Abstract
To investigate the influence of low-dose-rate irradiation on the growth of silkworms, Bombyx mori, eggs of silkworms were randomly divided into 2 groups and were grown on either low-dose-radiation-emitting sheets or control sheets. On the radiation-emitting sheets, the dose rate was measured as 66.0 (4.3) μSv/h (mean [standard deviation]) by a Geiger-Müller counter for α, β, and γ rays and 3.8 (0.3) μSv/h by a survey meter for γ rays. The silkworms became larger when bred on the radiation-emitting sheets, and their body weight was about 25% to 37% heavier on day 42 to 49 after starting the experiment. Continuous low-dose-rate irradiation promoted the growth of silkworms. It should be further investigated whether this phenomenon could be utilized by the silk industry.
Keywords: low-dose radiation, growth promotion, silkworm, hormesis
Introduction
Radiation exposure at high dose levels (usually >200 mSv for humans) is considered to be harmful and it increases the incidence of cancer. On the other hand, the effects of lower dose exposure remain controversial. Some consider radiation exposure below 200 mSv to also be hazardous based on the linear-no-threshold (LNT) hypothesis, while others consider low-dose exposure to have beneficial effects, which are known as radiation hormesis and the adaptive response.1–6 The former LNT concept is supported by several epidemiological studies, while the latter concept is supported by both basic, epidemiological and clinical studies.1–15 However, it should be noted that epidemiological studies inherently contain many and large biases.
Laboratory studies suggest that low-dose radiation exposure enhances immunological reactions and induces the production of DNA repair enzymes and radioprotective substances such as glutathione and superoxide dismutase.2,6,9–11 In various organisms including mammalians, insects, and plants, beneficial effects of low-dose irradiation have been reported.16–23 Elongation of the life span of irradiated flour beetles (Tribolium) was reported more than 40 years ago,16 and thereafter, the data showing biphasic responses of living organisms to irradiation (ie, beneficial effects at low doses and toxic or harmful effects at high doses) have been accumulating. However, more well-controlled laboratory studies are warranted to clarify the very important issue of the effect of low-dose radiation and to examine the adequateness of the LNT concept.6
Our group has been trying to use low-dose radiation to facilitate the growth of living organisms. Silkworms (Bombyx mori) produce silk, and we hypothesized that low-dose radiation administered to young silkworms may facilitate their growth and eventually increase silk production. First, we attempted to include radioisotopes in the feed, but this was unsuccessful. During the course of trial and error, we found that breeding silkworms on sheets containing low-level radioisotopes facilitates their growth. After confirming the effects in 3 preliminary experiments, we carried out a more controlled experiment, which is reported here.
Materials and Methods
Low-Dose-Radiation-Emitting Sheets
The sheets were manufactured at Aoyama Stein Co, Ltd (Kobe, Japan). Briefly, monazites containing radioisotopes were imported from Indian Rare Earths, Ltd (Mumbai, India). The main composites of the monazites were Ce2O3 (30.6%), P2O5 (29.2%), and La2O3 (15.7%). They were rubbed into sheets made of polyvinyl chloride (PVC) materials. These sheets are commercially available for use in the treatment of chronic pain. For control groups, PVC sheets with similar colors and physical characteristics containing no radioisotopes were also manufactured. Both sheets had a thickness of 3 mm, and they were cut to a 20 × 15 cm size to fit the breeding cages.
To measure radiation emitted from the sheets and analyze the contained radioisotopes, radiation dose rates from the sheets were measured with a Geiger-Müller (GM) counter Inspector USB (for α, β, and γ rays; Measure Works, Tokyo, Japan) and an ion chamber survey meter ICS-321B (Aloka, Tokyo, Japan) for γ rays. To obtain γ-ray spectra, emitted γ rays were collected for 1 hour by a portable γ-ray spectrometer GR-1 (Kromek, Durham, United Kingdom) and analyzed with a software GR-SPCTL (Version 1) developed by Dr Hideki Kato (Fujita Health University, Toyoake, Japan).
Breeding of Silkworms
Since silkworms are nonmammalian, the need for approval of the experiment by the Animal Ethics Committee of Nagoya City University was exempted. Two hundred silkworm eggs (4 packs of 50 eggs) and their feed containing mulberry powders, vitamins, and minerals were purchased from Kougensha Co, Ltd (Matsumoto, Japan). They were randomly divided into 2 groups and placed on the 6-cm-diameter, 3-mm-thick, humidified feeds that were laid on the radiation-emitting and control sheets. The eggs were incubated at 22°C ± 1°C under humidified atmosphere (65%-70% humidity), in accordance with a previous study on silkworms.20 After about 14 days, the eggs hatched and young silkworms emerged, with no apparent differences between the low-dose radiation and control groups. Thereafter, the young silkworms moved freely on the sheets. After hatching, the young silkworms were very small and fragile, so at 30 days after starting the experiment, 55 young silkworms in both groups were randomly chosen and placed into 3 cages (10, 20, and 25 silkworms in each cage) in both of the irradiated and nonirradiated groups. Using 3 cages per group was for the ease of measuring body weight, and the purpose of using different numbers of silkworms per cage was to examine the influence of insect density. At this time, the body weights of the silkworms were ≤0.1 g, and there was no difference between the 2 groups. Thereafter, the body weights of all silkworms were measured twice a week.
