Abstract
Combustion of biomass produces solid carbon particles, whereas their generation is highly unlikely when a biomass is heated instead of being burnt. For instance, in the Tobacco Heating System (THS2.2), the tobacco is heated below 350°C and no combustion takes place. Consequently, at this relatively low temperature, released compounds should form an aerosol consisting of suspended liquid droplets via a homogeneous nucleation process. To verify this assumption, mainstream aerosol generated by the heat-not-burn product, THS2.2, was assessed in comparison with mainstream smoke produced from the 3R4F reference cigarette for which solid particles are likely present. For this purpose, a methodology was developed based on the use of a commercial Dekati thermodenuder operating at 300°C coupled with a two-stage impactor to trap solid particles. If any particles were collected, they were subsequently analyzed by a scanning electron microscope and an electron dispersive X-ray. The setup was first assessed using glycerine-based aerosol as a model system. The removal efficiency of glycerin was determined to be 86 ± 2% using a Trust Science Innovation (TSI) scanning mobility particle sizer, meaning that quantification of solid particles can be achieved as long as their fraction is larger than 14% in number. From experiments conducted using the 3R4F reference cigarette, the methodology showed that approximately 80% in number of the total particulate matter was neither evaporated nor removed by the thermodenuder. This 80% in number was attributed to the presence of solid particles and/or low volatile liquid droplets. The particles collected on the impactor were mainly carbon based. Oxygen, potassium, and chloride traces were also noted. In comparison, solid particles were not detected in the aerosol of THS2.2 after passing through the thermodenuder operated at 300°C. This result is consistent with the fact that no combustion process takes place in THS2.2 and no formation and subsequent transfer of solid carbon particles is expected to occur in the mainstream aerosol.
Keywords: Solid particles, method development, no combustion
Introduction
Combustible tobacco products such as cigarettes release smoke composed of gas vapor and particulate phases1–3 upon tobacco combustion at temperatures around 850°C during puffing.1,4 While combustion progresses along the tobacco column in the course of the smoking experience, the location behind the combustion zone named the pyrolysis/pyro synthesis/distillation zone reaches temperatures in the order of 600°C. The region behind, the pyrolysis/pyrosynthesis/distillation zone, is heated below 350°C where distillation processes are kinetically favorable in comparison to pyrolysis. As a consequence, a limited number/quantity of harmful constituents is expected to form in this region. Based on physicochemical considerations, it is generally recognized5 that organic materials generate ultrafine organic particles in case of incomplete combustion or pyrolysis.6,7 Consequently, solid ultrafine particles are expected to be released in the mainstream cigarette smoke but not necessarily transferred completely to the consumer because of the smoke filtration process taking place in the tobacco column.8,9 In THS2.2, the tobacco is heated below 350°C instead of being burnt. At this relatively low temperature, only distillation processes can occur and no combustion will take place. Consequently, the release and transfer of solid carbon particles in the mainstream aerosol of THS2.2 are not expected.
The main objective of the current work was to compare 3R4F and THS2.2 solid particle number and chemical composition in their respective mainstream smoke and aerosol. The solid particle term refers to a possible combination of solid particulate matter and high boiling point droplets. Throughout the text, the term “solid particles” will be used.
