Abstract
Background: In phase III clinical studies, treatment with teduglutide was associated with clinically meaningful reductions (≥20% from baseline) in parenteral support (PS; parenteral nutrition and/or intravenous fluids) requirements in adult patients with intestinal failure associated with short bowel syndrome (SBS-IF). This analysis reports clinical characteristics of patients who achieved complete independence from PS during teduglutide treatment. Materials and Methods: Post hoc analysis of adult patients who achieved complete PS independence during treatment with teduglutide 0.05 mg/kg/d. Data were pooled from 5 teduglutide clinical trials (2 phase III placebo-controlled trials [NCT00081458 and NCT00798967] and their respective extension studies [NCT00172185, NCT00930644, NCT01560403]). Descriptive statistics were used; no between-group comparisons were performed because of the small sample size and lack of comparator. Results: Of 134 patients, 16 gained oral or enteral autonomy after a median of 5 years of PS dependence and 89 weeks of teduglutide treatment. Demographic and baseline disease characteristics varied among patients (median age, 55 years; 50% men; median baseline PS volume, 5.1 L/wk; median residual small intestine length, 52.5 cm). Most patients who achieved PS independence had colon-in-continuity; however, there was no significant difference in the frequency of PS independence among patients who maintained colon-in-continuity vs those who did not. Conclusion: Findings from this post hoc analysis suggest that oral or enteral autonomy is possible for some patients with SBS-IF who are treated with teduglutide, regardless of baseline characteristics and despite long-term PS dependence.
Keywords: intestinal failure, parenteral nutrition, nutrition, gastroenterology, short bowel syndrome, teduglutide
Clinical Relevancy Statement
Teduglutide, a glucagon-like peptide-2 analogue, increases intestinal adaptation and promotes intestinal absorption in patients with short bowel syndrome, based on several trials. A subset of patients was able to gain independence from parenteral support (parenteral nutrition and/or intravenous fluids). In most patients, oral or enteral autonomy was achieved after ≥1 year of teduglutide, suggesting accrued response to treatment. The wide range of disease and clinical characteristics is described for clinicians managing patients with short bowel syndrome considering treatment with teduglutide.
Introduction
Patients with intestinal failure associated with short bowel syndrome (SBS-IF) do not absorb sufficient fluid and nutrients through oral or enteral intake alone because of substantial intestinal resection or functional impairment. Many of these patients require complete or partial parenteral support (PS; parenteral nutrition [PN] and/or intravenous [IV] fluids).1 Although PS is lifesaving therapy, chronic PS dependence increases the risk of serious medical complications, including central venous catheter–related bloodstream infections and liver damage.2
Teduglutide is a recombinant analogue of the endogenous intestinotrophic hormone glucagon-like peptide-2 (GLP-2) and is the first targeted therapy to enhance absorptive capacity in patients with SBS-IF.3–5 In phase III, placebo-controlled studies and their long-term extensions, patients receiving teduglutide achieved and maintained clinical responses to treatment, as evidenced by reductions in weekly PS volume, additional days off from PS infusion, and total independence from PS for some patients.3,5–8 The safety profile of teduglutide during the placebo-controlled studies was consistent with its mechanism of action and with SBS-IF and its underlying causes.3,5,9 The most common adverse events (AEs) were gastrointestinal, including abdominal pain, nausea, and stoma enlargement; serious AEs (SAEs) included catheter-related complications, small intestinal obstruction, fever, and cholecystitis. Other AEs of special interest identified in the placebo-controlled studies and their long-term extensions were malignancy; colorectal polyps; gastrointestinal (GI) obstruction; gallbladder, biliary, and pancreatic disease; fluid overload; and increased absorption of concomitant oral medications.10
To gather more information about the efficacy and safety of teduglutide treatment and the conditional nature of PS dependence, this analysis was undertaken to report the characteristics of adult patients treated for up to 42 months with teduglutide at a subcutaneous dose of 0.05 mg/kg/d who have achieved oral or enteral autonomy in 2 phase III placebo-controlled studies and their respective extensions. Enteral autonomy was defined as complete independence from PS that occurred during treatment with teduglutide, subsequent to pretreatment standard-of-care optimization, including dietary and antidiarrheal therapy and stabilization of PS administration.3,5,7,8
