1B) and the brain to body weight ratio (P = 0.030, Fig. 1C) were significantly decreased in HS offspring. No significant difference was found in fasting plasma glucose (FPG) levels between the two groups (P = 0.903, Fig. 2A). The fasting plasma insulin (FINS) (P = 0.016, Fig. 2B) and insulin resistance index (Homa-IR) values (P = 0.002, Fig. 2C) were significantly increased in HS offspring, as compared with the control. The difference in glucose tolerance test between the two groups was significant following an intraperitoneal glucose load (F1, 14 = 4.756, P = 0.047, Fig. 2D), and the area under the time curve (AUC) was significantly greater in the HS group than that in the control (13.57 ± 0.519 versus 15.87 ± 0.819, P = 0.033).
Fig. 1.
The effect of prenatal HS diets on body weight (A), brain weight (B), the ratio of brain/body weight (C) in aged offspring. C: control group (n = 11), HS: prenatal HS group (n = 10); Results are presented as mean ± SEM; *, P < 0.05; **, P < 0.01 vs. the control.
Fig. 2.
The effect of prenatal HS diets on plasma glucose (A), plasma insulin (B), Homa-IR (C) and glucose tolerance test (D) in aged offspring. C: control group (n = 8), HS: prenatal HS group (n = 8); Results are presented as mean ± SEM; *, P < 0.05; **, P < 0.01 vs. the control.
2.2. The effect of prenatal HS on spatial memory performance in aged offspring
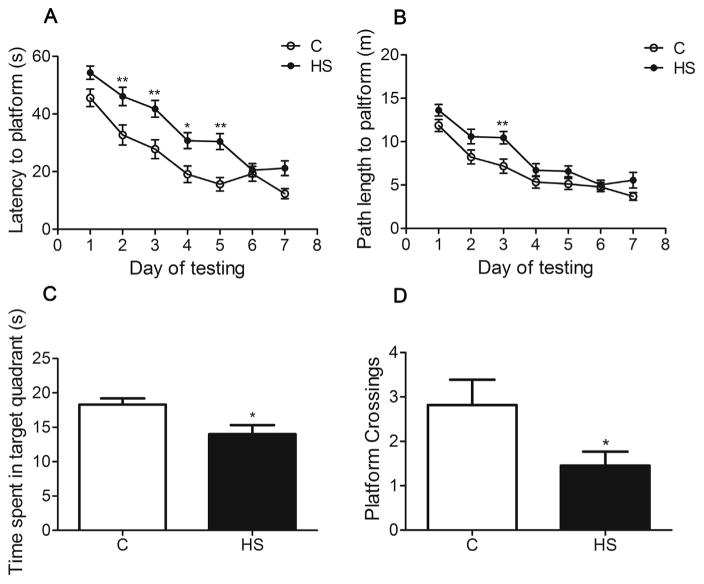
In the learning phase, aged HS offspring showed a significant increase in escape latency (F1, 20 = 15.572, P = 0.001, Fig. 3A), and the path length to the platform was prolonged (F1, 20 = 6.297, P = 0.021, Fig. 3B), as compared with the control. In the retention test, the time spent in the target quadrant (P = 0.0138, Fig. 3C) and the number of crossing the platform (P = 0.049, Fig. 3D) were significantly decreased in the HS group.
Fig. 3.
The effect of prenatal HS diets on behavior performance in aged offspring. The HS group showed longer escape latency and path length to the platform (A, B). The time spent in target quadrant was decreased in HS group in navigation test (C). The number of crossing the platform was significantly decreased in the HS group (D). C: control group (n = 11), HS: prenatal HS group (n = 11); Results are presented as mean ± SEM; *, P < 0.05; **, P < 0.01 vs. the control.
