Abstract
Background
We recently demonstrated that brain natriuretic peptide is expressed in the dorsal root ganglia, and that brain natriuretic peptide is required for normal detection of pruritogens. We further showed that the receptor for brain natriuretic peptide, natriuretic peptide receptor A, is present in the spinal cord, and elimination of these neurons profoundly attenuates scratching to itch-inducing compounds. However, the potential modulatory roles of brain natriuretic peptide in nociception, inflammation, and neuropathic mechanisms underlying the sensation of pain have not been investigated in detail.
Findings
To demonstrate the involvement of brain natriuretic peptide in pain, we compared the behavioral responses of brain natriuretic peptide knockout mice with their wild-type littermates. First, we showed that brain natriuretic peptide is not required in chemically induced pain responses evoked by the administration of capsaicin, allyl isothiocyanate, adenosine 5′-triphosphate, or inflammatory soup. We further measured pain behaviors and found no involvement of brain natriuretic peptide in hot, cold, or mechanical nociceptive responses in mice, nor did we find evidence for the involvement of brain natriuretic peptide in neuroinflammatory sensitization elicited by complete Freund’s adjuvant or in neuropathic pain.
Conclusions
These results demonstrate that brain natriuretic peptide is not essential for pain-related behaviors.
Keywords: Pain, gastrin-releasing peptide, brain natriuretic peptide, natriuretic peptide receptor A, inflammation, neuropathic
Introduction
Since the discovery of distinct sets of neurons responsible for itch and pain,1 numerous studies have been carried out to define and characterize the neurotransmitters responsible for itch and pain. Recent advances in the itch field have identified at least two major neurotransmitters responsible for the perception of itch, gastrin-releasing peptide (GRP) and brain natriuretic peptide (BNP).2–4 The expression pattern and location of these neurotransmitters within the neural itch circuitry have been the source of contention in recent years.5–7 Recent studies have implicated BNP as the primary neurotransmitter responsible for itch. BNP, a product of the gene Nppb, is a small peptide hormone that acts on the natriuretic peptide receptor A (NPRA) within the dorsal horn of the spinal cord.2 Studies have reported a loss of scratching response in mice lacking NPRA-expressing interneurons in the spinal cord under conditions that would normally induce itch, such as exposure to common pruritogens like histamine and chloroquine.2 On the contrary, some studies suggest that GRP is the primary neurotransmitter employed by itch sensory neurons and therefore argue against the BNP-NPRA pathway.6,7
Although many studies have investigated the role of BNP as a primary neurotransmitter involved in itch sensation, very few have been devoted to resolving the potential involvement of BNP in pain sensation. In recent years, some studies have presented evidence reporting the involvement of the BNP-NPRA pathway in the modulation of nociceptive signaling.8–10 These studies concluded an involvement of BNP in acute and chronic pain and suggested that BNP acts as a pain reliever and is a prime target for investigating the pathophysiology of chronic pain. These findings prompted us to investigate the role of BNP under acute, inflammatory, and neuropathic pain conditions.
In this study, we investigated whether BNP plays a role in mediating pain response. We carried out a detailed study of various types of nociceptive pain behaviors in wild-type and global BNP knockout (BNP-KO) mice. We demonstrated that BNP lacks functional involvement in mediating chemically induced, acute, inflammatory, and neuropathic pain responses. Our data strongly support the notion that BNP is not a neuropeptide/neurotransmitter involved in pain sensation and further supports our initial finding that BNP is specifically involved in itch sensation.
Materials and methods
Mice
Global BNP-KO mice were generated as previously described2 and maintained on the inbred strain with identical strain-matched wild-type littermates. Ten- to 12-week-old male mice were used for all experiments. All procedures were performed in accordance with North Carolina State University laboratory animal care.
Chemical sensitivity
Mice were habituated to the testing environment and subsequent behavior recorded by video camera for chemically induced pain caused by capsaicin or allyl isothiocyanate (AITC). Capsaicin- and AITC-induced eye wipes were counted for 1 min after delivery of 50 μl of solution (100 μM) capsaicin or 10 mM AITC in pharmaceutical grade phosphate buffer saline (PBS). These concentrations were chosen based on previous studies.11–13
Thermal responses
A Hargreaves’ test apparatus (Ugo Basile) was used to measure thermal responses to radiant heat. Mice were placed in individual chambers for 10 min for habituation. A radiant heat source was focused on the paws. A maximum cutoff of 20 s was used to prevent the injury. A single hot plate (Bioseb, France) set at 50℃ or 55℃ and dry ice were used to assess the acute temperature sensitivity.14 Each animal was tested at least twice to address the variability in responses.