Methods
Included Studies
Adult patients with SBS who required PS ≥3 times weekly for ≥12 months were enrolled in 2 phase III, multicenter, randomized controlled trials (RCTs)3,5 or their respective extension studies.6–8 Study NCT00081458 (ClinicalTrials.gov identifier; EudraCT, 2004-000438-35) and STEPS (NCT00798967; EudraCT, 2008-006193-15) were 24-week, double-blind RCTs of subcutaneous teduglutide (0.05 or 0.10 mg/kg/d in NCT00081458 and 0.05 mg/kg/d in STEPS).3,5 Study NCT00172185 was a double-blind, 28-week extension of NCT00081458.7 In study NCT00172185, patients who received teduglutide in NCT00081458 continued on the same teduglutide dose in the extension study, and patients who received placebo were randomized 1:1 to treatment with subcutaneous teduglutide 0.05 or 0.10 mg/kg/d. Total treatment time for patients who received teduglutide in NCT00081458 and completed the NCT00172185 extension was 1 year. STEPS-2 (NCT00930644; EudraCT, 2009-011679-65) was a 2-year, open-label extension of the phase III STEPS RCT.8 Patients were eligible to enroll in STEPS-2 if they had completed either the placebo or teduglutide arm of STEPS. In addition, patients who completed the fluid optimization and stabilization phase in STEPS but were not randomized to treatment because of full study enrollment could be enrolled directly into STEPS-2. All patients received subcutaneous teduglutide 0.05 mg/kg/d. STEPS-3 (NCT01560403) was a 1-year open-label extension of STEPS-2; total treatment time for patients who received teduglutide in STEPS and completed STEPS-2 and STEPS-3 was 42 months.6 Full eligibility criteria for these studies have been published previously.3,5,7
All studies were conducted in compliance with International Conference on Harmonisation Good Clinical Practice guidelines and the World Medical Association Declaration of Helsinki and its amendments. Local institutional review boards or medical ethics committees approved all protocols, and all study patients provided informed consent. Study authors had access to all study data, and all approved the final manuscript.
Protocol for Parenteral Support Optimization and Adjustments
Before randomization in the placebo-controlled studies, fluid balance was optimized and stabilized for up to 16 weeks using a standardized protocol to ensure adequate hydration status and urine output (target: 1–2 L/d) across all patients and clinical sites.3,5 Baseline values for PS requirements were determined following completion of the optimization/stabilization phases for all patients and at the start of teduglutide treatment (at the time of randomization for patients who received teduglutide in study NCT00081458 or STEPS and at the time of enrollment into the extension studies for all other patients). In all studies, patients were managed according to an algorithm that permitted PS reductions of 10%–30% of baseline volume if clinical status was stable and 48-hour urine output had increased by ≥10% over baseline.3,5,7,8 PS reductions were made at 2- to 4-week intervals in the placebo-controlled trials; the weaning algorithm was less aggressive in the extension studies, where PS reductions were possible at 2- to 12-week intervals.
Protocol for Antibody Assessment
Details regarding the detection methods of potential antiteduglutide antibody formation were previously published.3,7 Briefly, formation of antibodies to teduglutide was assessed using the Meso Scale Discovery method, and the neutralizing antibody assay was a functional adenosine 3′,5′-cyclic monophosphate accumulation assay.
End Points and Statistical Analysis
This post hoc analysis of pooled data evaluated the percentage of patients who achieved independence from PS during treatment with teduglutide 0.05 mg/kg/d, the dose approved by the U.S. Food and Drug Administration and the European Medicines Agency.10,11 Treatment-emergent AEs (TEAEs) and treatment-emergent SAEs (TESAEs) for patients who achieved independence from PS were coded using the Medical Dictionary for Regulatory Activities. A χ2 test and Fisher exact test were used to analyze the frequency of PS independence in patients who maintained colon-in-continuity and those who did not. All other end-point analyses were performed using descriptive statistics. No additional between-group comparisons or correlations were performed in this patient population because of the nature of the extension studies and small patient numbers.