2.3. The effect of prenatal HS on expression of NMDARs and Wnt signaling related genes in aged offspring
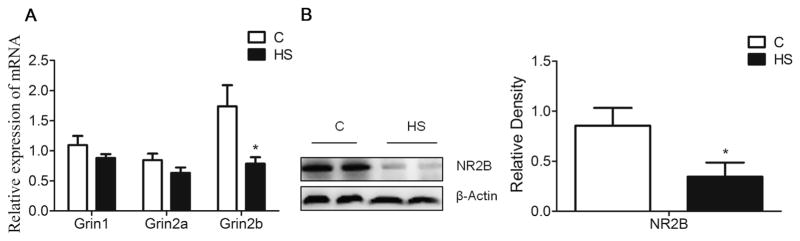
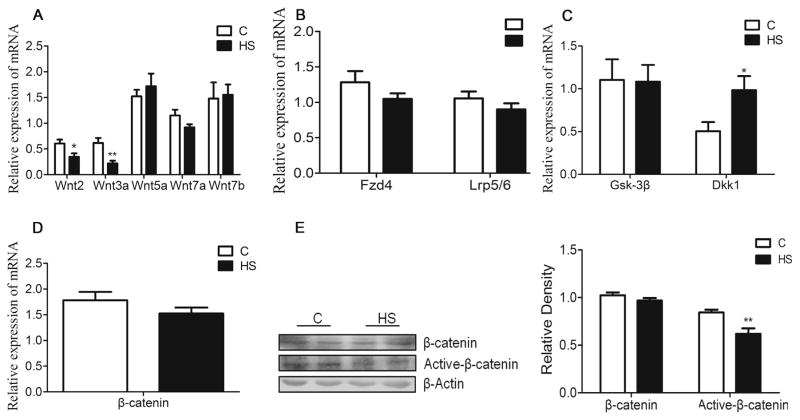
NMDARs-dependent synaptic plasticity is suggested to mediate several forms of learning. The q-PCR showed that mRNA abundance of Grin2b, but not Grin1 and Grin2a, was significantly decreased in the hippocampus of aged HS offspring (P = 0.024, Fig. 4A). Western blot analysis showed that NR2B protein abundance was decreased in aged HS offspring, as compared with the control (P = 0.044, Fig. 4B). Wnt signaling is necessary to maintain NMDARs transmission and synaptic plasticity. The q-PCR showed that mRNA abundance of Wnt2 and Wnt3a were significantly decreased (P = 0.03; P = 0.004, respectively, Fig.5A), whereas Dickkopf-related protein 1 (Dkk1) was increased (P = 0.044, Fig. 5C), as compared with the control. There were no significant differences in mRNA abundance of Fzd4, Lrp5/6, Gsk3β and β-catenin in between the control and HS offspring (P > 0.05, Fig. 5B, C). However, there was a significant decrease in active form of β-catenin protein in HS offspring, as compared with the control (P = 0.004, Fig. 5E).
Fig. 4.
The effect of prenatal HS diets on the expression on NMDARs subunits in the hippocampus in aged offspring. The q-PCR results showed Grin2b, not Grin1 and Grin2a expression level was decreased in the HS group compared with the control (A). Western blot analysis showed NR2B was decreased in HS group compared with the control group (B). C: control group (n = 7), HS: prenatal HS group (n = 7); Results are presented as mean ± SEM; *, P < 0.05; **, P < 0.01 vs. the control.
Fig. 5.
The effect of prenatal HS diets on the expression on Wnt/β-catenin signaling in the hippocampus in aged offspring. The mRNA expression of Wnt2 and Wnt3a were decreased in the hippocampus compared with the control (A). There were no differences in mRNA expression of Fzd4, Lrp5/6, Gsk3β and β-catenin, while Dkk1 was increased in HS group (B–D). The protein expression of β-catenin was no difference, but the form of Active-β-catenin protein was decreased compared with the control (E). C: control group (n = 7), HS: prenatal HS group (n = 7); Results are presented as mean ± SEM; *, P < 0.05; **, P < 0.01 vs. the control.
2.4. The effect of prenatal HS on lipid peroxidation and catalase in aged offspring
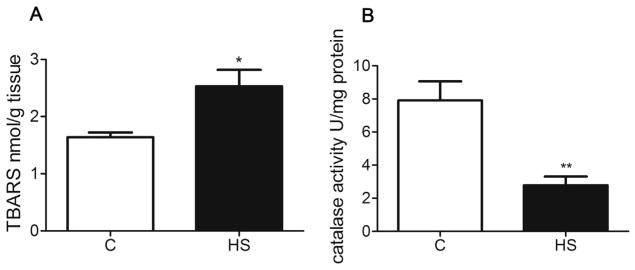
Thiobarbituricacid reactive substance (TBARS) is an index of lipid peroxidation in the hippocampus. Catalase is a metal protein that detoxifies H2O2 to H2O. TBARS was increased in the hippocampus of HS offspring (P = 0.011, Fig. 6A). In contrast, prenatal HS diets markedly decreased the activity of catalase in the hippocampus of aged offspring (P = 0.002, Fig. 6B).
Fig. 6.
The effect of prenatal HS diets on TBARS and catalase activity in aged offspring. C: control group (n = 7), HS: prenatal HS group (n = 7); Results are presented as mean ± SEM; *, P < 0.05; **, P < 0.01, vs. the control.