Mechanical responses
Threshold-force-induced paw withdrawal was recorded using a von Frey apparatus (Ugo Basile). Mice were kept in the testing chambers 10 min before commencing the experiment for habituation. A single metal filament was attached in place onto the force transducer located beneath the animal holding platform. The cutoff force was set at 50 g. Minimum four measurements were taken from the paws for each animal, and the average force at which paw withdrawal was observed was calculated accordingly. This was done to address variability in the recordings.
Algesic substances
Paw withdrawal, flinching, guarding, and lick responses were measured for 10 min after intraplantar injection of 10 µl of adenosine 5′-triphosphate (ATP; 1 nmol) or inflammatory soup (serotonin, histamine, prostaglandin E2, and bradykinin; 1 µmol each). PBS was used as a vehicle.
Complete Freund’s adjuvant-induced inflammation
Sterile complete Freund’s adjuvant (CFA; 10 µl) was injected into the plantar surface of the hind paw to induce inflammation. The site of injection and the surrounding area was observed 24 h post injection to visually confirm inflammation. Mice were then tested for thermal, cold, and mechanical pain sensitivity. As a control, PBS (10 µl) was injected into the plantar surface, and mice were then subjected to the same behavioral testing.13
Sciatic nerve ligation
Mice were anesthetized with Avertin (2,2,2-Tribromoethanol; 0.6 mg/g intraperitoneal). Body temperature was maintained between 35℃ and 37℃. The sciatic nerve was accessed through an incision on the lateral side of the right thigh. The biceps femoris was cut to expose the nerve. A partial nerve ligation was made by tying a tight ligature around approximately one-half of the diameter of the right sciatic nerve. The incision was then closed using sutures, and mice were allowed to recover fully from the anesthesia before putting them back in their cages. Mice were then tested for up to 14 days for mechanical pain sensitivity using von Frey apparatus. Baseline and presurgery testing was carried out for up to 14 days prior to surgery. No pain suppressants were administered to mice postsurgery to avoid any influence on pain behavior.15 Investigators were blinded during all behavioral analyses.
In situ hybridization on tissue sections
Preparation and pretreatment of tissue sections
Mice were decapitated and dorsal root ganglia (DRG) were removed quickly by dissection and frozen in Tissue Tek embedding medium (Miles, Elkhart, IN). Sections of 14 µm thickness were cut on a cryostat and mounted on silanized slides. Sections were then fixed using freshly prepared 4% paraformaldehyde solution (in PBS, pH 7.4) for 15 min. Slides were then washed thrice with PBS for 5 min each.
Prehybridization and hybridization treatments
Prehybridization was performed at room temperature with 200 µl hybridization buffer as in a chamber humidified with 5× saline-sodium citrate from 6 h to overnight. The hybridization mixture was prepared by adding 200 ng DIG-cRNA per ml hybridization buffer, then heated for 5 min at 85℃ to denature the probe and chilled on ice. The prehybridization solution was removed and 50 to 100 µl of hybridization mixture was spread over the sections with parafilm. The sections were covered with siliconized coverslips. In situ hybridization was carried out using digoxigenin labeled c-RNA probes. For detection, alkaline-phosphatase-conjugated antibodies were used, and manufacturer’s (Boheringer Mannheim and Amersham) protocols were followed.17–19
Primers for NPRA
5′-GGCGCGCCACCATGCCGGGTTCCCGACGC-3′
5′-GCTGACCAGAGAGAGGCTGCCCACCAAAGC-3′
Primers for Nppb in situ hybridization
5′-GGCGCGCCACCATGCCGGGTTCCCGACGC-3′
5′-GCTGACCAGAGAGAGGCTGCCCACCAAAGC-3′
Data analysis
Student’s t test was used to compare behavioral data between wild-type and BNP-KO mice. Data are presented as mean ± SEM, and p < 0.05 was considered significant.
Results
Several reports have suggested that BNP is involved in pain sensation via negative feedback mediated by activation of NPRA-expressing DRG sensory neurons.7–9 To examine the role of BNP in pain sensation, we used age-matched wild-type littermates and global BNP-KO mice to perform various nociceptive mouse behavior experiments.
