Abstract
Objective
Participation rates in clinical trials are low in teenagers and young adults (TYA) with cancer. Whilst the importance of clinical trials in informing best practice is well established, data regarding individual patient benefit are scarce. We have investigated the association between overall survival and trial recruitment in TYA patients with acute lymphoblastic leukaemia (ALL).
Design
Retrospective.
Setting
National (England) TYA patients treated for ALL.
Participants
511 patients aged 15–24 years diagnosed with ALL between 2004 and 2010 inclusive, of whom 239 (46.7%) participated in the UKALL2003 trial.
Outcome measures
Patients were identified using National Clinical Trial (UKALL2003) and Cancer Registry (National Cancer Data Repository, English National Cancer Online Registration Environment) Databases. Relative survival rates were calculated for trial and non-trial patients and observed differences were modelled using a multiple regression approach. The numbers and percentages of deaths in those patients included in the survival analysis were determined for each 3-month period, p values were calculated using the two-tailed z-test for difference between proportions and 95% CIs for percentage deaths were derived using the binomial distribution based on the Wilson Score method.
Results
Patients treated on the trial had a 17.9% better 2-year survival (85.4% vs 67.5%, p<0.001) and 8.9% better 1-year survival (90.8% vs 81.9%, p=0.004) than those not on the trial. 35 (14.6%) patients recruited to the trial died in the 2 years following diagnosis compared with 86 (32.6%) of those not recruited (p<0.001).
Conclusions
TYA patients recruited to the clinical trial UKALL 2003 in England had a lower risk of mortality and a higher overall survival than contemporaneous non-trial patients. These data underline the potential for individual patient benefit in participating in a clinical trial and the importance of international efforts to increase trial participation in the TYA age group.
Trial registration number
ISRCTN07355119.
Keywords: clinical trials, survival, teenage and young adult, acute lymphoblastic leukaemia
Strengths and limitations of this study.
The study asks a fundamental question regarding the value to an individual of recruitment to a clinical trial.
Large sample size including all TYA patients aged 15–24 years diagnosed with ALL in England between 2004 and 2010.
This retrospective study is possible because (1) the consent process for UKALL2003 included explicit consent for the trial data to be shared with regional and national cancer registries and (2) because the UK has full population coverage for cancer registration.
Clinically, significant outcomes of survival and mortality in those recruited to the clinical trial, UKALL2003, are compared with contemporaneous patients treated off trial.
Given the retrospective design of this study, there is an inherent risk of potential confounding variables influencing the observations, which are fully explored in the discussion; these include selection bias, centre effect, protocol used and recruitment to other clinical trials.
Introduction
Although survival rates in teenage and young adult (TYA) patients with cancer have improved over the last two decades,1 these outcome gains have been modest and cancer remains the leading cause of non-accidental mortality in the TYA age group.2 3
The barriers to improving survival are likely to be multifactorial and include suboptimal diagnostic pathways, complex tumour and host biology, access to age or site-specific specialist care and poor compliance with treatment.4 5 Importantly, recruitment rates to clinical trials in TYA patients with cancer are the lowest of any age group.5–8 In those patients for whom clinical trials are available, factors influencing participation include whether the treating physician is from a paediatric or adult background,5 9–12 the type of treating centre (academic, tertiary or other)9 and patient factors, notably the acceptability to and attitudes of TYA patients.13 In addition, there are often fewer clinical trials available to TYA patients than to younger or older patients with cancer,7 8 14 reflecting the unique distribution of tumours in this age group and the traditional separation between adult and paediatric clinical research programmes.5
While sequential clinical trials clearly inform evidence-based best practice in cancer therapy and have improved outcomes in specific disease types,15 the value of participation for an individual patient is less clear. The benefits may include access to a superior therapy not otherwise available, enhanced quality of care, access to a broader team of specialised professionals16 and stricter adherence to trial mandated treatment. However, to date, no study has demonstrated a survival advantage specifically associated with trial participation in TYA patients.17 18
The objective of this study was to report survival outcomes of TYA patients with acute lymphoblastic leukaemia (ALL) in England who were treated within or outside of the national clinical trial, UKALL2003.