Results
In the combined phase III studies, 16 (12%) of 134 patients treated with teduglutide 0.05 mg/kg/d achieved complete independence from any PS during the study treatment period. These patients gained oral or enteral autonomy after a median of 5 years (range, 2–18 years) of PS dependence. Table 1 reports the baseline demographics, baseline disease characteristics, exposure to teduglutide at the time of PS reductions, and exposure to teduglutide at the time of PS independence. The baseline demographics and disease characteristics of the 16 patients (50% men) varied: age was 34–69 years (median, 55 years), baseline PS volume was 3.5–13.4 L/wk (median, 5.1 L/wk), and baseline PS infusion days per week was 3–6 days (median, 4 days). Remaining small intestine length ranged from 26–250 cm (median, 52.5 cm). Causes of resection were mesenteric vascular events (n = 8), injury (n = 2), Crohn’s disease (n = 2), complications of surgery (n = 2), ulcerative colitis (n = 1), and radiation enteritis (n = 1). Most patients who achieved PS independence had at least partial colon-in-continuity (12/16; 75%); however, 4 patients (25%) had no colon-in-continuity. Among the 134 patients, there was no significant difference in the frequency of PS independence among patients who maintained colon-in-continuity vs those who did not (P = 0.3 and P = 0.4, χ2 and Fisher exact test, respectively). Of the 4 patients with no colon-in-continuity who achieved PS independence, their estimated remnant small bowel length was approximately 250 cm (n = 2) or was unknown (n = 2), and their baseline PS volume was 3.5, 13.0, 6.3, and 8.9 L/wk, respectively.
Table 1.
Characteristics of Patients Who Achieved Independence From PS With Teduglutide.
| Age (y), Sex | Colon-in- Continuity | Remaining Small Intestine, cm | Duration of PS Dependency at Start of TED, y | PS Requirement at Start of TED, L/wk | PS Infusion Requirement at Start of TED, Days per Week | Weeks on TED at PS Weaning | Cause for Resection |
|---|---|---|---|---|---|---|---|
| 61, M | Yes | 80 | 6 | 3.5 | 3.5 | 12 | Mesenteric infarction |
| 54, M | Yes | 28 | 16 | 5.4 | 3 | 16 | Mesenteric venous thrombosis |
| 46, F | No | 250 | 1 | 3.5 | 3.5 | 28 | Crohn’s disease |
| 50, M | No | 250 | 1 | 13.0 | 6 | 32 | Ulcerative colitis |
| 66, M | Yes | 48 | 2 | 10.6 | 4.7 | 53 | Ischemic bowel |
| 39, M | Yes | 50 | 13 | 6.8 | 3 | 75 | Injury |
| 60, F | No | Unknown | 2 | 6.3 | 3.5 | 79 | Complications of surgery |
| 66, F | Yes | 80 | 7 | 4.4 | 3 | 89 | Radiation enteritis |
| 46, M | No | Unknown | 6 | 8.9 | 4 | 89 | Crohn’s disease |
| 34, M | Yes | 50 | 2 | 4.6 | 3 | 90 | Mesenteric artery thrombosis |
| 69, F | Yes | 120 | 5 | 3.5 | 3 | 101 | Mesenteric arterial infarction |
| 61, F | Yes | 76 | 1 | 4.1 | 4.5 | 101 | Strangulated SI |
| 63, M | Yes | 26 | 16 | 13.4 | 4.5 | 115 | Mesenteric thrombosis |
| 43, F | Yes | 40 | 2 | 4.5 | 5 | 126 | Small bowel infarction |
| 56, F | Yes | 50 | 2 | 9.4 | 6 | 127 | Complications of surgery |
| 41, F | Yes | 55 | 3 | 4.7 | 5.5 | 130 | Injury |
F, female; M, male; PS, parenteral support (parenteral nutrition and/or intravenous fluids); SI, small intestine; TED, teduglutide.