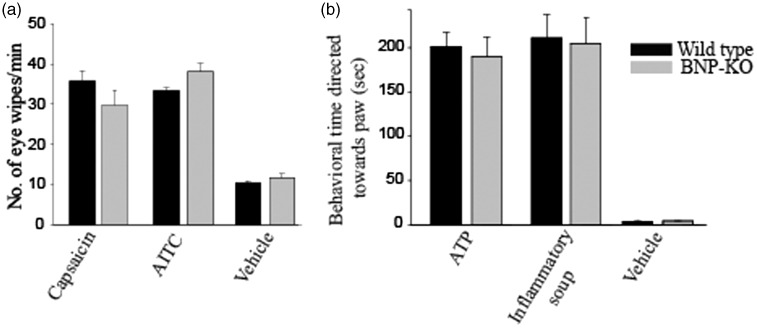
Nocifensive responses to algesic compounds were measured in mice. We first assessed the eye wipe response to both capsaicin and AITC, agonists of TRPV1- and TRPA1-receptors, respectively. Both are known to evoke nocifensive behavior in mice.2 Our data showed robust eye wipe responses to capsaicin and AITC in both wild-type and BNP-KO mice, confirming that BNP is not required for these acute behavioral responses (Figure 1(a)).
Figure 1.
BNP-KO mice do not exhibit any significant change upon exposure to chemical algesics compared to wild type. (a) 100 µM capsaicin and (b) 10 mM of AITC were applied directly onto the surface of the eyes in both wild-type (black bars) and BNP-KO mice (gray bars), and eye-wiping behavior was observed. Chemical algesia was introduced by (c) intraplantar injection of 1 nmol of ATP or (d) a mixture of compounds referred to as “inflammatory soup” (see Materials and Methods section). PBS was used as a vehicle control. No significant decrease in algesic response was observed in BNP-KO mice compared to wild-type control. Data are mean ± SEM, n ≥ 5 animals; Student’s t test was used to measure significant difference between genotypes. AITC: allyl isothiocyanate; ATP: adenosine 5′-triphosphate; BNP-KO: brain natriuretic peptide knockout.
ATP is an energy producer and modulator of cellular function throughout the body. ATP has been reported to produce fast excitatory potentials via purinergic receptor P2X3-expressing sensory neurons in the DRG.20 Intraplantar injection of ATP produced similar hind paw lifting and flinching behavior in both wild-type and BNP-KO mice (Figure 1(b)).
Tissue damage often results in nociceptive responses due to the release of chemicals into the area around the nociceptors. A mixture of compounds, referred to as the inflammatory soup, provides an acidic environment. Components of the inflammatory soup stimulate and sensitize sensory neurons, producing a pain response. We injected inflammatory soup into wild-type and BNP-KO mice and observed no significant differences in nociceptive behavioral response between genotypes (Figure 1(b)). No significant differences were found in wild-type and BNP-KO mice with PBS, which was used as a vehicle control (Figure 1(b)).
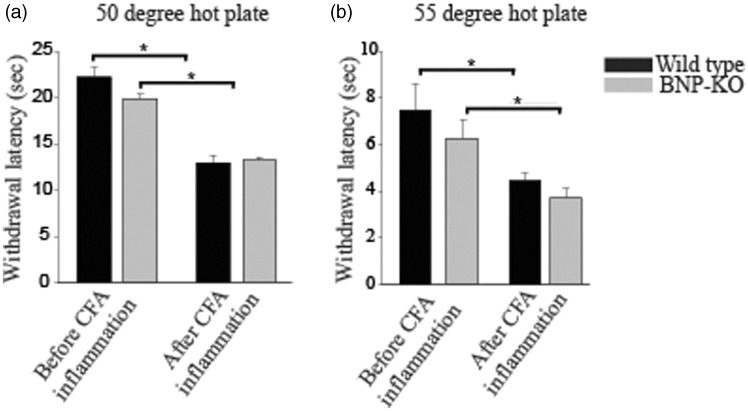
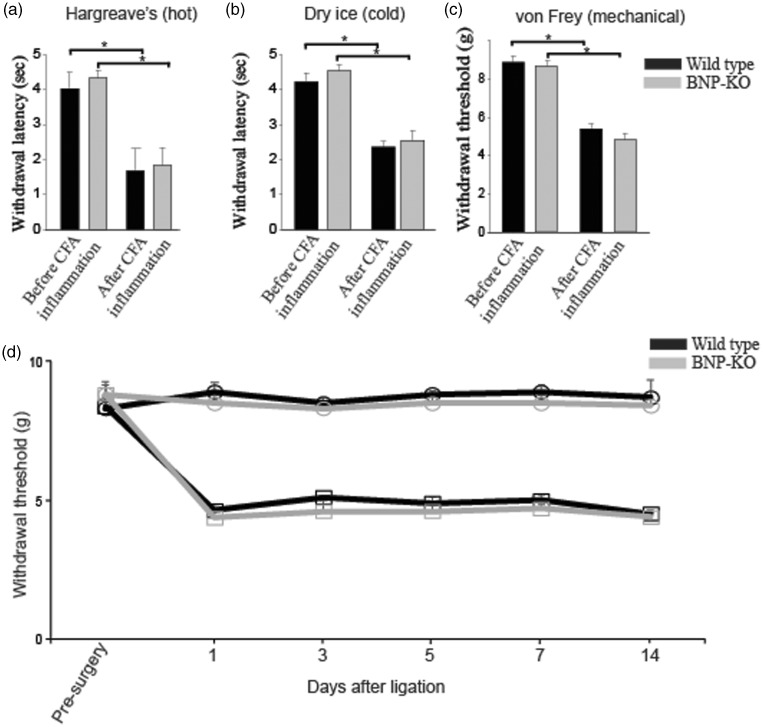
To demonstrate whether BNP-KO mice exhibit phenotypic differences in inflammation-induced pain response, we injected CFA, a well-known inflammatory agent, in both wild-type and BNP-KO mice. Once the inflammatory response to intraplantar CFA injection was confirmed visually (swollen and inflamed paws), after approximately 24 h, both wild-type and BNP-KO mice were subjected to hot (hot plate and Hargreaves Assay), cold, and mechanical pain sensitivity assays. As expected, we found that wild-type mice were hypersensitive to hot plate, Hargreaves, cold, and