Abstract
Although imatinib has dramatically improved major outcomes in patients with chronic myeloid leukemia (CML), there are newer tyrosine kinase inhibitors (TKIs) approved worldwide for the treatment of resistant cases, and two second-generation TKIs (dasatinib, nilotinib) are approved in some nations for treating patients in the upfront setting. Radotinib (IY5511HCL, Supect®) is a novel and selective second-generation BCR-ABL1 TKI, which is currently approved in Korea for the treatment of patients with CML both in the upfront and salvage settings. This review mainly focuses on the clinical potential of radotinib in patients with CML in chronic phase in terms of efficacy and safety.
Keywords: bosutinib, chronic myeloid leukemia, dasatinib, imatinib, nilotinib, ponatinib, radotinib, tyrosine kinase inhibitor
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic stem cell disease characterized by a translocation between chromosomes 9 and 22; the Philadelphia (Ph) chromosome is formed by the fusion of the breakpoint cluster region (BCR) and the Abelson murine leukemia (ABL) genes. The tyrosine kinase inhibitors (TKIs) revolutionized the treatment of CML, and currently in patients with CML in chronic phase (CML-CP) the first-line treatment is based on targeted therapy with TKIs. Imatinib is the first BCR-ABL1 TKI approved for the treatment of CML, and frontline treatment with imatinib has dramatically improved major outcomes including molecular and cytogenetic responses, and survival in patients with CML-CP.1 Although imatinib is beneficial in many patients, approximately 40% of patients with CML-CP quit receiving imatinib due to failure and/or intolerance. Second-generation TKIs (2GTKIs) (dasatinib and nilotinib) have been introduced to provide greater efficacy in patients who were resistant or intolerant to imatinib.2,3 Nilotinib and dasatinib were then approved for the upfront treatment of CML in some nations after Evaluating Nilotinib Efficacy and Safety in Clinical Trials – Newly Diagnosed Patients (ENESTnd) and Dasatinib Versus Imatinib Study in Treatment-Naive CML Patients (DASISION) trials.4,5 Another 2GTKI – bosutinib – and a pan-BCR-ABL1 kinase inhibitor – ponatinib – have recently been approved by the Food and Drug Administration (FDA) only for second- or later-line therapies.6,7 Most of these TKIs are utilized globally, whereas another 2GTKI, radotinib (IY5511HCL, Supect®) has been recently introduced locally in South Korea.8 Radotinib can be used in the second-line treatment of CML-CP,9 and it is approved by the Korean FDA in the upfront setting.10
Radotinib
Pharmacodynamics and pharmacokinetics
Radotinib is an oral, high-affinity BCR-ABL1 inhibitor that bears strong structural resemblance to imatinib and especially to nilotinib.9 According to recently conducted in vitro kinase assays, the IC50 (half maximal inhibitory concentration) value for radotinib against wild-type BCR-ABL1 kinase was 34 nm, which is relatively lower compared with the IC50 levels of c-kit (1324 nm), PDGFR (PDGFRα, 75.5 nm; PDGFRβ, 130 nm; and SRC (>2000 nm). Also, radotinib effectively inhibits the proliferation of common clones of BCR-ABL1, with the exception of T315I. In an off-target kinase assay to assess safety, DDR, EFHB, LYN, and PDGFR kinases were inhibited below the 180 nm level. Zabriskie and colleagues evaluated the efficacy of radotinib on mutant BCR-ABL1 clones, and they showed that radotinib could be used for patients with common BCR-ABL1 mutations, except T315I.9 They also remarked that the resistance pattern of radotinib was almost the same as nilotinib, which was attributed to bio-similarity between nilotinib and radotinib.9
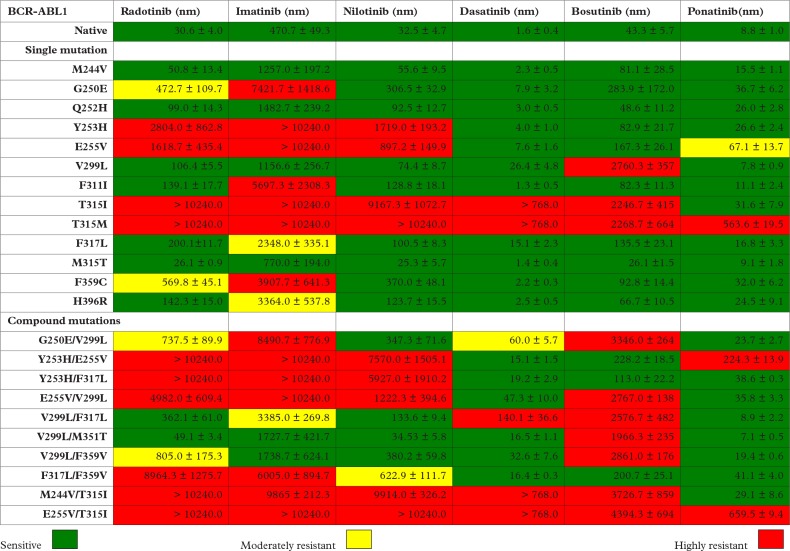
