Abstract
Background
Recently, accumulating studies have shown that ubiquitin associated protein 2-like (UBAP2L) is overexpressed in many kinds of malignant tumors, which is closely associated to tumor growth and metastasis. However, the correlations of UBAP2L expression with clinicopathological factors and prognosis of hepatocellular carcinoma (HCC) patients still remain unclear.
Material/Methods
Bioinformatics database (GEO and TCGA) and our own experimental results (including immunohistochemical staining, western blotting and real-time PCR) were analyzed to validate the expression levels of UBAP2L in HCC. Furthermore, Kaplan-Meier survival analysis and Cox multivariate regression model were used to demonstrate the associations of UBAP2L expression with clinicopathological factors and prognosis of HCC patients. Additionally, the potential underlying mechanisms associated to angiogenesis were preliminarily explored.
Results
Compared to the normal group, UBAP2L was significantly highly expressed in HCC cell lines and tissues. Kaplan-Meier survival analysis revealed that patients with high UBAP2L expression level had dramatically less survival time than those with low UBAP2L expression level (p=0.000). Moreover, multivariate Cox regression analysis showed that UBAP2L high expression was an independently unfavorable prognostic parameter for OS of HCC patients (p=0.000). Additionally, Pearson correlation analysis showed that the relationship between UBAP2L expression and VEGF or MVD was significantly positive, respectively (r=0.460, p=0.000 and r=0.387, p=0.000).
Conclusions
UBAP2L was overexpressed in HCC, and patients with high UBAP2L expression had unfavorable prognosis. UBAP2L could be a new potential therapeutic target for HCC in the future.
MeSH Keywords: Angiogenesis Inducing Agents; Carcinoma, Hepatocellular; Prognosis; Ubiquitin
Background
Hepatocellular carcinoma (HCC), as the most common pathological type, accounts for approximately 80% to 90% of primary liver cancer [1]. Surgical resection and liver transplantation are the most important treatments for HCC patients to achieve long-term survival [2–4]. But even if such a principle is available, the recurrence rate is rather high and the five-year recurrence rate is about 60% to 80%, which is the main obstacle to long-term survival of HCC patients [5,6]. The development and progression of HCC is the result of many factors. But until now, the detailed underlying mechanisms have been considered complicated and ambiguous. Accumulating studies have shown that angiogenesis plays a pivotal role in promoting tumor cell survival and accelerating the invasion and metastasis of HCC [7–13]. Therefore, to explore new biological targets to predict the prognosis of patients, prevent recurrence and metastasis, and develop new anti-angiogenic targeted therapeutic drugs were the main focus of this current study of HCC.
Ubiquitin associated protein 2-like (UBAP2L) is a molecule that binds to ubiquitin [14]. It can co-localize in the ubiquitinated protein accumulation region after inhibitors block the proteasome pathway, suggesting that UBAP2L may be involved in human cell pathophysiology process [15]. Recent studies have revealed that UBAP2L is overexpressed in some malignant tumors [16–19]. Inhibition of the UBAP2L expression will suppress tumor growth, induce cell apoptosis, and promote metastasis in these cancers. Until now, only Ye et al. [20] have shown that UBAP2L is highly expressed in HCC and downregulation of UBAP2L can inhibit the epithelial-mesenchymal transition (EMT) via snail1 regulation in HCC cells. However, the associations of UBAP2L expression with clinicopathological characteristics and prognosis of HCC patients still remain unclear.
Therefore, in the present study, we used bioinformatics databases and our own experimental results (including immunohistochemical staining, western blotting and real-time PCR) to analyze and validate the overexpression of UBAP2L in HCC. Furthermore, Kaplan-Meier survival analysis and Cox multivariate regression model were used to demonstrate the associations of UBAP2L expression with clinicopathological parameters and prognosis of HCC patients. Additionally, the potential underlying mechanisms associated with angiogenesis were preliminarily explored.
Material and Methods
Bioinformatics prediction
We initially used the Oncomine database (https://www.oncomine.org/resource/login.html) to predict the expression levels of UBAP2L mRNA in HCC and normal tissues. Then, the Cancer Genome Atlas (TCGA) database was employed to forecast the UBAP2L mRNA expression levels and the association between its expression levels with overall survival of HCC patients.