2.5. The effect of prenatal HS on expression of Sod/SOD subtypes in aged offspring
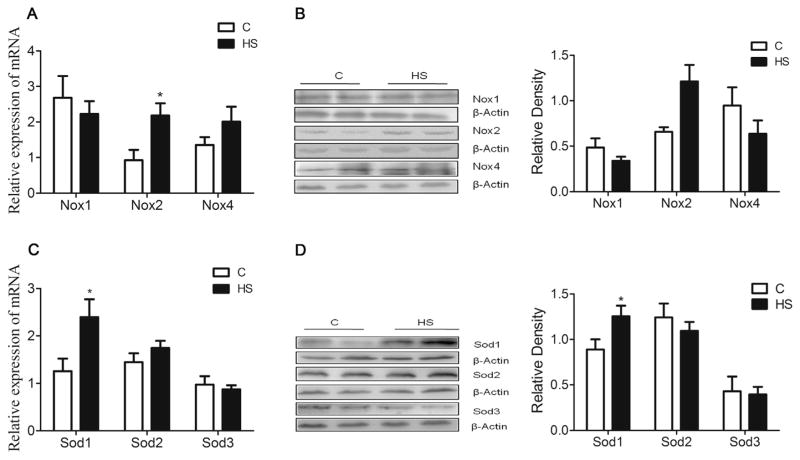
Superoxide dismutase (SOD) is one of the important antioxidant enzymes with three distinct isoforms: SOD1, SOD2, and SOD3. The q-PCR showed that mRNA abundance of Sod1, but not Sod2 and Sod3, was significantly increased in the hippocampus of HS offspring (P = 0.029, Fig. 7C). In addition, SOD1 protein abundance was increased as well in HS offspring, as compared with the control (P = 0.048, Fig. 7D).
Fig. 7.
The effect of prenatal HS diets on expression of Nox/NOX and Sod/SOD in the aged offspring. The mRNA expression and protein of NOX2 (A, B) and SOD1 (C, D) were significantly increased compared with the control. C: control group (n = 7), HS: prenatal HS group (n = 7); Results are presented as mean ± SEM; *, P < 0.05; **, P < 0.01, vs. the control.
2.6. The effect of prenatal HS on expression of Nox/NOX subtypes in aged offspring
NOX is a prominent source of ROS and is involved in various neurodegenerative disorders. The q-PCR showed that mRNA abundance of Nox2, but not Nox1 and Nox4, was significantly increased in the hippocampus of HS offspring, as compared with the control (P = 0.017, Fig. 7A). Furthermore, Western blot analysis demonstrated that NOX2 protein abundance was also increased in HS offspring (P = 0.015, Fig. 7B).
3. Discussion
The present study investigated the long-term effect of prenatal HS diets on hippocampal-dependent behavior and the underlying mechanisms in aged rat offspring. Previous studies reported that prenatal HS diets increased the blood glucose in both pregnant dams and fetuses. Increased birth weight was also found in the HS group as compared with the control (Wu et al., 2014). During the adolescence, the body weight of HS offspring remained higher than the control, while the brain weight was decreased (Kuang et al., 2014). At the adult stage (5 months old), there was no difference in the body weight between the two groups (Wu et al., 2014). In the present study, we showed that aged HS offspring has an increase in body weight and a decrease in brain weight at 18 months of age. In addition, aged HS offspring presented significantly higher FINS and Homa-IR levels than those of the control group.
It has been demonstrated that impaired glucose tolerance, hyperinsulinemia, and insulin resistance are the characteristics of the pre-diabetic state (Tabak et al., 2012). Epidemiological and experimental studies have indicated that people with diabetes show an increased risk in developing cognitive deficits and dementia as getting old (Cukierman et al., 2005). Oxidative stress has been suggested as a key player in diabetic encephalopathy with cumulative effects over a longer period to manifest cognitive decline (Soares et al., 2013).
To determine if prenatal HS causes oxidative stress in the brain, we measured hippocampal levels of TBARS, a marker of lipid lipoxidation. We found a significant increase in TBARS levels in aged HS offspring. Since oxidative stress depends not only on ROS production, but also on the antioxidant capacity, we determined two important antioxidant enzymes, SOD and catalase. The SOD family includes three isoforms. Cu/Zn-SOD1 is distributed throughout the cytoplasm, nucleus and inner membrane space of mitochondria, Mn-SOD2 is restricted to the mitochondrial matrix, and Cu/Zn- SOD3 is located in the extracellular space (Fridovich, 1983). Catalase is an important enzyme responsible for the decomposition of H2O2 to H2O and O2. We found that the expression of Sod1/SOD1 was increased, while the activity of catalase was decreased in the HS group. High concentration of SOD1 appears to lead to H2O2 formation and induces neuronal damage in the brain (Harris-Cerruti et al., 2004). It has been suggested that catalase may protect neurons from amyloid-induced cytotoxicity (Bruce et al., 1996). Our results indicated that prenatal HS intake induced excessive production of H2O2 in the hippocampus, which may induce impairment of neurons.
The present study further investigated whether prenatal HS diets might affect the expression of NOX, a family of ROS-producing enzymes. We found that the expression of Nox2/NOX2 in the hippocampus was significantly increased in aged HS offspring as compared to the control. Activation of NOX2 in neurons contributes to ROS production and apoptosis in the brain (Tammariello et al., 2000). Genetic deletion of Nox2 attenuated oxidative stress and neuronal cell death in mice (Nair et al., 2011). Our results suggest that prenatal HS diets may aggravate oxidative damage in the hippocampus probably via the abnormal activation of NOX2.