The BCR-ABL1 mutant sensitivity profile of radotinib and the other five approved TKIs are shown in Figure 1. There were BCR-ABL1 kinase domain (KD) mutations sensitive to radotinib, including M244V, Q252H, V299L, F311I, F317L, M351T and H396R, and V299L/M351T and V299L/F317L compound mutations (marked in green in Figure 1), whereas G250E, F359C, and G250E/V299L and V299L/F359V compound mutations were found to be moderately resistant to radotinib (marked in yellow in Figure 1). Y235H, E255V, T315I, T315M, and Y253H/E255V, Y253H/F317L, E255V/V299L, F317L/F359V, M244V/T315I, and E255V/T315I compound mutations were highly resistant to radotinib (marked in red in Figure 1).9
Figure 1.
BCR-ABL1 mutant sensitivity profile of all approved TKIs utilized in the treatment of CML, including radotinib.9 A color gradient from green to yellow to red denotes the IC50 sensitivity to each TKI: imatinib (green: <1000 nm; yellow: 1000–4000 nm; red: >4000 nm); nilotinib (green: <200 nm; yellow: 200–1000 nm; red: >1000 nm); radotinib (green: <200 nm; yellow: 200–1000 nm; red: >1000 nm); dasatinib (green: <25 nm; yellow: 25–150 nm; red: >150 nm); bosutinib (green: <150 nm; yellow: 150–1000 nm; red: >1000 nm); ponatinib (green: <25 nm; yellow: 25–150 nm; red: >150 nm).
Pre-clinical and clinical activities of radotinib
In a pre-clinical study, it was demonstrated that radotinib was superior to imatinib in both wild-type and mutant BCR-ABL1 positive CML cell lines.11 It was also shown that there was no dose-limiting toxicity with a dose up to 1000 mg/day of radotinib in a phase I study.12
Radotinib in patients with intolerance/resistance to imatinib
Efficacy of radotinib in the salvage setting
After the efficacy and safety profile of radotinib was shown in CML, Kim and colleagues performed a phase II trial for safety and efficacy of radotinib in the treatment of CML-CP patients with resistance and/or intolerance to former lines of TKI treatment.8 Seventy-seven Asian patients with CML-CP were enrolled in the study; the starting dose of the study drug was 400 mg twice daily. Major cytogenetic response (MCyR) was achieved in 50 (65%) patients, including 36 (47%) with complete cytogenetic response (CCyR) by 12 months. Rates of MCyR and CCyR were similar between imatinib-resistant and imatinib-intolerant patients, but these responses were superior in patients without BCR-ABL1 mutations.8 There were 12 patients with a BCR-ABL1 mutation [four P-loop (G250E, Y253F + E355G, E255K, E255V), F359V in two patients, and one each of M244V, M244V + H396R, L387M, F317L, M351T, E355G], and in two patients ABL1 KD abnormalities (between exons 8 and 9, and deletion of amino acids 363–386) were detected at baseline. During radotinib therapy, these findings were undetectable in only three patients, and baseline mutation(s) persisted in six cases after 12 cycles of treatment. On the other hand, 6 out of 63 patients without baseline KD mutation gained a new single-point mutation (E255V in two patients, and one each of F317L, T315I, F359V and E459K) during radotinib treatment.8 E255V, T315I and F359V are known to be highly and moderately resistant to radotinib, respectively, whereas, interestingly, F317L, which was detected in one patient, is known to be sensitive to radotinib (Figure 1).9 IC50 value against E459K has not yet been described for radotinib, as well as for other TKIs, including imatinib, nilotinib, dasatinib and bosutinib.13 However, in a patient harboring V299L and E459K compound mutations, with second-line bosutinib therapy E459K mutant was successfully suppressed and the patient gained hematologic, cytogenetic and molecular responses.13
In terms of efficacy, the results of this phase II study were comparable to those achieved with other 2GTKIs including nilotinib, dasatinib and bosutinib after imatinib resistance or intolerance.14–16
Toxicity of radotinib in the salvage setting