mechanical sensation 24 h post CFA application. Our BNP-KO mice also demonstrated the same hypersensitivity toward pain as seen in their wild-type counterparts post-CFA treatment. Although we saw a significant decrease in the withdrawal latency between pre- and post-CFA-induced inflammation conditions in both wild-type and BNP-KO mice, no significant difference in response was observed between the genotypes in either set of conditions. The hot plate assay was used to assess sensitivity to heat. We used temperature settings of 50℃ and 55℃ for both acute and CFA-induced inflammatory pain testing. We did not find any difference between genotypes for the conditions we tested (Figure 2(a) and (b)). The Hargreaves assay uses an intense beam of infrared light directed at hind paws to induce pain and to assess acute thermal sensitivity. Both wild-type and BNP-KO mice showed no significant difference in withdrawal latency in naive condition (before CFA inflammation) nor after CFA-induced inflammation (Figure 3(a)). The plantar cold test14 was used to assess acute sensitivity to cold in naive condition and after CFA-induced inflammation. As shown in Figure 3(b), the wild-type and BNP-KO mice demonstrated no difference in responses. Finally, we measured mechanical response by using automated von Frey and found no significant differences in mechanical pain sensitivity between BNP-KO and the wild-type littermate controls (Figure 3(c)). Additionally, we measured mechanosensory response after sensitization by sciatic nerve ligation in wild-type and BNP-KO mice. We found mechanical hypersensitization in both wild-type and BNP-KO mice following sciatic nerve ligation but observed no difference in mechanosensory response between genotypes in either pre- or postligation conditions (Figure 3(d)).
Figure 2.
BNP-KO mice retain sensitivity to thermal pain. Wild-type control and BNP-KO mice were subjected to thermal pain using hot plates set at (a) 50℃ and (b) 55℃ before and after intraplantar injection of 10 µl of CFA. No change in thermal pain sensitivity was observed when BNP-KO mice were compared to wild-type control mice. Data are mean ± SEM; n ≥ 5 animals; *p ≤ 0.01 was considered significant (Student’s t test). BNP-KO: brain natriuretic peptide knockout; CFA: complete Freund's adjuvant.
Figure 3.
BNP-KO mice exhibit normal cold, mechanical, thermal, and neuropathic pain responses. (a) Control and BNP-KO mice were exposed to acute cold using dry ice, (b) mechanical pain using von Frey test, (c) acute thermal pain using Hargreaves’ test, and (d) neuropathic pain induced by sciatic nerve ligation. Subsequent behavior analyses revealed no significant change in BNP-KO mice when compared to wild-type control. Data are mean ± SEM; n ≥ 5 animals; *p ≤ 0.01 was considered significant (Student’s t test). BNP-KO: brain natriuretic peptide knockout; CFA: complete Freund's adjuvant.