Patients and tissue samples
Human tissue microarray (TMA, catalog no. HLiv-HCC180Sur-04), including 90 cases of paired HCC tissues and adjacent normal tissues, was purchased from Shanghai Outdo Biotech Co., Ltd. (Shanghai, China). All HCC patients received radical surgery from August 2006 to November 2009. And the ultimate time of follow-up was September 2013. The present study was authorized by the Ethics Committee of Anhui Provincial Hospital and all patients signed the informed consent.
Immunochemical staining and evaluation
According to the manufacturer’s protocol, immunohistochemistry was performed with UBAP2L antibody (ab209105, Abcam, UK) at the dilution of 1: 500, anti-VEGF (1: 200, ab1316, Abcam, UK) and anti-CD34 (1: 200, ab81289, Abcam, UK). The results were determined using a blind method, and each slice was counted by two pathologists. The immunoreactive score (IRS) was calculated according to the staining intensity (SI) multiplied by the percentage of staining positive cells (PP). The detailed score standard were as follows: SI (ranged from 0–3 scores): 0 was negative, 1 was weak, 2 was moderate and 3 was strong; PP (ranged from 0–4): 0 for negative, 1 for 1% to 25%, 2 for 26% to 50%, 3 for 51% to 75% and 4 for 76% to 100%. IRS score was ranged from 0 to 12. If IRS >4, high expression of UBAP2L was considered and if IRS ≤4, low expression of UBAP2L was defined.
Evaluation of microvessel density (MVD)
Microvessel density (MVD counts were evaluated according to the report of Weidner et al. [21]. The microvessel distribution was initially selected at low magnification (40x), and then the number of blood vessels stained with CD34 was counted under high magnification (200×). The results were expressed as the mean of the number of vessels in five 200-fold visual fields.
Cell culture
HCC cell lines SMMC-7721, Huh7, Hep3B, and HepG2, and normal liver cell line L02 were purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, PR China). All the aforementioned cell lines were cultured according to the manufacturer’s instructions and our previously reported method [22].
Real-time PCR
According to the manufacturer’s protocol, total RNA was isolated from HCC cell lines by using TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). Then, PrimeScript™ RT reagent kit (Takara, Dalian, China) was used to do the reverse transcription. The mRNA levels were normalized against those of the GAPDH housekeeping gene. UBAP2L primer sequence was listed as follows:
forward primer: 5′-ATTCGCCTCACTCTCCACAC-3′,
reverse primer: 5′-TACCACCACACAACACAGCA-3′.
GAPDH primer sequence was listed below:
sense primer: 5′-TGACTTCAACAGCGACACCCA-3′,
antisense primer: 5′-CACCCTGTTGCTGTAGCCAAA-3′.
All samples were processed under the same experimental conditions. Then, SYBR Premix Ex Taq™ (Takara, Dalian, China) was used to perform the qRT-PCR base on the manufacturer’s scheme.
Western blot
HCC cell lines and four cases of HCC fresh specimens were used as the original sample to extract protein. Then, western blotting was performed as previously described [22]. Equivalent amounts of extracted proteins were used in the western blot experiments. Anti-UBAP2L (Abcam, UK) and anti-GAPDH antibodies (Abcam, UK) were used.
Statistical analysis
We used SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA) to do the statistical analysis. Quantitative data were expressed as mean ± standard deviation (SD). Pearson chi-square test or Fisher’s exact test was used to determine the relationship between UBAP2L expression and clinicopathological factors of HCC patients. Survival analysis was performed by the Kaplan-Meier and log-rank test. Then, parameters which were significant in univariate analysis were selected for Cox multivariate analysis to identify their prognostic significance. The difference was statistically significant if the p value was less than 0.05.
Results
Overexpression of UBAP2L mRNA in HCC predicted by bioinformatics
Initially, Oncomine database was used to predict the UBAP2L mRNA expression levels in HCC and normal tissues. Compared to the normal group, the expression level of UBAP2L mRNA was dramatically higher in HCC tissues (All p values <0.001, Figure 1A–1D). In addition, similar results were found through TCGA database as shown in Figure 2A (p<0.05).