Among the grade III/IV hematologic adverse events (AEs), thrombocytopenia and anemia were observed in 24.7% and 5.2% of the patients, respectively. Fatigue (3.9%), asthenia (3.9%), nausea (2.6%), myalgia (1.3%), rash (1.3%) and pruritus (1.3%) were the grade III/IV non-hematologic AEs.8 The most common (⩾10%) grade III/IV biochemical abnormalities were hyperbilirubinemia (23.4%), followed by hyperglycemia (19.5%), elevations of alanine transaminase (ALT) (11.7%) and lipase (10.4%). Overall elevations of ALT and aspartate transaminase (AST) were observed in 85.7% and 72.7% of patients, respectively. No grade III/IV diarrhea was observed; diarrhea was experienced in four patients (5.2%). QTc interval prolongation (>30 ms change from baseline) was reported in six (7.8%) patients, and in five (6.5%) cases a QTc interval over 450 ms was detected, but none of these patients had an interval over 480 ms.8
Almost half of the cases (33 patients; 42.9%) permanently quit the study drug within the 12 months of treatment, and dose reduction or dose interruption had to be carried out in 68.8% and 71.4% of the patients, respectively.8 The reasons for treatment discontinuation included abnormal laboratory tests (n = 15) and non-hematologic AEs (n = 3) (n = 18; 54.5%), disease progression (n = 8; 24.2%), death (n = 2; 6.1%, due to sepsis) and other reasons (n = 5; 15.2%). Overall radotinib discontinuation rates both in the original study8 and in the 24-month update17 were also comparable to other studies as concluded by Kim and colleagues.18
Relatively higher rates of AEs and biochemical abnormalities of radotinib in the salvage setting raised some concerns,19 and the authors concluded that as a starting dose the current 800 mg daily dose may be appropriate in the second-line setting, but the dose for frontline administration should be reduced; studies were ongoing for further dose optimization.18
Radotinib in newly diagnosed patients with CML-CP
Prior to the utilization of radotinib in the context of first-line therapy, there were some 2GTKIs tested in the frontline treatment of patients with CML-CP. Nilotinib and dasatinib induced faster and deeper responses than imatinib when used in the upfront setting among patients with CML-CP in two prospective, randomized, international, and company-sponsored trials.4,5 These two 2GTKIs were then approved in many countries for the treatment of newly diagnosed patients with CML-CP. Although bosutinib was proven to be efficient among imatinib-resistant or -intolerant cases and consequently approved for the salvage setting, it failed to demonstrate superior outcomes over imatinib in the Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia (BELA) trial.20 In the BELA trial, the primary endpoint was CCyR at 12 months. Although the rate of major molecular response (MMR) in the bosutinib arm was significantly superior than that of patients receiving imatinib (41% versus 27%, p < 0.001) as a secondary endpoint, the primary endpoint was not different for bosutinib (70%) versus imatinib (68%) (p = 0.601); consequently, bosutinib was not approved as a frontline treatment option for patients with CML-CP since the primary endpoint was not met.20 Also it was shown that the rate of discontinuation due to AEs was higher in patients receiving bosutinib 500 mg/day (19%) when compared to imatinib 400 mg daily (6%), which might be one of the reasons for the lower response rates in the bosutinib arm. Ponatinib is a potent TKI active against native and mutated forms of BCR-ABL1, including T315I, and it was approved after the phase II PACE study demonstrating that ponatinib was highly active in heavily pretreated Philadelphia chromosome-positive leukemia patients.21 EPIC was a multicenter, international, randomized, phase III trial in which ponatinib 45 mg daily was compared with imatinib 400 mg daily among newly diagnosed CML-CP patients.22 Although ponatinib was shown to have improved efficacy over imatinib, this study was terminated due to arterial thrombotic events after a median follow up of 5.1 months.