Differences between pairs of body weight curves were examined by factorial analysis of variance. Differences in the body weight at each time point were examined by Student t test. The statistical analyses were carried out using an open source software R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
The radiation dose rate at the surface of the radiation-emitting sheets was 66.0 (4.3) μSv/h (mean [standard deviation]) when measured by the GM counter and 3.8 (0.3) μSv/h when measured by the survey meter. These dose rates did not change before and after the experiments. The dose rates for the control sheets were around the background level: 0.2 μSv/h by the GM counter and 0.1 μSv/h by the survey meter. Figure 1 shows γ-ray spectra of the sheets. From the spectra, it was considered that the sheet contained 228Ac and 77Br.
Figure 1.
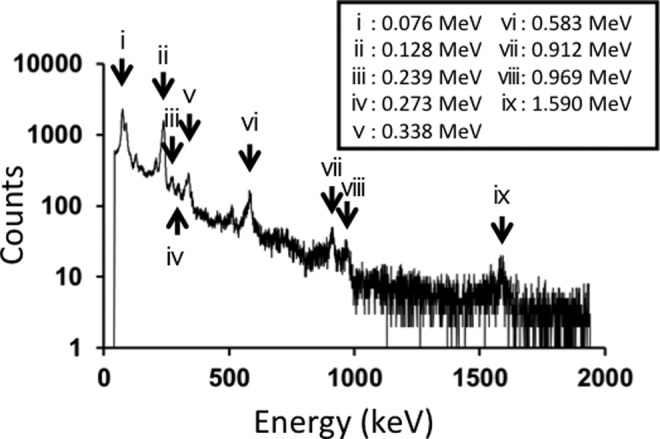
Spectra of γ rays emitted from the sheets. Peak energies iv, v, vii, viii, and ix corresponded to those of 228Ac; and peak energies ii, iii, and vi corresponded to those of 77Br.
Body weights of the silkworms did not change by the insect density (ie, number of silkworms per cage), so all silkworms in each group were analyzed together (n = 55 in both groups). Figure 2 shows changes in the body weight of the silkworm larvae over time. The silkworms grown on the radiation-emitting sheets became larger than those of the control groups on day 39 and thereafter (P < .001). On day 42, the mean body weight was 2.5 (0.2) g for the radiation sheet group and 2.0 (0.2) g for the control group (P < .001). On day 49, they were 4.5 (0.5) and 3.6 (0.4) g, respectively (P < .001).
Figure 2.
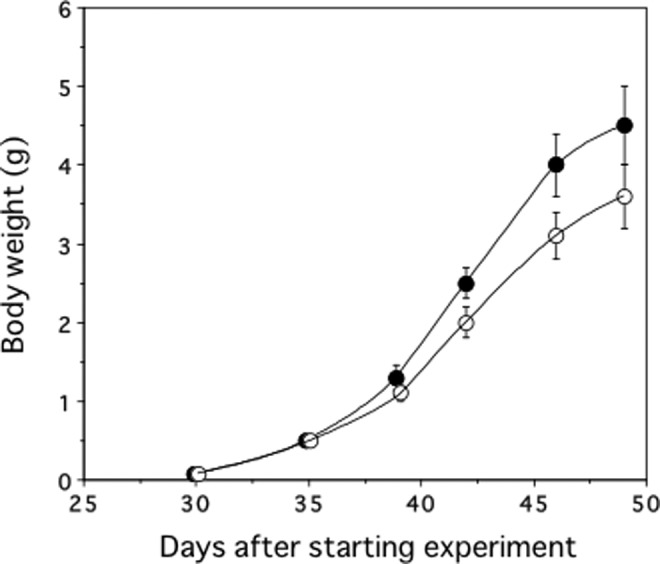
Changes in body weight of silkworms. ^ indicate control group; •, radiation-emitting sheet group. n = 55 for both groups. Data represent mean (standard deviation).
On day 50, some of the larvae started to produce silk, and body weight measurement was terminated, because it was difficult to completely separate the silk and silkworm’s body. For this reason, it was difficult to quantify the amount of silks produced by the silkworms.