Figure 1.
UBAP2L mRNA overexpression in HCC predicted by the Oncomine database. UBAP2L mRNA levels in (A) Roessler Liver (GEO: GSE 14520/GPL571), (B) Chen Liver (GEO: GSE 3500), (C) Roessler Liver2 (GEO: GSE 14520/GPL3921), and (D) Wurmbach Liver (GEO: GSE 6764) grouped by HCC and normal liver in Oncomine database.
Figure 2.
UBAP2L mRNA overexpression and its correlation with overall survival time of HCC patients predicted by the TCGA database. TCGA database mining analysis of (A) UBAP2L mRNA levels grouped by HCC and normal liver and (B) correlation between UBAP2L mRNA and the OS of HCC patients, * p<0.05.
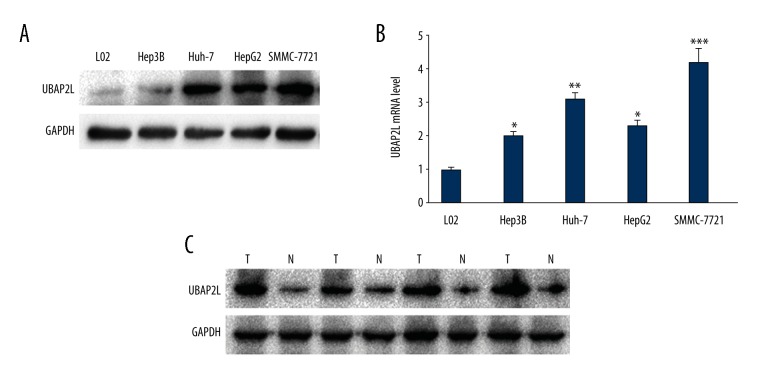
Verification of UBAP2L mRNA and protein overexpression in human HCC cell lines and tissues
In order to verify the aforementioned predictive findings, four kinds of HCC cell lines (Hep3B, Huh-7, HepG2, and SMMC-7721), four cases of fresh HCC tissues, and 90 cases of HCC paraffin specimens were chosen to examine the expression levels of UBAP2L mRNA and protein, respectively. Compared to the corresponding normal groups (L02 hepatic cell line and the matched adjacent normal HCC tissues), either protein or mRNA expression levels of UBAP2L in HCC cell lines (Figure 3A, 3B) or HCC fresh and paraffin tissues (Figure 3C) were dramatically higher (all p values <0.05), respectively.
Figure 3.
UBAP2L expression levels in HCC cell lines and tissues. (A) High expression of UBAP2L protein in four kinds of HCC cell lines (Hep3B, Huh-7, HepG2, and SMMC-7721) and hepatic normal cell line (L02). (B) Relative high UBAP2L mRNA level in HCC cell lines compared to the normal cell line, * p<0.05, ** p<0.01, *** p<0.001. (C) High expression of UBAP2L protein in four cases of fresh paired HCC and adjacent normal tissues, T – HCC, N – adjacent normal tissues.
Relationship between UBAP2L differential protein expression levels and clinicopathological parameters of HCC patients
To explore the relationship between UBAP2L differential expression levels and clinicopathological parameters of HCC patients, the immunochemical results were statistically analyzed further. The results showed that UBAP2L staining was localized both in nucleus and cytoplasm (Figure 4C, 4D). Compared to that in the matched normal tissues, UBAP2L protein expression was dramatically higher in HCC tissues (Figure 4A, 4B, Table 1, p=0.000). Besides, we observed the relationship between UBAP2L protein differential expression and clinicopathological factors of HCC patients (Table 2). There were no significant relationships between the differential expression levels of UBAP2L protein and clinicopathological parameters in HCC patients.
Figure 4.
Representative immunohistochemical images of UBAP2L in 90 cases of paired HCC and adjacent normal tissues: (A) High expression of UBAP2L in HCC tissue; (B) Low expression of UBAP2L in matched adjacent normal tissue; (C) Local magnification of (A); (D) Low expression of UBAP2L in HCC tissue. Bar=100 um.