Efficacy of radotinib in the upfront setting
After being tested in patients refractory/intolerant to prior TKI therapies,8 radotinib was planned to be utilized in the first-line treatment of patients with CML-CP. In the clinical phase III Radotinib Versus Imatinib in Newly Diagnosed Philadelphia Chromosome and Chronic Myeloid Leukemia Chronic Phase Patients (RERISE) trial, which was first reported at the 57th American Society of Hematology meeting in 2015, Kwak and colleagues compared radotinib with imatinib among newly diagnosed Asian patients with CML-CP in terms of efficacy and safety.10 There were three study groups, including radotinib at 300 mg twice daily (n = 79), radotinib 400 mg twice daily (n = 81) and imatinib at 400 mg once daily (n = 81). The study groups were equally balanced regarding age, gender, race and Sokal risk scores. After a minimum follow up of 12 months, the rates of MMR were significantly higher in patients receiving the radotinib 300 mg dose (52%) and the radotinib 400 mg dose (46%) when compared to imatinib (30%), and the MR4.5 rates by 12 months were also higher for both radotinib 300 mg (15.2%) and 400 mg (13.6%) than imatinib (8.6%). The CCyR rates by 12 months were also significantly higher for radotinib 300 mg bid (91.1%) compared with imatinib (76.5%).10
In a recently published systematic review and meta-analysis of randomized controlled trials, the authors compared newer TKIs (nilotinib, dasatinib, bosutinib, ponatinib and radotinib) with imatinib in terms of efficacy among patients with CML-CP in the upfront setting.23 The authors concluded that the newer TKIs were associated with greater MMR rates and lower rates of progression to advanced disease phases, but not with significant differences in CCyR, progression-free survival (PFS), overall survival (OS), or ABL1 KD mutation rates relative to imatinib at 12 months of TKI therapy.23 Also in this meta-analysis, it was shown that in the subgroup analyses performed among the newer TKIs, the treatment response was significantly better with nilotinib, dasatinib and radotinib than bosutinib or ponatinib (p = 0.01).23
Toxicity of radotinib in the upfront setting
The rates of grade III/IV thrombocytopenia were 16.5%, 13.6% and 19.8% in patients receiving radotinib 300 mg bid, radotinib 400 mg bid and imatinib, respectively.10 Grade III/IV neutropenia occurred in 19.0%, 23.5% and 29.6% for radotinib 300 mg bid, 400 mg bid and imatinib arms, respectively. The most common non-hematologic AEs (all grades) were skin rash (35.4% and 33.3%), nausea/vomiting (22.8% and 23.5%), headache (19.0% and 30.9%), and pruritus (19.0% and 30.0%) in radotinib 300 mg bid and radotinib 400 mg bid, respectively; AEs in the imatinib group were edema (34.6%), myalgia (28.4%), nausea/vomiting (27.2%) and skin rash (22.2%).10 Discontinuation due to AEs or laboratory abnormalities occurred in 7 (8.8%), 16 (19.8%) and 5 (6.2%) patients for radotinib 300 mg, radotinib 400 mg, and imatinib, respectively.
Concluding remarks
The management of patients with CML-CP has undergone an evolution with the advent of imatinib. This drug has significantly changed the natural history of the disease, increasing 10-year OS from 10–20% to 80–90%.24 However, a subset of patients may have resistance and/or intolerance to imatinib and these patients require further treatment options including 2GTKIs and ponatinib.
Nilotinib, dasatinib and bosutinib are the 2GTKIs approved in many countries for the treatment of resistant/intolerant cases following the international multicenter trials conducted among different racial or ethnic groups including Asians.14–16 However, the radotinib trial, which was conducted in the salvage setting, only included Asian patients,8 and this drug is currently approved only in Korea for this indication.
In addition to the use in the salvage setting, two 2GTKIs (nilotinib, dasatinib) are approved in some countries for the treatment of patients with CML-CP in the upfront setting after the ENESTnd and DASISION trials, which also included patients from different ethnic groups including Asians.4,5 Following the RERISE trial,10 radotinib was also approved by the Korean FDA for the management of CML-CP in newly diagnosed patients.