Equipment and methodology
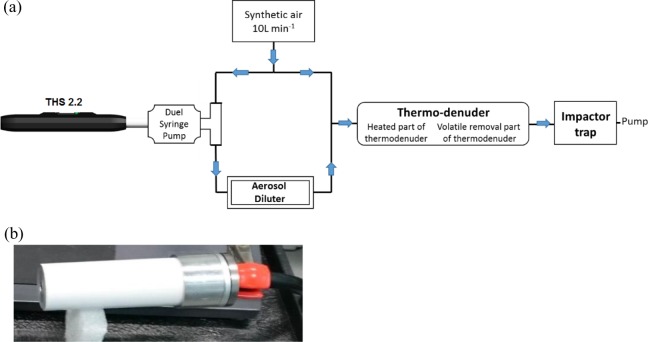
A test item (3R4F or a tobacco stick) was inserted in a cigarette holder connected to the inlet of a programmable dual syringe pump, while its outlet was connected to a T-junction positioned upstream to a TSI dilution unit 3302A shown in Figure 1(a). This dilution was set to fractionate the puff-by-puff mainstream aerosol by a factor of 100 for THS2.2 and 10,000 for 3R4F to avoid sensor and thermodenuder saturation. An additional dilution factor of 100 was used when testing 3R4F because smoke has a higher amount of particulate matter per cubic centimeter than aerosol generated from THS2.2. Prior to starting aerosol generation under Health Canada regimen, a constant pure synthetic air gas flow rate of 10 L min−1 was introduced to supply two gas transport lines (see Figure 1(a)). The first line was used to dilute the puff-by-puff mainstream aerosol with 5 L min−1, while the second line was carrying a flow of 5 L min−1 that was recombined with the diluted mainstream aerosol positioned downstream to the TSI dilution unit. This step was necessary to feed the diluter with the required flow rate of 5 L min−1 and to ensure a flow rate of 10 L min−1 positioned upstream to the thermodenuder to meet its optimal operating conditions. At the outlet of the thermodenuder, the efficiency of the setup was evaluated using model aerosols and a TSI scanning mobility particle sizer. Subsequently, a TSI condensation particles counter (CPC) was operated for the potential particle detection from 3R4F and THS2.2. Finally, a two-stage impactor trap was used to deposit the aerosols on a collection substrate (see Figure 1(b))10 for further scanning electron microscope (SEM)/energy dispersive X-ray (EDX) analyses.
Figure 1.
(a) Experiment design: mainstream aerosol collection setup. (b) Impactor trap.
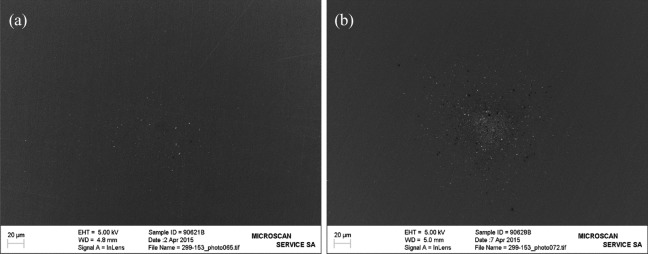
When the aerosol reaches the entrance of the thermodenuder maintained at 300°C, it is transported to a second section consisting of a gas stripper used to remove the gas vapor phase from evaporated droplets. It has been demonstrated that glycerin droplets were removed at a yield of 86% considering that the 100% was obtained when the thermodenuder temperature was set at 30°C. Additionally, the temperature-dependent aerosol losses resulting from thermal diffusion were determined using sodium chloride crystals when the thermodenuder temperature was set at 300°C. In fact, a quantification could be achieved when the removal yield of the aerosol number was comprised between 80% and 14% (considering together wall losses and the removal efficiency of droplets). Prior to starting aerosol generation experiments, it was necessary to ensure that any solid particles detected or deposited on the impactor trap would be from the test items. For that reason, blank experiments were also carried out in collection impactor traps. One blank experiment was performed prior to starting aerosol generation to verify whether the experiment setup was clean and did not release solid particles from the system. A second blank experiment was conducted at the end of the aerosol generation experiments to demonstrate that the thermodenuder did not reemit particles from the gas stripper. In all cases, the blank experiments showed very few accumulated solid particles. This is shown in Figure 2 where sparse solid particles were observed. From these results, the blanks were considered clean enough to conduct the investigations on 3R4F mainstream smoke and the THS2.2 mainstream aerosol.
Figure 2.