Table 1.
UBAP2L overexpression in 90 cases of HCC compared to the paired adjacent normal tissues.
| Samples | UBAP2L expression levels (cases, %) | P | |
|---|---|---|---|
| Low | High | ||
| HCC tissues | 35 (38.9) | 55 (61.1) | 0.000 |
| Adjacent normal tissues | 69 (76.7) | 21 (23.3) | |
Table 2.
Correlation of UBAP2L with clinicopathological parameters of HCC patients.
| Variables | Cases (N) | UBAP2L expression | P value | |
|---|---|---|---|---|
| Low | High | |||
| Age at surgery (years) | ||||
| ≤60 | 66 | 24 | 42 | 0.415 |
| >60 | 24 | 11 | 13 | |
| Gender | ||||
| Male | 81 | 30 | 51 | 0.282 |
| Female | 9 | 5 | 4 | |
| Edmondson classification | ||||
| I–II | 57 | 22 | 35 | 0.940 |
| III–IV | 33 | 13 | 20 | |
| Tumor size (cm) | ||||
| ≤5 | 38 | 17 | 2P | 0.331 |
| >5 | 52 | 18 | 34 | |
| Vascular invasion | ||||
| Yes | 7 | 1 | 6 | 0.167 |
| No | 83 | 34 | 49 | |
| Cirrhosis | ||||
| Yes | 33 | 16 | 18 | 0.331 |
| No | 57 | 20 | 37 | |
| TNM stage | ||||
| I–II | 46 | 20 | 26 | 0.361 |
| III–IV | 44 | 15 | 29 | |
Overexpression of UBAP2L was closely associated with poor prognosis of HCC patients
Furthermore, we used the Kaplan-Meier method and log-rank test to examine the overall survival rate of HCC patients based on high- or low-expression level of UBAP2L protein (Table 3). The results showed that patients with high levels of UBAP2L protein had remarkably shorter survival time than those with low levels of UBAP2L protein (p=0.000, Figure 5A). In addition, tumor size, vascular invasion, and TNM stage were also significantly related to the OS of HCC patients (Figure 5B–5D, Table 3). Consistently, Kaplan-Meier survival analysis from the TCGA database showed that HCC patients with high expression level of UBAP2L mRNA had worse prognosis than those with low UBAP2L mRNA level (p<0.001, Figure 2B). Additionally, multivariate Cox regression analysis revealed that high expression of UBAP2L protein was an independently unfavorable prognostic parameter for OS of HCC patients (p=0.000, Table 4).
Table 3.
Kaplan-Meier survival analysis of UBAP2L and other clinicopathological parameters in HCC patients.
| Variables | Mean survival time (months) | 95% CI | P value |
|---|---|---|---|
| UBAP2L expression | |||
| Low | 59.169 | 50.608–67.730 | 0.000 |
| High | 27.875 | 20.313–35.437 | |
| Age at surgery (years) | |||
| ≤60 | 37.431 | 29.688–45.173 | 0.261 |
| >60 | 47.917 | 36.072–59.762 | |
| Gender | |||
| Male | 39.778 | 32.863–46.693 | 0.652 |
| Female | 41.556 | 22.570–60.541 | |
| Edmondson classification | |||
| I–II | 41.856 | 33.717–49.996 | 0.493 |
| III–IV | 36.774 | 26.070–47.477 | |
| Tumor size (cm) | |||
| ≤5 | 51.725 | 41.821–61.629 | 0.005 |
| >5 | 31.687 | 23.789–39.586 | |
| Vascular invasion | |||
| Yes | 16.286 | 3.395–29.176 | 0.017 |
| No | 42.079 | 35.254–48.904 | |
| Cirrhosis | |||
| Yes | 41.028 | 29.907–52.148 | 0.965 |
| No | 39.801 | 31.625–47.977 | |
| TNM stage | |||
| I–II | 50.092 | 41.272–58.912 | 0.001 |
| III–IV | 29.639 | 21.018–38.259 | |
Figure 5.