Until now, the total number of patients receiving radotinib 400 mg twice daily (both upfront and salvage settings) are 158, while 79 newly diagnosed patients received 300 mg twice daily.8,10 These numbers are relatively small when compared to most of the other 2GTKI trials, so as the cumulative ‘real-life’ data on the use of radotinib in daily care patients with CML mature, most probably we will be able to compare this 2GTKI with others more appropriately.
AEs may differ between different BCR-ABL1 TKIs,25,26 and the rates of these AEs are not the same when these TKIs are administered in different lines of treatment (first-line versus second-line or higher) and in different phases of the disease (CP versus advanced phases). Since the trials that led to the approval of other TKIs were conducted worldwide, the rates of AEs can vary if radotinib is administered in CML-CP patients across different racial/ethnic groups in different countries. Vascular adverse events (VAEs) are an emerging problem in patients with CML receiving 2GTKIs, especially in patients with pre-existing risk factors and comorbidities. TKI-related VAEs include peripheral, cerebral and coronary artery changes in patients receiving nilotinib, venous and arterial occlusive events during ponatinib therapy, and pulmonary hypertension in patients receiving dasatinib.27 In two radotinib trials,8,10 no such VAEs were reported to date, but the median follow-up durations of these trials are relatively shorter than those of nilotinib and dasatinib studies. So with extended radotinib exposure, some of these VAEs may be observed among these patients in long-term follow up.
The improved outcome achieved first with imatinib and later with newer TKIs resulted in a substantial increase in therapeutic expenses,28 and the healthcare systems and reimbursement authorities most probably could not afford the expense of the original TKIs in the near future even in developed countries; generics at a lower price could improve treatment penetration.29 For example, in 2016 a group of experts anticipated that the per-patient, per-month cost of imatinib will drop 60–90% once it loses patent protection in the United States, while 2GTKIs dasatinib and nilotinib will hold their high costs steady.30 They concluded that from a US perspective, a generic imatinib-first strategy compared with the current standard of care would save an average of $91,163 per patient in direct medical costs over five years among CML-CP patients, and imatinib will be the most cost-effective initial treatment strategy for CML-CP compared with dasatinib and nilotinib.30 Similarly, the advent of radotinib helped to overcome the relatively high cost of TKI treatment, and in South Korea the annual price of TKI treatment has dropped to between $21,000 and $28,000 in 2013, most probably due to the approval of radotinib by the Korean health authorities (the annual price of radotinib was approximately $21,500).28 In the same time period, the annual cost of TKI treatment was much higher (annual prices range from $92,000 to $138,000) in the US prior to the patent expiration of imatinib.
To conclude, radotinib can be a reasonable treatment option in the management of patients with CML-CP both in the salvage setting and in newly diagnosed cases. As it gives both the physicians and patients another treatment option, the utilization of radotinib might further reduce the cost of TKI treatment in patients with CML-CP worldwide. However, the experience with radotinib gained to date is relatively limited when compared to other 2GTKIs, and there is still a need for international, multicenter trials recruiting more patients of different racial and ethnic groups with longer follow up in terms of evaluating the efficacy and safety of this 2GTKI.
Acknowledgments
We would like to thank Prof. Teoman Soysal for his continuous support, encouragement and great mentorship.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: AEE has received honoraria from Novartis and Bristol-Myers Squibb. DK has no relevant conflict of interest to declare.
Contributor Information
Ahmet Emre Eskazan, Division of Hematology, Department of Internal Medicine, Cerrahpasa Faculty of Medicine, Istanbul University, Kocamustafapasa/Fatih, 34303 Istanbul, Turkey.
Dilek Keskin, Division of Hematology, Department of Internal Medicine, Cerrahpasa Faculty of Medicine, Istanbul University, Istanbul, Turkey.