Methods
UKALL2003
UKALL2003 was the UK clinical trial of minimal residual disease (MRD)-directed chemotherapy for Philadelphia chromosome negative ALL in children and young people aged 1–24 years, which opened in 2003 and closed in 2011. The maximum age for trial entry was the 18th birthday when the trial began, but was increased to the 20th birthday in 2006 and the 25th birthday in 2007. Details of the protocol and outcomes of the trial have been published previously.19–21 The consent form for UKALL2003 included explicit consent for data sharing with national cancer registries. Patients with Philadelphia chromosome positive ALL were eligible for post-induction recruitment to the European intergroup study on post-induction treatment of Philadelphia positive ALL (ESPHALL) trial if aged less than 18 years (until 2009) or UKALL XII if aged 18 years or older (until 2006). The lower age limit for recruitment of patients with Philadelphia negative ALL to UKALL XII was sequentially increased according to the change in the upper age limit for UKALL2003 to avoid overlapping age eligibility for the two trials. These changes to age eligibility for UKALL2003 and UKALL XII were communicated in newsletters circulated by the clinical trials unit and presented at both paediatric and adult annual national leukaemia trials update meetings. The National Institute for Health Research also hosts a trial database, which summarised all of these trials and was searchable by any clinician. The Strengthening the reporting of observational studies in epidemiology (STROBE) guidelines for the reporting of observational studies was followed.
Study population
The study population for this analysis was defined as any patient diagnosed with ALL in 2004–2010 (the seven complete years during which UKALL2003 was recruiting), aged 15–24 years and resident in England at the time of diagnosis. The diagnosis reported to the registry and trial was made in the laboratories of treating centre, without central verification. Details were obtained on the 337 TYA patients who took part in the UKALL2003 Trial including name, date of birth and hospital of treatment, but not address or country of residence. Details of missing NHS numbers on UKALL2003 were obtained using the NHS Strategic Tracing Service (NSTS), which contains details of all individuals registered with a GP in England or Wales. The National Cancer Data Repository (NCDR) was the database used to undertake cancer analyses at the time this study commenced. It included details of all patients diagnosed with cancer who were resident in England. A frozen copy of NCDR was compiled from the eight regional registries then in existence, when cancer data for a given calendar year were considered to be near complete—a process which took considerable time. The version used in this study was that produced when 2010 data were considered complete. The patients in UKALL2003 were matched against those on the NCDR database who were diagnosed in 2004–2010 aged 15–24 years with any cancer. Patients recorded on NCDR as having ALL and who were not on the UKALL database were included in our study as the non-trial arm.
The move to a single cancer registry for England, which was completed while this study was underway, resulted in the production of the English National Cancer Online Registration Environment (ENCORE). This is a live database, which is updated regularly. It is used by registry staff to determine if a given patient has already been registered. It contains details of all residents of England who have been diagnosed with cancer, including patients reported to the National Cancer Registration and Analysis Service (NCRAS) but with insufficient details to be counted as a case of cancer; these are called provisional registrations and are upgraded to full registrations when further details are obtained. ENCORE also contains details of patients with cancer managed at a hospital in England but resident elsewhere. Patients on the UKALL database not found on NCDR were manually checked on ENCORE, in order to determine the reasons why these patients were not on NCDR and to identify any patients reported to NCRAS after the NCDR was compiled. Those whose records were found on ENCORE and who fulfilled the study definition were added to patients on both UKALL and NCDR to make up the trial arm in our study.
Details of patients on the UKALL database but not found on ENCORE were checked against the NSTS to determine whether living in England or Wales. The UKALL database was interrogated for the country of the treating hospital for those patients not found on NSTS.
Consent
Registry data was routinely submitted to the national cancer registry under legal permissions that were initially included under Section 60 of the Health and Social Care Act 2001 and more recently Section 251 of the NHS Act 2006. This permission is renewed annually. Identifiable trial data were shared with explicit patient consent obtained during trial registration.