It was previously suggested that the NPRA receptor is expressed in the DRG and trigeminal ganglia.7–9 We used in situ hybridization, a sensitive and specific assay for gene expression, to determine whether NPRA is found in sensory neurons. We made both antisense and sense probes. Although we could not detect NPRA receptor expression in the DRG, we did find expression of NPRA receptor in the dorsal layer of the spinal cord. We used kidney as a positive control to detect the expression of NPRA receptor (Figure 4).
Figure 4.
Distribution and arrangement of BNP-expressing neurons ((a) and (b)), NPRA receptor ((c) and (d)) in sections of dorsal root ganglion and dorsal horn of spinal cord respectively were analyzed by in situ hybridization. DRG sections show high expression of BNP neurons and almost no NPRA expression while dorsal horn of the spinal cord shows very high expression of NPRA and no BNP expression. Kidney sections with sense probe (e) and with NPRA antisense probe ((f) and (g)) are included as a positive control for NPRA expression. Scale bar = 100 µm. BNP: brain natriuretic peptide; NPRA: natriuretic peptide receptor A.
Discussion
In our previous study,2 we identified BNP, encoded by the gene Nppb, as one of the key neurotransmitters involved in the transmission of itch from the periphery to the spinal cord. Based on the findings of this study, we proposed a model where BNP is released by primary afferent neurons in the DRG in response to pruritogens binding to its receptor, NPRA, which is expressed in the dorsal horn of the spinal cord. Activation of NPRA-expressing interneurons, in turn, leads to the release of GRP activating its receptor, gastrin-releasing peptide receptor. Though some studies have indicated GRP to be the primary neurotransmitter6,7 in both the periphery and the spinal cord, recent studies have proven the involvement of BNP in itch at the periphery.3,5,21 In addition to being involved in the propagation of itch, some studies suggest that BNP partakes in pain modulation and transmission.8,9
To investigate this, we carried out a detailed study using BNP-KO mice. Capsaicin, a known natural ligand of TRPV1, and AITC, a known ligand of TRPA1, have been used to study acute pain in rodents.11,22,23 Studies have shown that activation of TRPA1 leads to the release of calcitonin gene-related peptide (CGRP), a known neurotransmitter involved in pain.22 We used these algogens in wild-type and BNP-KO mice but failed to see any significant difference between the genotypes in terms of acute pain behavior during eye wipe assays. Although our previous study2 showed a percentage of BNP neurons expressing known pain-producing neurotransmitters—CGRP and neuromedin B—we failed to see any effect on the nociceptive behavior of BNP-KO mice. We attribute this to corneal application of capsaicin and AITC sufficient to produce a peripheral response but insufficient to cause central sensitization that would lead to the release of neurotransmitters. Furthermore, the expression of CGRP in many different neurons in the DRG, and its role in neuroinflammation, is not clear. Further studies are required to probe the role of CGRP release in pain. Studies have shown that ATP, bradykinin, serotonin, prostaglandins, and histamine produce pain when injected into tissue.15,24 Although the receptors targeted by these algogens have been identified, the neuronal populations involved have not been established. To see if BNP-expressing neurons play any role in chemically induced pain, we injected a mixture of these compounds, called inflammatory soup, into the plantar surface of wild-type and BNP-KO mice. We did not see any change in nociceptive behavior between both genotypes.
Neurogenic inflammation has been shown to increase expression of P2X3 receptors in DRG and heightened sensitization to noxious stimuli.24 To address this, we injected CFA into the plantar surface of wild-type and BNP-KO mice and tested their phenotype for thermal, cold, and mechanical pain stimuli. Although the overall sensitivity to these noxious stimuli was elevated in both genotypes, no significant change in nociceptive behavior was seen between wild-type and BNP-KO mice. Neuropathic pain has been shown to produce touch-evoked allodynia (mechanical hyperalgesia) in rodents.11,24 To study this, we created a nerve injury model by ligating the sciatic nerve in wild-type and BNP-KO mice. We did not observe any significant difference between either genotypes.16
Studies by Zhang et al.8 and Li et al.9 have shown BNP to have an antinociceptive effect in rats when injected into the spine (intrathecal injection). This effect could be due to overstimulation of NPRA receptors by excessive BNP, which could possibly decrease nociception. However, none of these studies used a knockout model to corroborate the antinociceptive effect. In our study, we used BNP-KO mice, where endogenous BNP was absent. Another explanation for why we were unable to see an antinociceptive effect could be due to differences in the rat and mouse model systems used in these two independent studies, presumably suggesting plasticity between excitatory and inhibitory neurotransmission in the spinal cord. Based on these findings, we fail to see any functional role for BNP in acute, inflammatory, or neuropathic pain.