Kaplan-Meier analysis of overall survival (OS) curves of HCC patients based on UBAP2L expression and other significantly meaningful indicators. (A) OS curve based on UBAP2L expression (high versus low); (B) OS curve based on tumor size (≤5c m versus >5 cm); (C) OS curve based on vascular invasion (Yes versus No); (D) OS curve based on TNM stage (I–II versus III–IV).
Table 4.
Cox multivariate analysis of UBAP2L and other clinicopathological parameters in HCC patients.
| Covariates | HR | 95% CI for HR | P value |
|---|---|---|---|
| UBAP2L expression (low vs. high) | 3.429 | 1.868–6.296 | 0.000 |
| Tumor size (≤5 vs. >5 cm) | 1.430 | 0.775–2.637 | 0.252 |
| Vascular invasion (No vs. Yes) | 1.967 | 0.812–4.761 | 0.134 |
| TNM stage (I–II vs. III–IV) | 2.051 | 1.142–3.685 | 0.016 |
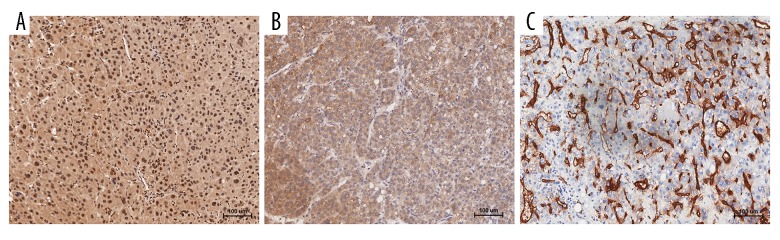
Potential mechanism of UBAP2L overexpression in HCC associated to angiogenesis
To explore the underlying mechanism of UBAP2L overexpression in HCC, we then examined the expression levels of UBAP2L, VEGF, and MVD (labeled by CD34 staining) and analyzed the relationship among them. The results showed that there was a positive correlation expression trend among them (Figure 6). Based on the low- or high-expression levels of UBAP2L, MVD in the two groups had statistical significance (p=0.000, Figure 7). Moreover, Pearson correlation analysis showed that the relationship between UBAP2L expression and VEGF or MVD was significantly positive, respectively (r=0.460, p=0.000 and r=0.387, p=0.000; Table 5).
Figure 6.
High co-expression of UBAP2L, VEGF, and CD34 in HCC tissues examined by immunohistochemistry. (A) UBAP2L high expression; (B) VEGF high expression; (C) CD34 high expression. Bar=100 um.
Figure 7.
Relationship between UBAP2L expression and microvessel density (MVD) in HCC. (A) Differential MVD values grouped by UBAP2L high- or low-expression; (B) representative image of MVD in group of UBAP2L high expression; (C) representative image of MVD in group of UBAP2L low expression. MVD was calculated by the CD34 immunohistochemical staining. Bar=100 um.
Table 5.
Correlations of UBAP2L expression levels with VEGF and MVD in HCC tissues.
| Immunoreactivity | UBAP2L expression levels (cases) | |||
|---|---|---|---|---|
| Low | High | r | P | |
| VEGF expression | ||||
| Low | 23 | 11 | 0.460 | 0.000 |
| High | 12 | 44 | ||
| MVD value | ||||
| Low | 24 | 16 | 0.387 | 0.000 |
| High | 11 | 39 | ||
Discussion
Recently, accumulating studies have found that UBAP2L overexpressed in some types of malignancies, such as prostate cancer [16], glioma [17], colorectal carcinoma [18], and lung adenocarcinoma [19]. Moreover, inhibition of UBAP2L expression will suppress the tumor growth, induce cell apoptosis, and promote the metastasis in these cancers. In HCC, only one study revealed that UBAP2L was highly expressed and suppressed its gene expression level could inhibit the EMT via regulation of snail in HCC cell lines [20]. But until now, the relationship between UBAP2L expression level and clinicopathological factors of HCC patients and its potentially prognostic significance have still remained still unclear. Therefore, in order to clarify the aforementioned issues, the present study was arranged.