Most patients (12/16, 75%) had received ≥1 year of teduglutide therapy before PS could be eliminated (Figure 1). Oral or enteral autonomy was achieved after 12–130 weeks (median, 89 weeks) of teduglutide treatment; PS decreases were observed between 1 and 827 days after initiation of teduglutide. The change in body weight, body mass index (BMI), plasma citrulline (a biomarker of intestinal mucosa mass), serum albumin, and serum creatinine between baseline and end of teduglutide treatment is reported in Table 2. Median percentage change at end of teduglutide treatment was 0.3% (range, −16% to 12%) in body weight/BMI, 28.3% (range, −34% to 198%) in plasma citrulline, 2.3% (range, −8% to 27%) in serum albumin, and −6.1% (range, −25% to 50%) in serum creatinine.
Figure 1.
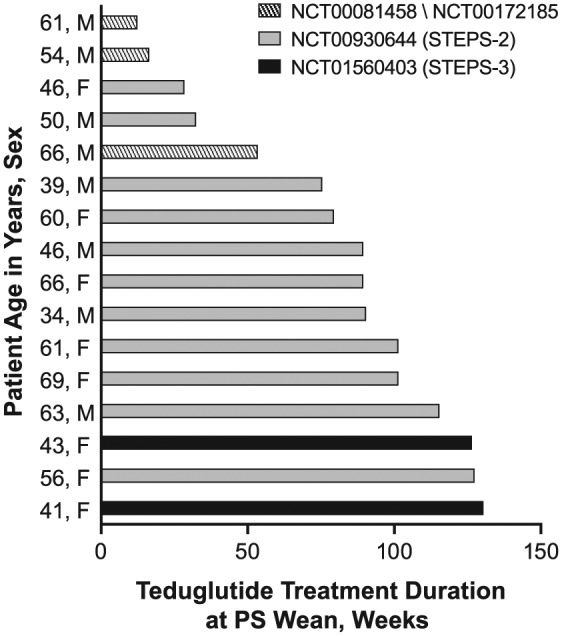
Duration of teduglutide treatment at time of PS independence. Bars represent the length of time in weeks that a patient was on teduglutide when independence from PS was achieved. Shading on the bars represents the clinical study treatment period that the patient was enrolled and receiving teduglutide when PS independence was obtained. PS, parenteral support (parenteral nutrition and/or intravenous fluids).
Table 2.
Nutrition and Clinical Characteristics of Patients at Baseline and Time of PS Independence.
| Patient |
Body Weight |
BMI |
Plasma Citrulline |
Serum Albumina |
Serum Creatinineb |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (y), Sex | BL, kg | EOT, kg | Change From BL, % | BL, kg/m2 | EOT, kg/m2 | Change From BL, % | BL, µmol/L | EOT, µmol/L | Change From BL, % | BL, g/L | EOT, g/L | Change From BL, % | BL, µmol/L | EOT, µmol/L | Change From BL, % |
| 61, M | 76.9 | 78.3 | 1.8 | 26.3 | 26.8 | 1.8 | 20.9 | 48.9 | 134.0 | 39 | 37 | −5.1 | 151 | 140 | −7.3 |
| 54, M | 67.2 | 66.4 | −1.3 | 21.9 | 21.7 | −1.2 | 27.9 | 29.1 | 4.3 | 45 | 43 | −4.4 | 106 | 80 | −24.5 |
| 46, F | 76.6 | 67.1 | −12.4 | 29.6 | 25.9 | −12.4 | 20.0 | 31.1 | 55.5 | 41 | 44 | 7.3 | 102 | 97 | −4.9 |
| 50, M | 74.8 | 79.3 | 6.0 | 26.5 | 28.1 | 6.0 | 17.0 | 16.7 | −1.8 | 41 | 45 | 9.8 | 106 | 159 | 50.0 |
| 66, M | 68.2 | 64.5 | −5.4 | 25.4 | 24.0 | −5.4 | NA | 25.0 | NA | 33 | 31 | −6.1 | 71 | 106 | 49.3 |
| 39, M | 63.8 | 67.0 | 5.0 | 22.6 | 23.7 | 5.0 | 12.0 | 15.4 | 28.3 | 47 | 47 | 0 | 74 | 83 | 12.2 |