Discussion
We observed growth promotion of silkworm larvae by continuous low-dose irradiation. Silkworm is a radioresistant insect, and its lethal radiation dose seems to exceed 100 Gy.24 However, growth retardation of the larvae was reported after irradiation at 10 Gy,20 and defects on wing formation were observed after pupation following 50- or 100-Gy irradiation.24 Therefore, high-dose irradiation appears harmful to silkworms, and thus, the finding obtained in this study would indicate a hormetic effect of radiation in silkworms.
Various stimuli are known to accelerate the growth of insects and plants. These stimuli include ultraviolet radiation, magnetic and electromagnetic fields, microwaves, ultrasound, and low-dose irradiation.16–23 In silkworms, shortening of the embryogenesis period by several hours was reported after chronic radiation, 100, 1000, and 4000 times exceeding the natural background radiation.20 The group also reported an increase in the mass of larvae after a single dose of 2 Gy. In the present study, we employed lower doses and the silkworm eggs were placed on the feed that should absorb radiation, so shortening of the embryogenesis period was not observed, but growth promotion after hatching was observed. In Drosophila melanogaster, elongation of life span and enhancement of locomotive behavior after low-dose irradiation (∼400 mGy) together with gene expression changes have been reported.18,19 In plants, stimulating effects on enzymatic activity and nucleic acid and protein synthesis and reduction of oxidative stress have been proposed as possible mechanisms for growth promotion.21,23 Thus, low-dose stress effects on growth promotion are being increasingly reported.
In view of the radioresistance of silkworms, the optimal dose or dose rate to promote growth may be higher than we used. Our sheets emit α, β, and γ rays. The γ-ray doses may be too low (3.8 μSv/h or 33 mSv/year upon close contact), so α and β rays might play a role; the penetration of these rays, especially β rays, may be sufficient for the small silkworm larvae. In our experience, single-dose irradiation is less likely to induce such beneficial effects,11,12 and such effects may be more likely to be induced by continuous low-dose irradiation. We did not observe radioadaptive response after a single 50 mGy dose in cultured cells,25 but we did find the adaptive response in the same cell line after culture on the low-dose emitting sheets used in this study (C. Sugie, MD, unpublished data, October 2015). Manufacturing the sheet-emitting radiation at higher dose rates is not easy, but we are planning to investigate the optimal radiation dose by using multiple stacked sheets in the near future.
The primary limitation of this investigation was the inability to identify the mechanism for this effect since we have had no method for doing it. However, many investigations, including the changes in gene expression, are conceivable in future. Although it is unclear whether such effects can be detected using larger animals due to the penetration issue of α and β rays, we are conducting similar experiments using young mice, for which blood sample analysis is possible. Second, silk production could not be quantitated, but in a previous study,20 silk production was estimated from the weights of cocoons and their shells, and larger larvae were considered to produce greater amounts of silk. Therefore, low-dose stimulation to silkworm eggs and larvae may contribute to harvesting more and better silk.
In conclusion, breeding on low-dose-radiation-emitting sheets promoted the growth of silkworms. This finding may contribute to the development of the silk industry, which should be tested in future studies.
Acknowledgments
The authors would like to thank Dr Chikao Sugie for his helpful advice on statistical analysis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Natsuto Aoyama is a president of a company manufacturing radiation hormesis products.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a research grant for manufacturing from Hyogo Prefecture, Japan.
References
- 1. Luckey TD, Lawrence KS. Radiation hormesis: the good, the bad, and the ugly. Dose Response. 2006;4(3):169–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pollycove M, Feinendegen LE. Biologic responses to low doses of ionizing radiation: detriment versus hormesis. Part 2. Dose responses of organisms. J Nucl Med. 2001;42(9):26N–32N. [PubMed] [Google Scholar]
- 3. Calabrese EJ, O’Connor MK. Estimating risk of low radiation doses—a critical review of the BEIR VII report and its use of the linear no-threshold (LNT) hypothesis. Radiat Res. 2014;182(5):463–474. [DOI] [PubMed] [Google Scholar]
- 4. Baldwin J, Grantham V. Radiation hormesis: historical and current perspective. J Nucl Med Technol. 2015;43(4):242–246. [DOI] [PubMed] [Google Scholar]