The pictures correspond to the resulting submicron solid particles collection (a) prior to the start of aerosol generation and (b) right after the 3R4F collection were completed (blank experiments).
Based on the determined thermodenuder removal efficiency and wall losses, the smoke from 3R4F cigarettes and the aerosol from THS2.2 were tested to verify whether solid particles were present in their respective mainstream. On a test item basis, the smoke and the aerosol were generated in separate experiments using two test items of the same type.
Results and discussions
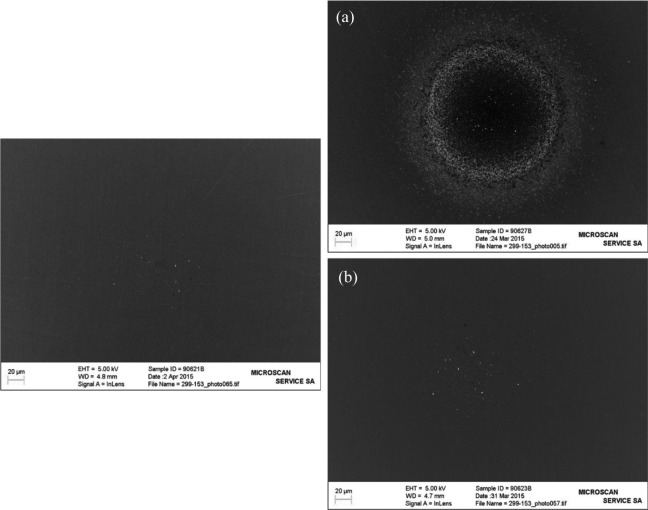
It was estimated that approximately 1012 solid particles were generated in the mainstream smoke of 3R4F over 11 puffs. In the light of this result, the CPC was replaced by an impactor trap in view of performing particle trapping and image analyses. Two 3R4F test items were smoked to accumulate in total 22 puffs in a given impactor trap to ensure a sufficient amount of material could be accumulated. The impactor trap was subsequently disassembled and the impactor substrates analyzed with an SEM/EDX. The same experimental approach was used for THS2.2 mainstream aerosol. However, the use of the CPC revealed an aerosol concentration ratio smaller than 14%, which did not allow detection and thus subsequent quantification of solid particles. As in the case of 3R4F, the THS2.2 thermodenuder-treated mainstream aerosol was collected on an impaction trap and analyzed by SEM/EDX. Figure 3(a) and (b) shows pictures obtained of the impactor trap substrates related to 3R4F and THS2.2 experiments. Figure 3(b) clearly shows that no solid particles were accumulated from the mainstream aerosol of THS2.2 (right pictures) in comparison to the blank (left picture). This shows that no additional solid particles were released and transferred in the mainstream aerosol of THS2.2 when compared to the blank.
Figure 3.
Pictures showing accumulated submicron particles from the mainstream of (a) 3R4F and (b) THS2.2. The picture on the left side is the related blank prior aerosol collection started.