| 60, F | 64.3 | 61.2 | −4.8 | 24.2 | 23.0 | −5.0 | 28.0 | 25.8 | −7.9 | 42 | 43 | 2.4 | 97 | 80 | −17.5 |
| 66, F | 48.8 | 46.0 | −5.7 | 22.0 | 20.7 | −5.7 | 18.0 | 43.1 | 139.4 | 42 | 49 | 16.7 | 72 | 78 | 8.3 |
| 46, M | 84.6 | 93.0 | 9.9 | 27.9 | 30.7 | 9.9 | 34.0 | 57.8 | 70 | 48 | 44 | −8.3 | 118 | 100 | −15.3 |
| 34, M | 67.5 | 75.3 | 11.6 | 24.5 | 27.3 | 11.6 | 20.0 | 14.2 | −29 | 45 | 44 | −2.2 | 66 | 65 | −1.5 |
| 69, F | 79.2 | 82.0 | 3.5 | 29.8 | 30.9 | 3.5 | 21.0 | 18.4 | −12.4 | 37 | 47 | 27.0 | 53 | 65 | 22.6 |
| 61, F | 51.0 | 42.7 | −16.3 | 22.0 | 18.4 | −16.4 | 20.0 | 40.4 | 102 | 38 | 35 | −7.9 | 88 | 80 | −9.1 |
| 63, M | 59.0 | 58.1 | −1.5 | 19.9 | 19.6 | −1.5 | 11.0 | 24.1 | 119.1 | 40 | 41 | 2.5 | 105 | 96 | −8.6 |
| 43, F | 87.9 | 97.7 | 11.1 | 30.4 | 33.8 | 11.2 | 13.0 | 16.6 | 27.7 | 32 | 39 | 21.9 | 88 | 88 | 0 |
| 56, F | 62.0 | 54.0 | −12.9 | 24.5 | 21.4 | −12.9 | 14.0 | 41.7 | 197.9 | 45 | 46 | 2.2 | 77 | 70 | −9.1 |
| 41, F | 55.2 | 56.9 | 3.1 | 20 | 20.6 | 3.0 | 28.0 | 18.4 | −34.3 | 40 | 42 | 5 | 71 | 62 | −12.7 |
BL, baseline; BMI, body mass index; EOT, end of treatment; F, female; M, male; NA, not available; PS, parenteral support (parenteral nutrition and/or intravenous fluids).
Reference range, 32–50 g/L.
Reference range, 40–119 µmol/L.
All patients who achieved independence from PS experienced >1 TEAE, the most common of which were abdominal pain (n = 9), nausea (n = 7), diarrhea (n = 5), nasopharyngitis (n = 5), pain in extremity (n = 4), stoma complications (swelling, protrusion, or increased intermittent flow, n = 3), and injection site complication (erythema, hematoma, or infection, n = 4). Central line infections occurred in 2 patients; none of the 16 patients were hospitalized. Nineteen TESAEs occurred in 13 patients. The only TESAEs reported in >1 patient were catheter-related infections (n = 4). TESAEs occurring in 1 patient each were acute cholecystitis, hemorrhoids, catheter sepsis, abdominal pain, superior vena cava stenosis, urinary tract infection, deep vein thrombosis, lactic acidosis, implant site extravasation, delusional disorder (unspecified type), pneumonia, hydronephrosis, nephrolithiasis, catheter-related complication, and gastroenteritis. Teduglutide-specific antibodies were detected in 3 of 16 (19%) patients; no neutralizing antibodies to teduglutide were detected.