References
- 1. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004. [DOI] [PubMed] [Google Scholar]
- 2. Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood 2007; 109: 2303–2309. [DOI] [PubMed] [Google Scholar]
- 3. Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood 2007; 110: 3540–3546. [DOI] [PubMed] [Google Scholar]
- 4. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010; 362: 2251–2259. [DOI] [PubMed] [Google Scholar]
- 5. Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260–2270. [DOI] [PubMed] [Google Scholar]
- 6. Cortes JE, Kantarjian HM, Brümmendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011; 118: 4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cortes JE, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med 2012; 367: 2075–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S-H, Menon H, Jootar S, et al. Efficacy and safety of radotinib in chronic phase chronic myeloid leukemia patients with resistance or intolerance to BCR-ABL1 tyrosine kinase inhibitors. Haematologica 2014; 99: 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zabriskie MS, Vellore NA, Gantz KC, et al. Radotinib is an effective inhibitor of native and kinase domain-mutant BCR-ABL1. Leukemia 2015; 29: 1939–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kwak J-Y, Kim H, Kim JA, et al. Efficacy and safety of radotinib compared with imatinib in newly diagnosed chronic phase chronic myeloid leukemia patients: 12 months result of phase 3 clinical trial. Blood 2015; 126: abstract 476. [Google Scholar]
- 11. Lee J, Han BC, Goh HG, et al. IY551, a novel BCR-ABL tyrosine kinase inhibitor, is highly active compound in inhibition of CrkL phosphorylation and in xenograft animal chronic myeloid leukemia model. ISH-APD. APBMT meeting abstract 2007; 02-061. [Google Scholar]
- 12. Lee J, Kim HJ, Sohn S-K, et al. Safety and efficacy of radotinib, a tyrosine kinase inhibitor. Korean J Hematol 2011; 46(Suppl. 2): 152.22065968 [Google Scholar]
- 13. Kim D, Kim DW, Cho BS, et al. Structural modeling of V299L and E459K Bcr-Abl mutation, and sequential therapy of tyrosine kinase inhibitors for the compound mutations. Leuk Res 2009; 33: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 14. Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood 2011; 117: 1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica 2010; 95: 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cortes JE, Kantarjian HM, Brümmendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 2011; 118: 4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim SH, Menon H, Jootar S, et al. Early response of radotinib therapy may predict long-term outcomes in chronic phase chronic myeloid leukemia patients with resistance or intolerance to BCR-ABL1 TKIs: 24 month update of radotinib phase 2 trial. Haematologica 2014; 99(s1): 77 [abstract]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim SH, Kim DW. Comment on ‘efficacy and safety of radotinib in chronic phase chronic myeloid leukemia patients with resistance or intolerance to BCR-ABL1 tyrosine kinase inhibitors’. Haematologica 2015; 100: e120–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eskazan AE, Soysal T. Radotinib in the treatment of chronic phase chronic myeloid leukemia patients. Haematologica 2015; 100: e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol 2012; 30: 3486–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med 2013; 369: 1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lipton JH, Chuah C, Guerci-Bresler A, et al. Epic: a phase 3 trial of ponatinib compared with imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CP-CML). Blood 2014; 124: abstract 519. [Google Scholar]
- 23. Yun S, Vincelette ND, Segar JM, et al. Comparative effectiveness of newer tyrosine kinase inhibitors versus imatinib in the first-line treatment of chronic-phase chronic myeloid leukemia across risk groups: a systematic review and meta-analysis of eight randomized trials. Clin Lymphoma Myeloma Leuk 2016; 16: e85–e94. [DOI] [PubMed] [Google Scholar]
- 24. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer 2012; 118: 3123–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Apperley JF. Chronic myeloid leukemia. Lancet 2015; 385: 1447–1459. [DOI] [PubMed] [Google Scholar]
- 26. Kirkizlar O, Eskazan AE. Adverse events of tyrosine kinase inhibitors and their impact on quality of life in patients with chronic myeloid leukemia. Expert Rev Qual Life Cancer Care 2016; 1: 353–359. [Google Scholar]
- 27. Valent P, Hadzijusufovic E, Hoermann G, et al. Risk factors and mechanisms contributing to TKI-induced vascular events in patients with CML. Leuk Res 2017; 59: 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood 2013; 121: 4439–4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soysal T, Eskazan AE, Ar MC. Generics in chronic myeloid leukemia: current arguments for and against and the established evidence. Expert Rev Hematol 2014; 7: 697–699. [DOI] [PubMed] [Google Scholar]
- 30. Padula WV, Larson RA, Dusetzina SB, et al. Cost-effectiveness of tyrosine kinase inhibitor treatment strategies for chronic myeloid leukemia in chronic phase after generic entry of imatinib in the United States. J Natl Cancer Inst 2016; 108 DOI: 10.1093/jnci/djw003. [DOI] [PMC free article] [PubMed] [Google Scholar]