Morris water maze was widely used to evaluate hippocampus-dependent spatial learning and memory in rodents. During adolescence, HS offspring exhibited prolonged escape latency and path length in a hidden platform-learning phase, but did not affect the memory retention (Kuang et al., 2014). In the present study, the performance in the spatial acquisition test became worse, and both the time spent in the target quadrant and the number of crossing the platform in retention test were decreased in aged HS offspring, as compared with the control, indicating that the prenatal insult plus aging may cause worse performance on learning and memory task.
NMDARs are a subtype of ionotropic glutamate receptors gated by the neurotransmitter glutamate (Mayer and Westbrook, 1987). We tested three important subunits of NMDARs (NR1, NR2A and NR2B) and found that only Grin2b/NR2B subtype was decreased in aged HS offspring. NR2B subunit is expressed at higher levels throughout the brain during early stages of development and declines after birth, while NR2A subunit-containing NMDARs increase through the life span (Laurie et al., 1997; Law et al., 2003), suggesting different expression patterns during various life periods. Previous study showed that maternal high fat diets decreased the expression of NR2B and the ratio of NR2B/NR2A in adult offspring (Page et al., 2014). NR2B subunit plays an important role in recruiting relevant molecules important for learning and memory (Foster et al., 2010). The finding of decreased Grin2b/NR2B in the present study suggests a possible involvement of NMDARs in the damaged brain function in aged HS offspring.
In addition to NMDARs, the expression levels of Wnt2 and Wnt3a, as well as the active form of β-catenin protein were decreased, while Dkk1 was increased in aged HS offspring. Wnt2 and Wnt3a facilitate the activation of FZD and co-receptor LRP5/6 and ultimately lead to the activation of Wnt/β–catenin signaling (Ai et al., 2007). Abnormal Wnt/β–catenin signaling resulted in cognitive decline (Ivanova et al., 2016). Increased Wnt2 or Wnt3a may enhance dendritic arbors and synaptic plasticity (Wayman et al., 2006), while the down-regulation of Wnt signaling in the hippocampus has been related to cognitive decline associated with neuronal loss (Bayod et al., 2015). The present study showed unchanged Wnt5a and Wnt7a in aged offspring, suggesting a selective effect of Wnt signaling by prenatal HS. Wnt5a and Wnt7a mainly rely on non-β–catenin-dependent pathways and pertain to intracellular calcium release (Patapoutian and Reichardt, 2000). DKK1 is a secreted protein that acts as an inhibitor of Wnt signaling by binding to LRP5/6 (Semenov et al., 2001). The expression of DKK1 was found significantly increased in the hippocampus of AD mouse models, while reduced expression of DKK1 counteracted the age-related decrease in both neurogenesis and cognitive function (Caricasole et al., 2004; Seib et al., 2013). The finding of decreased Wnt2, Wnt3a, active β-catenin, and increased Dkk1 in the present study suggests that prenatal HS may selectively influence the molecules of NMDARs/Wnt signaling pathway in the aged brain. However, to confirm impairment of cognitive functions, more motor and sensory tests of hippocampus function are needed in the future study.
In summary, the present study demonstrated that maternal HS diets during pregnancy is a risk factor for the development of age-related diseases in offspring. Cognitive impairments induced by prenatal HS diets may be correlated with oxidative stress and abnormal NMDARs/Wnt signaling during aging in the hippocampus. These results suggest that prenatal influence may affect health and disease during various life stages. Postnatal influence such as aging may be co-operated with prenatal influence to produce further damage to the brain function in old offspring. Thus, the findings provide new information for further understanding the causes and possible mechanisms of cognitive problems in the old age, as well as new insight in early prevention of the diseases initiated from early developmental stages.
4. Materials and methods
4.1. Experimental animals
Pregnant Sprague-Dawley rats (250–280 g) were obtained from Soochow University Animal Center. Rats were housed in a climate-controlled light-regulated facility with 12:12 h day-night cycles and allowed to free access to food and water. Pregnant rats were randomly divided into two groups (N = 11 each group). From gestational 1 to 21 day, one group was fed with 20% sucrose solution and the other was fed with fresh tap water (C). Both groups were provided with the same standard rat food containing 39.1% carbohydrates, 19.3% protein, 4.0% fat, 0.6% NaCl, 1.00% calcium, 0.70% phosphate and 0.68% potassium (Slaccas, Shanghai, China). During pregnancy, there was no difference in daily fluid intake (36.2 ± 1.4 ml/day versus 36.3 ± 1.5 ml/day). After delivery, all rats were provided with fresh tap water. An average litter size was 9–14. After weaning, male offspring from both groups (n = 1 from each dam) were randomly selected and raised with standard food until 18 month old. All procedures and protocols were approved by the Institutional Animal Care and Use Committee and conform to the Guidelines for the Care and Use of Laboratory Animals.