Discussion
Teduglutide therapy (0.05 mg/kg/d) was associated with complete independence from PS for 16 of 134 (12%) treated patients with SBS-IF during their respective active treatment periods. All 16 patients in this post hoc analysis of pooled clinical study data achieved PS independence only after treatment with teduglutide; no patient in the placebo groups within the subset of randomized controlled studies was weaned of PS.5,8 Seventy-five percent of patients received at least 1 year of teduglutide therapy before PS could be eliminated, including 1 patient who weaned off PS after 2.5 years of treatment. In fact, the patient who achieved oral or enteral autonomy after 2.5 years (130 weeks) had the initial benefit in PS reductions begin after 118 weeks of teduglutide treatment. Considered in the context that PS volume reductions were associated with continued teduglutide treatment for up to 3.5 years in long-term clinical studies,6–8 the PS independence data also suggest that teduglutide is associated with PS benefits accrued over time; thus, regular monitoring as part of individualized patient management is recommended.
The adult patients with SBS who enrolled in the phase III teduglutide studies had a wide range of underlying disease characteristics, including varying remnant bowel anatomy. The prognostic factors previously associated with spontaneous adaptation, such as remnant bowel length or colon-in-continuity, did not clearly predict teduglutide-induced enteral autonomy in this study.12,13 The remaining small intestine length ranged from 26–250 cm. Although most patients who achieved independence from PS had colon-in-continuity (12/16), χ2 and Fisher exact tests did not show a significant association between the presence or absence of colon-in-continuity and PS weaning, perhaps because of small patient numbers.
The possible role of citrulline as a biomarker remains unclear. In this report, the percentage change from baseline in citrulline levels ranged from −34% to 198% in the 16 patients who achieved oral or enteral autonomy while on teduglutide therapy. Furthermore, no clear pattern emerges when the duration of teduglutide exposure before PS independence and the change in citrulline is examined in the individual patient.
Median body weight and BMI remained stable in patients who were weaned from PS during the study period (+0.3% change from baseline). Although 6 patients experienced weight loss >5% (range in weight variation for all patients, −16.3% to 11.6%), all patients in the study maintained a BMI >18.5 kg/m2. Based on median body weight, BMI, serum albumin, and creatinine levels, PS independence was achieved with a noncompromised hydration and nutrition status. Median serum albumin was steady during the study (+2.3% change from baseline). At end of treatment, only 1 patient had serum albumin below 35 g/L, the lower limit of normal; this individual began teduglutide treatment with low serum albumin. Median serum creatinine was normal in 14 of 16 patients who achieved oral or enteral autonomy while on teduglutide therapy (2 patients had high but not clinically significant levels). Collectively, median serum creatinine decreased during teduglutide treatment (−6.1% change from baseline).
The safety profile of teduglutide in patients who achieved independence from PS was generally consistent with the safety profile of the phase III clinical study populations as a whole.3,5–8 The most frequent TEAEs in this cohort of patients were GI symptoms, which are common among patients with SBS-IF.9 The only TESAEs that occurred in >1 patient were catheter-related infections, highlighting the need for careful catheter management in patients who are dependent on chronic PS. Patients in these studies achieved oral or enteral autonomy despite a relatively high incidence of TEAEs and TESAEs, suggesting that AEs can be managed to achieve optimal results with teduglutide.