- 5. Marcus CS. Destroying the linear no-threshold basis for radiation regulation: a commentary. Dose Response. 2016;14(4). doi:10.1177/1559325816673491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sacks B, Meyerson G, Siegel JA. Epidemiology without biology: false paradigms, unfounded assumptions, and specious statistics in radiation science (with commentaries by Inge Schmitz-Feuerhake and Christopher Busby and a reply by the authors). Biol Theory. 2016;11(2):69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sakai K, Hoshi Y, Nomura T, et al. Suppression of carcinogenic processes in mice by chronic low dose rate gamma-irradiation. Int J Low Radiation. 2003;1(1):142–146. [Google Scholar]
- 8. Ina Y, Tanooka H, Yamada T, Sakai K. Suppression of thymic lymphoma induction by life-long low-dose-rate irradiation accompanied by immune activation in C57BL/6 mice. Radiat Res. 2005;163(2):153–158. [DOI] [PubMed] [Google Scholar]
- 9. Otsuka K, Koana T, Tauchi H, Sakai K. Activation of antioxidative enzymes induced by low-dose-rate whole-body gamma irradiation: adaptive response in terms of initial DNA damage. Radiat Res. 2006;166(3):474–478. [DOI] [PubMed] [Google Scholar]
- 10. Yamaoka K, Mitsunobu F, Kojima S, et al. The elevation of p53 protein levels and SOD activity in the resident blood of the Misasa radon hot spring district. J Radiat Res. 2005;46(1):21–24. [DOI] [PubMed] [Google Scholar]
- 11. Ito M, Shibamoto Y, Ayakawa S, Tomita N, Sugie C, Ogino H. Low-dose whole-body irradiation induced radioadaptive response in C57BL/6 mice. J Radiat Res. 2007;48(6):455–460. [DOI] [PubMed] [Google Scholar]
- 12. Ito M, Shibamoto Y, Ayakawa S, Tomita N, Sugie C, Ogino H. Effect of low-dose total-body irradiation on transplantability of tumor cells in syngeneic mice. J Radiat Res. 2008;49(2):197–201. [DOI] [PubMed] [Google Scholar]
- 13. Kojima S, Tsukimoto M, Shimura N, Koga H, Murata A, Takara T. Treatment of cancer and inflammation with low-dose ionizing radiation: three case reports. Dose Response. 2017;15(1). doi:10.1177/1559325817697531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cuttler JM, Feinendegen LE, Socol Y. Evidence that lifelong low dose rates of ionizing radiation increase lifespan in long- and short-lived dogs. Dose Response. 2017;15(1). doi:10.1177/1559325817692903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siegel JA, Sacks B, Pennington CW, Welsh JS. Dose optimization to minimize radiation risk for children undergoing CT and nuclear medicine imaging is misguided and detrimental. J Nucl Med. 2017;58(6):865–868. [DOI] [PubMed] [Google Scholar]
- 16. Ducoff HS. Form of the increased longevity of Tribolium after X-irradiation. Exp Gerontol. 1975;10(3-4):189–193. [DOI] [PubMed] [Google Scholar]
- 17. Moskalev AA, Plyusnina EN, Shaposhnikov MV. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: the role of cellular stress-resistance mechanisms. Biogerontology. 2011;12(3):253–263. [DOI] [PubMed] [Google Scholar]
- 18. Zhikrevetskaya S, Peregudova D, Danilov A, et al. Effect of low doses (5-40 cGy) of gamma-irradiation on lifespan and stress-related genes expression profile in Drosophila melanogaster . PLoS One. 2015;10(8):e01338 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim CS, Seong KM, Lee BS, et al. Chronic low-dose γ-irradiation of Drosophila melanogaster larvae induces gene expression changes and enhances locomotive behavior. J Radiat Res. 2015;56(3):475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yusifov NI, Kuzin AM, Agaev FA, Alieva SG. The effect of low level ionizing radiation on embryogenesis of silkworm, Bombyx mori L. Radiat Environ Biophys. 1990;29(4):323–327. [DOI] [PubMed] [Google Scholar]
- 21. Araujo Sde S, Paparella S, Dondi D, Bentivoglio A, Carbonera D, Balestrazzi A. Physical methods for seed invigoration: advents and challenges in seed technology. Front Plant Sci. 2016;7:646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tezuka T, Hotta T, Watanabe I. Growth promotion of tomato and radish plants by solar UV radiation reaching the Earth’s surface. J Photochem Photobiol B: Biology. 1993;19(1):61–66. [Google Scholar]
- 23. Hajnorouzi A, Vaezzadeh M, Ghanati F, Jamnezhad H, Nahidian B. Growth promotion and a decrease of oxidative stress in maize seedlings by a combination of geomagnetic and weak electromagnetic fields. J Plant Physiol. 2011;168(10):1123–1128. [DOI] [PubMed] [Google Scholar]
- 24. Takahashi M, Lee JM, Mon H, Kawaguchi Y, Koga K, Kusakabe T. Cell cycle arrest induced by radiation in cultured silkworm cells. J Insect Biotechnol Sericol. 2006;75(1):23–30. [Google Scholar]
- 25. Miyamoto A, Shibamoto Y, Sugie C, Ito M, Ayakawa S. Absence of radioadaptive response in four cell-lines in vitro as determined by colony formation assay. Kurume Med J. 2006;53(1):1–5. [DOI] [PubMed] [Google Scholar]