4.2. Task of Morris water maze
The water maze was modified from the standard version of the Morris test (Morris, 1984). It contained a circular pool (0.46 m in depth and 1.2 m in diameter) and a submerged platform (26 cm in height and 10 cm in diameter). The pool was divided into four virtual quadrants, and each quadrant had different sign on the wall. During testing, the pool was filled with opaque water (26 °C ± 0.5 °C) by addition of nontoxic white paint. There were 4 acquisition trials each day with training lasting for 7 days and the position of the cues was not changed during testing. Each trial was started by placing a rat facing toward the wall. A trial was ended until the rat climbed on the hidden platform with all 4 paws or until 60 s elapsed. If the rats couldn’t find the platform, they were guided to the platform and sat on it for 15 s before being removed and dried with a towel. In the end, the latency and path length for swimming to the platform were measured.
The navigational task was tested at the end of the day of acquisition training finished after the platform was removed. All rats were placed in the pool in a randomly determined quadrant only once and were allowed to swim within a limited time (60 s). The numbers of crossing the platform and the time spent in target quadrant were measured. All activities of rats in the testing were monitored, recorded, and analyzed using MT- 200 water maze video tracking system (Taimeng, Chengdu, China).
4.3. Intraperitoneal glucose tolerance test and sample preparation
Glucose tolerance test was performed in fasted rats (16 h). Blood for glucose determination was drawn from the tail at intervals of 0, 30, 60, and 120 min after giving a glucose solution (2 g/kg body weight) by intraperitoneal injection.
Offspring rats were sacrificed using sodium pentobarbital (100 mg/kg; Jiangsu Henrui) intraperitoneally. Blood samples were collected from abdominal aorta and placed into anti-coagulation tubes. Rat brains were removed by cutting at the edge of vermis for measuring weight. Then, hippocampal tissues were collected and were frozen at −80 °C for analysis.
4.4. Blood sample assay
Plasma glucose was determined using Glucose Assay Kit (Shanghai Rongsheng Cat: 361500) according to manufacturer’s instructions, and plasma insulin was assayed with radioimmunoassay (RIA) by Huaying Biotechnology Institute (Beijing, China). The sensitivity for insulin was 0.01 mIU/L. The intra-assay and inter-assay CV were about 3.0–6.7% and 7.5–9.9%, respectively. Insulin sensitivity of individual rats was evaluated using the homeostasis model assessment (Homa). The fomular used as follows: FPG (mmol/L) × FINS (mIU/L)/22.5. Samples and data were handled in a blind manner.
4.5. TBARS and catalase assay
The measurement of TBARS is a well-established method for monitoring lipid peroxidation. Levels of TBARS in the hippocampus were measured using TBARS Assay Kit (Cayman Item No. 10009055). The reaction mixture contained 0.1 ml hippocampus homogenate (25 mg protein), 530 mg thiobarbituric acid, 2 ml acetic acid solution, (pH 3.5, 20%), 2 ml sodium hydroxide, and 0.1 ml SDS (0.1%). Samples were quantitatively analyzed by reading the absorbance at 532 nm (Dawn-Linsley et al., 2005). Catalase activity was assayed by calculating the rate decomposed H2O2 colorimetrically at a wavelength of 240 nm using Assay Kit (Nanjing Jiancheng, Cat: A007-1) (Sinha, 1972). The reaction mixture (1.5 ml) contained 1 ml phosphate buffer (pH 7.0), 0.1 ml of hippocampus homogenate (supernatant), and 0.4 ml H2O2. The reaction was stopped by acetic acid reagent. The levels of TBARS and enzyme activities of catalase were expressed as units per milligrams of protein (U/mg protein). Total protein concentration was determined using the Bicinchoninic acid (BCA) assay (Beyotime).
4.6. Quantitative real-time PCR
Total RNA was isolated from the hippocampus tissues using RNA plus (RNA extraction reagent, TaKaRa). RNA was reverse transcribed using Revert Aid First-Strand cDNA Synthesis Kit (TaKaRa) according to the manufacturer’s instructions. Q-PCR was performed in 20 μL of the reaction mixture contained 10 μL SYBR Premix Ex Taq (TaKaRa), 5 μL cDNA (50 ng), and 5 μL primers (400 nM). The reaction was analyzed on iCycler Real-Time PCR Detection System (Bio-Rad) with the following conditions: 95 °C for 5 min and 45 cycles of 95 °C 5 s/62 °C 15 s/72 °C 15 s. The analysis was repeated three times for each sample and the expression of mRNA was normalized by each β-Actin mRNA and used 2−ΔΔCT method. The primer sequences are shown in Table 1.
Table 1.