This cohort of patients who obtained oral or enteral autonomy while receiving teduglutide all continued teduglutide treatment past the point of PS weaning until the end of the clinical study period. This pooled clinical trial post hoc analysis was unable to add to the limited available data regarding outcomes of patients who discontinue teduglutide. However, 3 patients in this cohort who participated in the NCT00081458 and NCT00172185 studies were included in the Compher et al14 post hoc analysis that reported that all remained independent of PS 12 months after withdrawal of 0.05 mg/kg/d teduglutide.7 Two of these patients had colon-in-continuity, and one had an end-jejunostomy. However, other evidence from the same analysis suggests that teduglutide discontinuation may be associated with a rebound in PS requirements. Twelve of 25 patients who responded to teduglutide with a ≥20% reduction in PS volume required increases in PS after stopping teduglutide.14 Compared with drug responders who maintained or decreased PS volume following teduglutide discontinuation, these 12 patients had shorter remnant small bowel (58 vs 35 cm, respectively) and were less likely to have colon-in-continuity (92% vs 57%), but neither observation reached statistical significance. In the current phase III extension study group as a whole, patients who received teduglutide 0.05 mg/kg/d required a mean increase in PS of 1.5 L/wk between end of treatment and the 4-week follow-up visit.7 These findings highlight the importance of individualized patient management with teduglutide and continued monitoring after PS weaning. Patients who obtain oral or enteral autonomy still have their underlying condition of SBS and will likely continue to require specialized diets, vitamin and other micronutrient supplementation, concomitant antidiarrheal and other medications, and fluid optimization after weaning.15
This study was limited by the small patient sample size, which precluded performance of regression analyses for predictive factors associated with PS independence. In addition, the studies pooled for this analysis included minor protocol differences that may have influenced rates of PS weaning. For example, the weaning algorithm in NCT00081458/NCT00172185 permitted a 10% maximum reduction from baseline in PS volume during a PS adjustment vs a 30% maximum reduction in the STEPS study and its extension.3,5,7,8 The intervals between study visits were also shorter in the RCTs compared with the extension studies (2–4 vs 2–12 weeks, respectively). Furthermore, the influence of spontaneous intestinal adaptation on PS weaning during the extension studies is currently unclear. Although most spontaneous intestinal adaptation is thought to occur during the first 2 years following small bowel resection, recent studies indicate that adaptation can advance over longer time periods.12,16 Finally, the patients described here were managed under strict clinical trial protocols and may not fully reflect the experiences of patients treated in general clinical practice. The ongoing teduglutide global registration study (ClinicalTrials.gov identifier: NCT01990040) will provide critical data regarding the efficacy and safety of teduglutide treatment under real-world conditions.
Together, these data indicate that oral or enteral autonomy is possible for some patients with SBS-IF who are treated with teduglutide, regardless of baseline characteristics and despite long-term dependence on PS. Studies involving larger patient populations are required to establish factors that may predict PS independence associated with teduglutide treatment. Oral or enteral autonomy in patients with SBS may be conditional, particularly during periods of metabolic stress and if teduglutide treatment is discontinued. Careful monitoring and individualized patient management can allow patients to obtain optimal results.
Acknowledgments
Editorial support was provided by Heather Heerssen, PhD, of Complete Healthcare Communications, LLC (Chadds Ford, Pennsylvania, USA) and was funded by NPS Pharmaceuticals, Inc, which is a wholly owned indirect subsidiary of Shire.
Footnotes
Financial disclosure:This research was funded by NPS Pharmaceuticals, Inc, Lexington, Massachusetts, USA. NPS Pharmaceuticals, Inc, is a wholly owned indirect subsidiary of Shire.
Conflicts of interest:KRI has served as a site investigator, advisory board member, and consultant for NPS Pharmaceuticals, Inc. MK and TRZ have served as site investigators for NPS Pharmaceuticals, Inc. JIB, SG, and U-FP have served as site investigators and advisory board members for NPS Pharmaceuticals, Inc. MNVC has served as a site investigator for NPS Pharmaceuticals, Inc, and an advisory board member for Shire. KF has served as a consultant, speaker, and study investigator for NPS Pharmaceuticals, Inc. FJ and SMS have served as speakers, study investigators, and advisory board members for NPS Pharmaceuticals, Inc. BL and NNY were employees of NPS Pharmaceuticals, Inc, at the time the studies were conducted. PBJ received research support and was a consultant, advisory board member, and study investigator for NPS Pharmaceuticals, Inc.
This article originally appeared online on November 23, 2016.

Download a QR code reader on your smartphone, scan this image, and listen to the podcast for this article instantly. Or listen to this and other JPEN podcasts at http://journals.sagepub.com/page/pen/podcasts.