Conclusion
In summary, our data do not support a tonic role for BNP in modulating pain in mice. Similarly, the BNP-dependent population of peptidergic neurons, which we have shown to be critical for mediating a chemically induced itch, appears normal for both acute and chemically induced pain. Inflammation-induced hot, cold, and mechanical sensation were normal between genotypes. In addition, sciatic nerve ligation-induced neuropathic pain evoked similar pain responses in wild-type and BNP-KO mice. Therefore, these findings are consistent with the hypothesis that BNP does not play a functional role in mediating pain, and more study needs to be done to elucidate the spinal complexity for pain and itch sensation.
Acknowledgments
The BNP-KO mice used in this study were provided by Dr Mark Hoon, National Institutes of Health. We are grateful to Drs Mark Hoon, Thierry Olivry, Duncan Lascelles, Troy Ghashghaei, and Wolfgang Baeumer for their helpful and critical advice on the manuscript. Also, we are grateful to Mishra lab members for critically reading the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the startup fund from the college of Veterinary Medicine, North Carolina State University.
References
- 1.Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science 2013; 340: 968–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci 2014; 17: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun YG, Zhao ZQ, Meng XL, et al. Cellular basis of itch sensation. Science 2009; 325: 1531–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goswami SC, Thierry-Mieg D, Thierry-Mieg J, et al. Itch-associated peptides: RNA-Seq and bioinformatic analysis of natriuretic precursor peptide B and gastrin releasing peptide in dorsal root and trigeminal ganglia, and the spinal cord. Mol Pain 2014; 10: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 2007; 448: 700–703. [DOI] [PubMed] [Google Scholar]
- 7.Liu XY, Wan L, Huo FQ, et al. B-type natriuretic peptide is neither itch-specific nor functions upstream of the GRP-GRPR signaling pathway. Mol Pain 2014; 10: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang FX, Liu XJ, Gong LQ, et al. Inhibition of inflammatory pain by activating B-type natriuretic peptide signal pathway in nociceptive sensory neurons. J Neurosci 2010; 30: 10927–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li ZW, Wu B, Ye P, et al. Brain natriuretic peptide suppresses pain induced by BmK I, a sodium channel-specific modulator, in rats. J Headache Pain 2016; 17: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solorzano C, Villafuerte D, Meda K, et al. Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns. J Neurosci 2015; 35: 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwan KY, Allchorne AJ, Vollrath MA, et al. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006; 50: 277–289. [DOI] [PubMed] [Google Scholar]
- 12.Bautista DM, Jordt SE, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006; 124: 1269–1282. [DOI] [PubMed] [Google Scholar]
- 13.Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci 2010; 43: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner DS, Golden JP, Gereau RW., IV A novel behavioral assay for measuring cold sensation in mice. PLoS One 2012; 7: e39765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishra SK, Tisel SM, Orestes P, et al. TRPV1-lineage neurons are required for thermal sensation. EMBO J 2011; 30: 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 1990; 43: 205–218. [DOI] [PubMed] [Google Scholar]
- 17.Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 1993; 100: 431–440. [DOI] [PubMed] [Google Scholar]
- 18.Hoon MA, Adler E, Lindemeier J, et al. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell 1999; 96: 541–551. [DOI] [PubMed] [Google Scholar]
- 19.Ryba NJ, Tirindelli R. A new multigene family of putative pheromone receptors. Neuron 1997; 19: 371–379. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. Br J Pharmacol 1999; 126: 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 2015; 18: 145–153. [DOI] [PubMed] [Google Scholar]
- 22.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lotsch J, Dimova V, Oertel BG. Reply to “Can topical capsaicin induce a neuropathic pain?”. Pain 2015; 156: 1369–1370. [DOI] [PubMed] [Google Scholar]
- 24.North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol 2004; 554: 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]