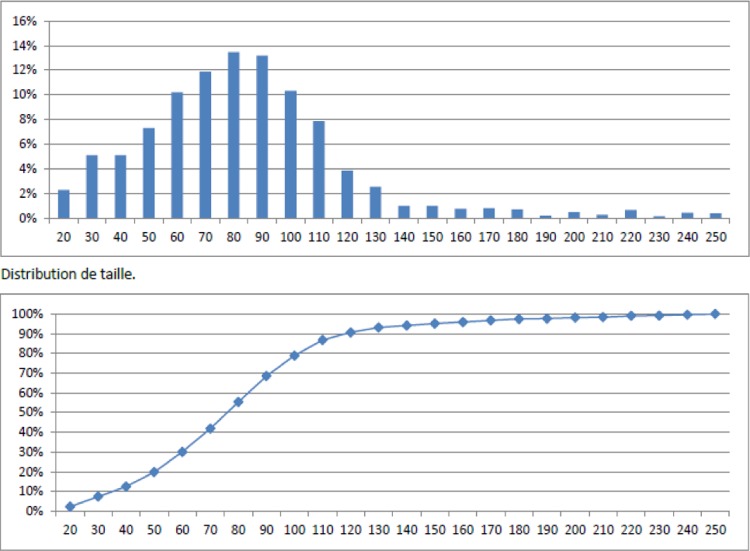
From Figure 3(a), it was observed that a statistically significant greater number of solid particles were collected from the mainstream smoke of 3R4F (right picture) in comparison to the blank (left picture). This result is in line with the measurements obtained from the coupling of the thermodenuder and the CPC revealing that approximately 1012 solid particles per 3R4F test item were quantified in the mainstream smoke. Because of the very high number of solid particles accumulated on the impactor substrate (Figure 3(a), right picture), an automatic counting/sizing of the deposited particulate matter was performed. The resulting size distribution is displayed in Figure 4. The size distribution (upper insert in Figure 4) and the cumulative distribution (lower insert in Figure 4) revealed that the count median diameter (CMD) was approximately 75 nm. This size corresponds to the definition of ultrafine particles,11 typically found in combustion/pyrolysis processes occurring for instance in candle burning,12 biomass fuel combustion13 or during cooking activities such as grilling bacon.14 It has to be noted that the presence of solid organic particles in the mainstream smoke of 3R4F is an indication that pyrolysis/combustion took place while their absence in THS2.2 mainstream aerosol indicates that no combustion occurred.6,15,16
Figure 4.
Size distribution of deposited solid particles from 3R4F mainstream using the SEM. The upward insert represents the solid particle size distribution whereas the downward insert the cumulative size distribution. The x-axis represents the particles size in nanometers. SEM: scanning electron microscope.
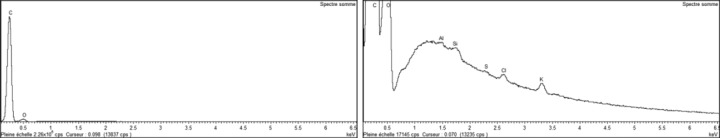
Owing to the significant number of solid particles found in the mainstream smoke of 3R4F, it was not possible to determine their individual elemental composition. Consequently, an average elemental composition was determined. The spectrum in Figure 5 shows that the elemental composition of the deposited solid particles from 3R4F mainstream smoke consisted mainly of carbon-based material with oxygen. In addition, potassium and chlorine were found. Also, to a lesser extent, traces of aluminum, sulfur, and silicon were detected, likely released from the experimental setup.
Figure 5.
X-rays spectra for deposited solid particles from 3R4F mainstream.
Conclusions
The methodology described here allows the detection of solid particulate matter and droplets containing high boiling point species if they are present. In agreement with the results obtained using the CPC, SEM analysis showed that no solid particles were observed in the mainstream aerosol of THS2.2. As a comparison, 3R4F cigarettes were used to represent combustible tobacco products. Because combustion takes place during smoking of 3R4F, the release and transfer of solid particles in the mainstream smoke are likely to take place. This hypothesis was tested using the thermodenuder/CPC and subsequently the thermodenuder/SEM. Both techniques showed a significant amount of solid particles to the order of magnitude of 1012 per 3R4F test item and the related CMD was determined to be smaller than 100 nm. The observed ultrafine size distribution is generally consistent with combustion processes of organic materials such as candle burning or cooking activities. The X-ray analyses also showed that the resulting solid particles were composed mainly of carbon-based material and oxygen. Furthermore, traces of potassium and chlorine were also found. This is consistent with the work from Fariss et al.17 who observed amorphous carbon particulate matter, from cigarettes, characterized by a median size diameter smaller than 100 nm, part of the ultrafine fraction.