First, we used the bioinformatics methods, including data from GEO and TCGA databases, to find that UBAP2L mRNA expression levels were remarkably overexpressed in HCC tissues compared to those in normal tissues. Then, in order to verify the above predictive results, we examined the mRNA and protein levels of UBAP2L in HCC cells and tissues. The experimental findings were consistent with the predictive results and both of UBAP2L mRNA and protein levels were overexpressed in HCC.
Then, the relationship between UBAP2L expression levels and clinicopathological parameters of HCC patients was examined. Our results showed that there were no significant difference between UBAP2L expression and clinicopathological parameters of HCC patients based on low- or high-levels of UBAP2L. But in the Kaplan-Meier survival analysis, we found that HCC patients with high UBAP2L levels had significantly worse overall survival time than those with low UBAP2L levels. Moreover, Cox multivariate analysis revealed that the expression level of UBAP2L was an independently unfavorable prognostic factor of HCC patients.
Lastly, the potentially underlying mechanisms were preliminarily explored. Obviously, the development of HCC is the result of a combination of various factors, in which angiogenesis plays an important role. It can not only impact tumor tissue oxygen and nutrient supply, but also provide a shortcut for tumor cell spread. A large number of studies have demonstrated that aggravation of angiogenesis is a pivotal element to the tumor growth, invasion and metastasis of HCC [7–13]. In the present study, we also found that UBAP2L expression level was positively associated with both VEGF expression and MVD in HCC. In addition, further analysis revealed that HCC patients with high UBAP2L expression had dramatically higher MVD than those with low UBAP2L expression. As we known, VEGF and MVD labeled by CD34 staining are considered to be commonly representative indicators of tumor angiogenesis [23–25]. Thus, together with the aforementioned, it is suggested that UBAP2L may exert its biological function through promoting angiogenesis in HCC.
However, there were some limitations in this study. First, we selected discontinuous cases, which may have led to selection bias and affected the final statistical results. Furthermore, the number of clinical cases was relatively small and some pathological parameters were not complete (such as AFP, HBsAg status, etc.), which could have a certain impact on the overall analysis results. In addition, whether UBAP2L can mediate the angiogenesis of HCC and the exact detailed molecular mechanisms have not been observed and verified. Therefore, these issues will be explored in more detail in our future studies.
Conclusions
Our preliminary study demonstrated that UBAP2L was overexpressed in HCC, and patients with high UBAP2L expression had unfavorable prognosis. UBAP2L could be a new potential therapeutic target for HCC in the future.
Footnotes
Source of support: This research was partly supported by the Natural Science Foundation of Anhui Province (No. 1708085QH177) and National Natural Science Foundation of China (No. 81201906)
Conflicts of interest
None.
References
- 1.Tunissiolli NM, Castanhole-Nunes MMU, Biselli-Chicote PM, et al. Hepatocellular carcinoma: A comprehensive review of biomarkers, clinical aspects, and therapy. Asian Pac J Cancer Prev. 2017;18:863–72. doi: 10.22034/APJCP.2017.18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erstad DJ, Tanabe KK. Hepatocellular carcinoma: Early-stage management challenges. J Hepatocell Carcinoma. 2017;4:81–92. doi: 10.2147/JHC.S107370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745–61. doi: 10.1053/j.gastro.2016.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: Outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203–17. doi: 10.1038/nrgastro.2016.193. [DOI] [PubMed] [Google Scholar]
- 5.Dai WC, Cheung TT. Strategic overview on the best treatment option for intrahepaitc hepatocellular carcinoma recurrence. Expert Rev Anticancer Ther. 2016;16:1063–72. doi: 10.1080/14737140.2016.1226136. [DOI] [PubMed] [Google Scholar]