Primer sequences.
| Gene | Forward primer(5′–3′) | Reverse primer(3′-5′) | NCBI Ref. Seq. |
|---|---|---|---|
| β-actin | CCTAAGGCCAACCGTGAAAAG | GCTCGAAGTCTAGGGCAACATAG | NM_031144.3 |
| Nox1 | TTTTCAGTTTGCCCTTTGCT | TCCAAGAGCTGAAGCAGGTT | NM_053683.1 |
| Nox2 | TCTTCAGCCATTCACACCAT | CAGAGAAGGGAGGCTCACC | NM_023965.1 |
| Nox4 | CGGGGTGGCTTGTTGAAGTAT | CCTCCAGGCAAAGATCCATG | NM_053524.1 |
| Sod1 | CGTCATTCACTTCGAGCAGA | AAAATGAGGTCCTGCAGTGG | NM_017050.1 |
| Sod2 | CTGGACAAACCTGAGCCCTA | GAACCTTGGACTCCCACAGA | NM_017051.2 |
| Sod3 | AGGCTCTTTCTCAGGCCTCT | CACCAGTAGCAGGTTGCAGA | NM_012880.1 |
| Grin1 | ATACAGATGGCCCTGTCAGTGT | AGTGAAGTGGTCGTTGGGAGTA | NM_001270606.1 |
| Grin2a | TGTCCAGAATCCTAAAGGCACA | CTGTACTTCCATTGGGTACCGTC | NM_012573.3 |
| Grin2b | CACCTTCATCTGTGAGCATCTG | TATGGCTACCCCATGGATACAG | NM_012574.1 |
| Wnt2 | CAACATTGACTACGGGATCAAA | CATGACACTTGCATTCTTGTT | XM_575397.6 |
| Wnt3a | AACCGTCACAACAATGAGGC | GCAGGTCTTCACTTCGCAAC | NM_001107005.2 |
| Wnt5a | AAGCAGGTCGCAGGACAGTA | GAGTTGAAGCGGCTGTTGAC | NM_022631.2 |
| Wnt7a | CCGAGAGATCAAGCAGAATGC | TCCAGTTTCATGTTCTCCTCCA | NM_001100473.1 |
| Wnt7b | CAAGAGCTCCGAGTAGGGAGTC | ACAGCCACAGTTGCTCAGATTG | NM_001009695.1 |
| Fzd4 | GCTACAACGTGACCAAGATGC | ATGGGGATGTTGATCTTCTCTG | NM_022623.2 |
| Lrp6 | GTTGTAAGCTCGGTTCCAAATC | CAATTTTGGGAGATCTTTCCTG | NM_001107892.1 |
| GSK-3β | GTTTCATGTGCTTCCTTTGC | GGTACTTCCACAAGAACAGCC | NM_032080.1 |
| Dkk1 | CATTTCATTTGATTCCTGGCG | CTTGAGTTGCTCACCAGTTCG | NM_001106350.1 |
| β-catenin | GCTTTGCTCAACAAAACAAACG | ACAGACAGCACCTTCAGCACTC | NM_053357.2 |
4.7. Western blotting
Hippocampal tissues (50 mg) were lysed by 0.25 ml RIPA (Beyotime). Protein homogenates were separated on SDS-PAGE gels eletrophoretically and transferred onto PVDF membranes. The membranes were incubated with the antibodies: NOX1 (Santa Cruz; sc-5821), NOX2 (Santa Cruz; sc74514), NOX4 (Santa Cruz; sc21860), SOD1 (Santa Cruz; sc-11407), SOD2 (Santa Cruz; sc- 30080), SOD3 (Santa Cruz; sc-67088), NR2B (Millipore; AB15362), β-catenin (1:2000, Millipore), active-β-catenin (1:2000, Millipore) and β-Actin (Beyotime; AA128) overnight at 4 °C. Then membranes were washed in Tris-buffered saline with Tween (TBS-T) and incubated with secondary antibodies (1:1000) for an hour. After washing with TBS-T; the bands were visualized after exposed to Hyperfilm (Amersham). Results were quantified using a UVP Bio-imaging system EC3 apparatus (UVP, Upland, CA, USA). The relative density of the bands was normalized to β-Actin.
4.8. Statistics analysis
Data were analyzed using Prism (GraphPad) and SPSS (version 17.0). Unpaired, two tailed Student’s t-test or Mann-Whitney’s test was used to determine differences between the control and HS groups for biochemical, hormonal, and physiological parameters, as well as for q-PCR and western blot data. Repeated measures ANOVA was conducted for analyzing the data from glucose tolerance and water maze tests, Pos-hoc comparisons were made using the LSD method. Difference among groups were considered statistically significant if P < 0.05.
Acknowledgments
Acknowledgments/funding
The authors thank Animal Center of Soochow University for experimental support.
This work is supported by Ministry of Science and Technology of China (2013CB945400), (2012CB947600) and (2013BAI04B05); National Natural Science Foundation of China (81320108006) and (81570960); Jiangsu Province’s Key Discipline/Laboratory of Fetal Medicine.