Based on the current findings, it was concluded that heated tobacco products neither generate nor transfer solid particles in the mainstream aerosol when considering our experimental conditions. The absence of carbon-based solid particle can be explained by the absence of combustion/pyrolysis in THS2.2. The strict control of heating power, by design, delivered to the product does not allow the tobacco to reach temperatures where combustion can occur.
Acknowledgements
We would like to thank Microscan Service SA (Rue de la Blancherie 17, 1022 Chavannes-près-Renens, Suisse) to have performed the SEM/EDX analyses and provided scientific expertise and SEM pictures.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All authors were Philip Morris Products S.A employees at the time of the studies that were funded by Philip Morris Products S.A.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Davies CN. Cigarette smoke: generation and properties of the aerosol. J Aerosol Sci 1988; 4: 463–469. [Google Scholar]
- 2. Fuoco FC, Buonanno G, Stabile L, et al. Influential parameters on particle concentration and size distribution in the mainstream of e-cigarettes. Eviron Pollut 2014; 184: 523–529. [DOI] [PubMed] [Google Scholar]
- 3. Bernstein DM. A review of the influence of particle size, puff volume,and inhalation pattern on the deposition of cigarette smoke particles in the respiratory tract. Inhal Toxicol 2004; 16: 675–689. [DOI] [PubMed] [Google Scholar]
- 4. Baker RR. Smoke generation inside a burning cigarette: modifying combustion to develop cigarettes that may be less hazardous to health. Prog Energy Combust Sci 2006; 32: 373–385. [Google Scholar]
- 5. Butler KM, Mulholland GW. Generation and transport of smoke components. Fire Technol 2004; 40: 49–176. [Google Scholar]
- 6. Oha KC, Leea CB, Leeb EJ. Characteristics of soot particles formed by diesel pyrolysis. J Anal Appl Pyrol 2011; 92: 456–462. [Google Scholar]
- 7. Hildemann LM, Markowski GR, Jones MC, et al. Submicrometer aerosol mass distributions of emissions from boilers, fireplaces, automobiles, diesel trucks, and meat-cooking operations. Aerosol Sci Technol 1991; 14: 138–152. [Google Scholar]
- 8. Ishizu Y, Ohta K, Okada T. Changes in the particle size and the concentration of cigarette smoke through the column of a cigarette. J Aerosol Sci 1977; 9: 25–29. [Google Scholar]
- 9. Song CB, Park HS. Analytic solutions for filtration of polydisperse aerosols in fibrous filter. Powder Technol 2006; 170: 64–70. [Google Scholar]
- 10. Jalanti T, Henchoz P. Analytical scanning electron microscopy: a most important aid for solving microcontamination problems. Swiss Contam Control 1990; 3(4a): 428–432. [Google Scholar]
- 11. Hinds WC. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. 2nd Edition Wiley. [Google Scholar]
- 12. Wright MD, Fews AP, Keitch PA, et al. Small-ion and nano-aerosol production during candle burning: size distribution and concentration profile with time. Aerosol Sci Technol 2007; 41: 475–484. [Google Scholar]
- 13. Hata M, Chomanee J, Thongyen T, et al. Characteristics of nanoparticles emitted from burning biomass fuels. J Environ Sci 2014; 26: 1913–1920. [DOI] [PubMed] [Google Scholar]
- 14. Buonanno G, Stabile L, Morawska L. Particle emission factors during cooking activities. Atmos Environ 2009; 43(20): 3235–3242. [Google Scholar]
- 15. Shurupov SV. Some factors that govern particulate carbon formation during pyrolysis of hydrocarbon. Proc Combust Inst 2000; 28: 2507–2514. [Google Scholar]
- 16. Butller KM, Mulholland GW. Generation and transport of smoke components. Fire Technol 2004; 40: 149–176. [Google Scholar]
- 17. Fariss MW, Gilmour MI, Reilly CA, et al. Emerging mechanistic targets in lung injury induced by combustion-generated particles. Toxicol Sci 2013; 132(2): 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]