- 6.Allaire M, Nault JC. Advances in management of hepatocellular carcinoma. Curr Opin Oncol. 2017;29:288–95. doi: 10.1097/CCO.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 7.Berretta M, Rinaldi L, Di Benedetto F, et al. Angiogenesis inhibitors for the treatment of hepatocellular carcinoma. Front Pharmacol. 2016;7:428. doi: 10.3389/fphar.2016.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong XL, Qin SK. Progress in systemic therapy of advanced hepatocellular carcinoma. World J Gastroenterol. 2016;22:6582–94. doi: 10.3748/wjg.v22.i29.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taketomi A. Clinical trials of antiangiogenic therapy for hepatocellular carcinoma. Int J Clin Oncol. 2016;21:213–18. doi: 10.1007/s10147-016-0966-0. [DOI] [PubMed] [Google Scholar]
- 10.Lin D, Wu J. Hypoxia inducible factor in hepatocellular carcinoma: A therapeutic target. World J Gastroenterol. 2015;21:12171–78. doi: 10.3748/wjg.v21.i42.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks EI, Yee NS. Molecular genetics and targeted therapy in hepatocellular carcinoma. Curr Cancer Drug Targets. 2016;16:53–70. doi: 10.2174/1568009615666150916092903. [DOI] [PubMed] [Google Scholar]
- 12.Sampat KR, O’Neil B. Antiangiogenic therapies for advanced hepatocellular carcinoma. Oncologist. 2013;18:430–38. doi: 10.1634/theoncologist.2012-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnoni A, Santini D, Scartozzi M, et al. Hepatocellular carcinoma treatment over sorafenib: epigenetics, microRNAs and microenvironment. Is there a light at the end of the tunnel? Expert Opin Ther Targets. 2015;19:1623–35. doi: 10.1517/14728222.2015.1071354. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann K, Bucher P. The UBA domain: A sequence motif present in multiple enzyme classes of the ubiquitination pathway. Trends Biochem Sci. 1996;21:172–73. [PubMed] [Google Scholar]
- 15.Wilde IB, Brack M, Winget JM, Mayor T. Proteomic characterization of aggregating proteins after the inhibition of the ubiquitin proteasome system. J Proteome Res. 2011;10:1062–72. doi: 10.1021/pr1008543. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Huang Y. Knockdown of ubiquitin associated protein 2-like inhibits the growth and migration of prostate cancer cells. Oncol Rep. 2014;32:1578–84. doi: 10.3892/or.2014.3360. [DOI] [PubMed] [Google Scholar]
- 17.Zhao B, Zong G, Xie Y, et al. Downregulation of ubiquitin-associated protein 2-like with a short hairpin RNA inhibits human glioma cell growth in vitro. Int J Mol Med. 2015;36:1012–18. doi: 10.3892/ijmm.2015.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chai R, Yu X, Tu S, Zheng B. Depletion of UBA protein 2-like protein inhibits growth and induces apoptosis of human colorectal carcinoma cells. Tumour Biol. 2016;37:13225–35. doi: 10.1007/s13277-016-5159-y. [DOI] [PubMed] [Google Scholar]
- 19.Aucagne R, Girard S, Mayotte N, et al. UBAP2L is amplified in a large subset of human lung adenocarcinoma and is critical for epithelial lung cell identity and tumor metastasis. FASEB J. 2017 doi: 10.1096/fj.201601219RRR. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Ye T, Xu J, Du L, et al. Downregulation of UBAP2L inhibits the epithelial-mesenchymal transition via SNAIL1 regulation in hepatocellular carcinoma cells. Cell Physiol Biochem. 2017;41:1584–95. doi: 10.1159/000470824. [DOI] [PubMed] [Google Scholar]
- 21.Weidner N. Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat. 1995;36:169–80. doi: 10.1007/BF00666038. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Jia WD, Hu B, Pan YY. RAB10 overexpression promotes tumor growth and indicates poor prognosis of hepatocellular carcinoma. Oncotarget. 2017;8:26434–47. doi: 10.18632/oncotarget.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Z, Zhou X, Lin Z, et al. Surgical treatment of hepatocellular carcinoma and related basic research with special reference to recurrence and metastasis. Chin Med J (Engl) 1999;112:887–91. [PubMed] [Google Scholar]
- 24.Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–92. doi: 10.3748/wjg.v8.i3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: A review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513. doi: 10.1007/s00432-004-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]