Abbreviations
- AD
Alzheimer’s disease
- DKK1
Dickkopf-related protein 1
- FINS
fasting plasma insulin
- HS
high sucrose
- FPG
fasting plasma glucose concentration
- FZD
Frizzled
- Homa-IR
insulin resistance index
- LRP5/6
low-density lipoprotein-related protein receptors5/6
- NMDARs
N-methyl-D-aspartate receptors
- NADPH
nicotinamide adenine dinucleotide phosphate
- NOX
NADPH oxidases
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBARS
thiobarbituricacid reactive substance
Footnotes
Author contributions
A.H., M.S., and Z.X. designed research; A.H., Y.Z., Y.Y., J.L., X.F. B. W., D.Z., L.W., Y.L. conducted research; A.H., L.Z., M.S., and X.Z. analyzed data or wrote the paper; A.H. had primary responsibility for final content. All authors read and approved the final manuscript.
Conflict of interest/disclosures
None.
References
- Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci USA. 2007;104:9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, Scheff SW. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic Biol Med. 2008;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of adult disease. BMJ. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayod S, Felice P, Andres P, Rosa P, Camins A, Pallas M, Canudas AM. Downregulation of canonical Wnt signaling in hippocampus of SAMP8 mice. Neurobiol Aging. 2015;36:720–729. doi: 10.1016/j.neurobiolaging.2014.09.017. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, White CL, Gupta S, Knight AG, Pistell PJ, Ingram DK, Morrison CD, Keller JN. NOX Activity in Brain Aging: exacerbation by High Fat Diet. Free Radic Biol Med. 2010;49:22–30. doi: 10.1016/j.freeradbiomed.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AJ, Malfroy B, Baudry M. Beta-Amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci USA. 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, Storto M, Gaviraghi G, Terstappen GC, Nicoletti F. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caruso A, Caraci F, Iacovelli L, Sortino MA, Terstappen GC, Nicoletti F. The Wnt pathway, cell-cycle activation and beta-amyloid: novel therapeutic strategies in Alzheimer’s disease? Trends Pharmacol Sci. 2003;24:233–238. doi: 10.1016/s0165-6147(03)00100-7. [DOI] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- Dawn-Linsley M, Ekinci FJ, Ortiz D, Rogers E, Shea TB. Monitoring thiobarbituric acid-reactive substances (TBARs) as an assay for oxidative damage in neuronal cultures and central nervous system. J Neurosci Methods. 2005;141:219–222. doi: 10.1016/j.jneumeth.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SA, Yamada KA, Butterfield DA, Abdul HM, Xu L, Miller NE, Banks WA, Morley JE. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010;30:2676–2685. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases: regularities and irregularities. Harvey Lect. 1983;79:51–75. [PubMed] [Google Scholar]
- Harris-Cerruti C, Kamsler A, Kaplan B, Lamb B, Segal M, Groner Y. Functional and morphological alterations in compound transgenic mice overexpressing Cu/Zn superoxide dismutaze and amyloid precursor protein. Eur J Neurosci. 2004;19:1174–1190. doi: 10.1111/j.1460-9568.2004.03188.x. [DOI] [PubMed] [Google Scholar]
- Ivanova OY, Dobryakova YV, Salozhin SV, Aniol VA, Onufriev MV, Gulyaeva NV, Markevich VA. Lentiviral modulation of Wnt/beta-catenin signaling affects in vivo LTP. Cell Mol Neurobiol. 2016 doi: 10.1007/s10571-016-0455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida M, Hino S, Michiue T, Yamamoto H, Kishida S, Fukui A, Asashima M, Kikuchi A. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase Iepsilon. J Biol Chem. 2001;276:33147–33155. doi: 10.1074/jbc.M103555200. [DOI] [PubMed] [Google Scholar]
- Kuang H, Sun M, Lv J, Li J, Wu C, Chen N, Bo L, Wei X, Gu X, Liu Z, Mao C, Xu Z. Hippocampal apoptosis involved in learning deficits in the offspring exposed to maternal high sucrose diets. J Nutr Biochem. 2014;25:985–990. doi: 10.1016/j.jnutbio.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A. The emerging role of dietary fructose in obesity and cognitive decline. Nutr J. 2013;12:114. doi: 10.1186/1475-2891-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DJ, Bartke I, Schoepfer R, Naujoks K, Seeburg PH. Regional, developmental and interspecies expression of the four NMDAR2 subunits, examined using monoclonal antibodies. Brain Res Mol Brain Res. 1997;51:23–32. doi: 10.1016/s0169-328x(97)00206-4. [DOI] [PubMed] [Google Scholar]