Statement of Authorship: K. R. Iyer, M. Kunecki, J. I. Boullata, K. Fujioka, F. Joly, S. Gabe, U.-F. Pape, S. M. Schneider, M. N. V. Casas, T. R. Ziegler, and P. B. Jeppesen were study investigators; B. Li was the lead statistician for the analysis; and N. N. Youssef was the clinical lead for NPS Pharmaceuticals, Inc. The study design was approved by NPS Pharmaceuticals, Inc. K. R. Iyer, M. Kunecki, J. I. Boullata, K. Fujioka, F. Joly, S. Gabe, U.-F. Pape, S. M. Schneider, M. N. V. Casas, T. R. Ziegler, and P. B. Jeppesen contributed to the study concept and design, study supervision, and acquisition of data; and all authors participated in the analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. All authors had access to the study data, reviewed and approved the final manuscript, and agree to be fully accountable for ensuring the integrity and accuracy of the work.
References
- 1. Jeppesen PB. Spectrum of short bowel syndrome in adults: intestinal insufficiency to intestinal failure. JPEN J Parenter Enteral Nutr. 2014;38:8S-13S. [DOI] [PubMed] [Google Scholar]
- 2. Winkler MF, Smith CE. Clinical, social, and economic impacts of home parenteral nutrition dependence in short bowel syndrome. JPEN J Parenter Enteral Nutr. 2014;38:32S-37S. [DOI] [PubMed] [Google Scholar]
- 3. Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473-1481. [DOI] [PubMed] [Google Scholar]
- 4. Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O’Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iyer K, Fujioka K, Boullata JI, Ziegler TR, Youssef NN, Seidner D. Long-term safety and efficacy with teduglutide treatment in patients with intestinal failure associated with short bowel syndrome (SBS-IF): the STEPS-3 study. Clin Nutr. 2014;33:S167-S168. [DOI] [PubMed] [Google Scholar]
- 7. O’Keefe SJ, Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol. 2013;11:815-823. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz LK, O’Keefe SJ, Fujioka K, et al. Long-term teduglutide for the treatment of patients with intestinal failure associated with short bowel syndrome. Clin Transl Gastroenterol. 2016;7:e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalaitzakis E, Carlsson E, Josefsson A, Bosaeus I. Quality of life in short-bowel syndrome: impact of fatigue and gastrointestinal symptoms. Scand J Gastroenterol. 2008;43:1057-1065. [DOI] [PubMed] [Google Scholar]
- 10. GATTEX (teduglutide [rDNA origin]) [full prescribing information]. Lexington, MA: Shire-NPS Pharmaceuticals, Inc; 2016. [Google Scholar]
- 11. Revestive (teduglutide) [EMA summary of product characteristics]. Dublin, Ireland: NPS Pharma Holdings Limited; 2016. [Google Scholar]
- 12. Amiot A, Messing B, Corcos O, Panis Y, Joly F. Determinants of home parenteral nutrition dependence and survival of 268 patients with non-malignant short bowel syndrome. Clin Nutr. 2013;32:368-374. [DOI] [PubMed] [Google Scholar]
- 13. Carbonnel F, Cosnes J, Chevret S, et al. The role of anatomic factors in nutritional autonomy after extensive small bowel resection. JPEN J Parenter Enteral Nutr. 1996;20:275-280. [DOI] [PubMed] [Google Scholar]
- 14. Compher C, Gilroy R, Pertkiewicz M, et al. Maintenance of parenteral nutrition volume reduction, without weight loss, after stopping teduglutide in a subset of patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2011;35:603-609. [DOI] [PubMed] [Google Scholar]
- 15. Matarese LE. Nutrition and fluid optimization for patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2013;37:161-170. [DOI] [PubMed] [Google Scholar]
- 16. Tappenden KA. Intestinal adaptation following resection. JPEN J Parenter Enteral Nutr. 2014;38:23S-31S. [DOI] [PubMed] [Google Scholar]