- Law AJ, Weickert CS, Webster MJ, Herman MM, Kleinman JE, Harrison PJ. Changes in NMDA receptor subunit mRNAs and cyclophilin mRNA during development of the human hippocampus. Ann N Y Acad Sci. 2003;1003:426–430. doi: 10.1196/annals.1300.043. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Ma MW, Wang J, Zhang Q, Wang R, Dhandapani KM, Vadlamudi RK, Brann DW. NADPH oxidase in brain injury and neurodegenerative disorders. Mol Neurodegener. 2017:12. doi: 10.1186/s13024-017-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolopoulos KN, Klotz LO, Korsten P, Bornstein SR, Barthel A. Linking Alzheimer’s disease to insulin resistance: the FoxO response to oxidative stress. Mol Psychiatry. 2010;15:1046–1052. doi: 10.1038/mp.2010.17. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Davis S, Butcher SP. Hippocampal synaptic plasticity and NMDA receptors: a role in information storage? Philos Trans R Soc London Ser B: Biol Sci. 1990;329:187–204. doi: 10.1098/rstb.1990.0164. [DOI] [PubMed] [Google Scholar]
- Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One. 2011;6:e19847. doi: 10.1371/journal.pone.0019847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S. Nutritional management of older adults with cognitive decline and dementia. Geriatr Gerontol Int. 2014;14(Suppl 2):17–22. doi: 10.1111/ggi.12252. [DOI] [PubMed] [Google Scholar]
- Page KC, Jones EK, Anday EK. Maternal and postweaning high-fat diets disturb hippocampal gene expression, learning, and memory function. Am J Physiol Regul Integr Comp Physiol. 2014;306:R527–537. doi: 10.1152/ajpregu.00319.2013. [DOI] [PubMed] [Google Scholar]
- Park H, Popescu A, Poo MM. Essential role of presynaptic NMDA receptors in activity-dependent BDNF secretion and corticostriatal LTP. Neuron. 2014;84:1009–1022. doi: 10.1016/j.neuron.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol. 2000;10:392–399. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedova L, Seda O, Kazdova L, Chylikova B, Hamet P, Tremblay J, Kren V, Krenova D. Sucrose feeding during pregnancy and lactation elicits distinct metabolic response in offspring of an inbred genetic model of metabolic syndrome. Am J Physiol Endocrinol Metab. 2007;292:E1318–E1324. doi: 10.1152/ajpendo.00526.2006. [DOI] [PubMed] [Google Scholar]
- Seib DR, Corsini NS, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T, Martin-Villalba A. Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell. 2013;12:204–214. doi: 10.1016/j.stem.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, Kuhl M, Sokol S, He X. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–961. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer’s. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- Soares E, Prediger RD, Nunes S, Castro AA, Viana SD, Lemos C, De Souza CM, Agostinho P, Cunha RA, Carvalho E, Fontes Ribeiro CA, Reis F, Pereira FC. Spatial memory impairments in a prediabetic rat model. Neuroscience. 2013;250:565–577. doi: 10.1016/j.neuroscience.2013.07.055. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, Panza F, Frisardi V, Seripa D, Logroscino G, Imbimbo BP, Pilotto A. Diet and Alzheimer’s disease risk factors or prevention: the current evidence. Expert Rev Neurother. 2011;11:677–708. doi: 10.1586/ern.11.56. [DOI] [PubMed] [Google Scholar]
- Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379:2279–2290. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammariello SP, Quinn MT, Estus S. NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci. 2000;20:Rc53. doi: 10.1523/JNEUROSCI.20-01-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino S, Aguilar-Alonso P, Flores Hernandez JA, Brambila E, Guevara J, Flores G, Lopez-Lopez G, Munoz-Arenas G, Morales-Medina JC, Toxqui V, Venegas B, Diaz A. A high calorie diet causes memory loss, metabolic syndrome and oxidative stress into hippocampus and temporal cortex of rats. Synapse. 2015;69:421–433. doi: 10.1002/syn.21832. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Marks D, Saneyoshi T, Grant WF, Derkach V, Soderling TR. Activity-dependent dendritic arborization mediated by CaM-kinase I activation and enhanced CREB-dependent transcription of Wnt-2. Neuron. 2006;50:897–909. doi: 10.1016/j.neuron.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Wimo A, Jonsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimers Dement. 2013;9:1–11.e13. doi: 10.1016/j.jalz.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Wu C, Li J, Bo L, Gao Q, Zhu Z, Li D, Li S, Sun M, Mao C, Xu Z. High-sucrose diets in pregnancy alter angiotensin II-mediated pressor response and microvessel tone via the PKC/Cav1.2 pathway in rat offspring. Hypertens Res. 2014;37:818–823. doi: 10.1038/hr.2014.94. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Zhao X, Rosenke R, Kronemann D, Brim B, Das SR, Dunah AW, Magnusson KR. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience. 2009;162:933–945. doi: 10.1016/j.neuroscience.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]