Analyses
We determined the number and percentage of patients who participated in UKALL2003 by year of diagnosis and age group. Survival rates were calculated for patients in the trial and not in the trial, excluding patients on whom the only available data were from death certificates—death certificate only (DCO) registrations. Death details were obtained from NCDR up to the end of 2012, with follow-up starting from the date of diagnosis recorded on NCDR; the equivalent data were obtained from ENCORE for those patients not found on NCDR. One year, 2 year and 2 year conditional on 1 year relative survival rates were calculated for trial and non-trial patients aged 15–24, 15–19 and 20–24 years. Relative survival was estimated from life tables stratified by age, sex and time using the Stata strs programme.22 Expected survival was estimated using the Ederer II method.
Differences in relative survival were modelled using a multiple regression approach based on generalised linear models, assuming a Poisson distribution for the observed number of deaths.23 Differences were considered statistically significant if two-sided p values were <0.05. All statistical analyses were conducted using Stata V.13.
The numbers of deaths in those patients included in the survival analysis were determined for each 3-month period during the 2 years of follow-up and percentages calculated based on the number of patients alive at the beginning of each 3-month period. p Values were calculated using the two-tailed z-test for difference between proportions. The 95% CIs for percentage deaths were derived using the binomial distribution based on the Wilson Score method.24
Completion of TYAC form
During 2009 and 2010, enhanced cancer registration forms were in use throughout the English TYA Principal Treatment Centres (PTCs). The forms were developed by the professional organisation Teenagers and Young Adults with Cancer (TYAC) (http://www.tyac.org.uk) and were collected and matched with registry data by the lead UK regional registry for TYA cancer at the time, the North West Cancer Intelligence Service (now part of Public Health England (PHE)). Receipt of a TYAC form by the registry was used as a proxy that the patient’s management had been provided by or at least discussed with the regional TYA PTC. We calculated the percentage of patients in the trial for whom a TYAC form was completed to explore the relationship between access to TYA specialist services and participation in the trial.
Results
Study population
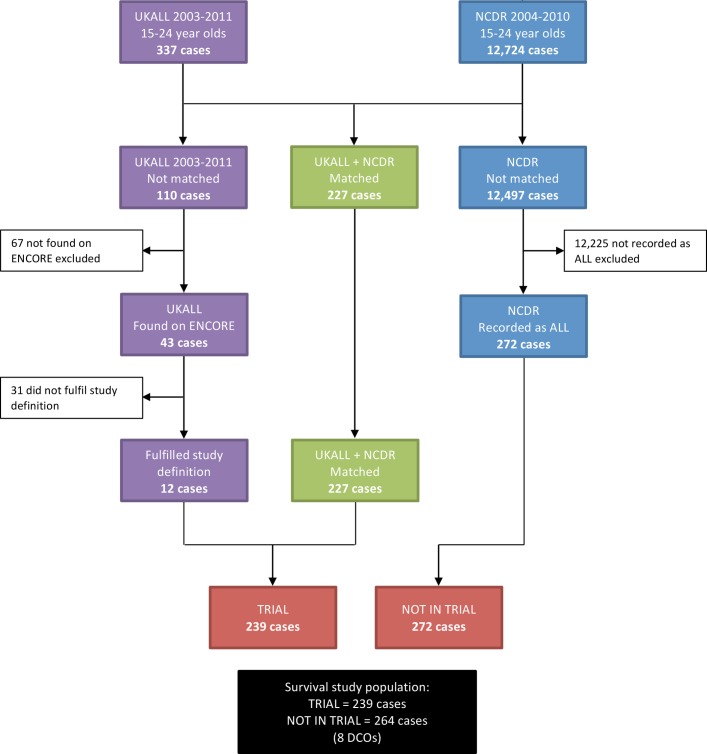
The process by which the study population was selected is shown in figure 1. Details on 227 of the 337 patients on the UKALL database were found on NCDR; 20 of whom had a diagnosis other than ALL on NCDR. The most common diagnoses were leukaemia (5), acute leukaemia (4), acute myeloid leukaemia (4) and non-Hodgkin’s lymphoma (4). Two hundred and seventy-two patients with a diagnosis of ALL on NCDR were not on the UKALL database and these constituted the non–trial arm of this study.
Figure 1.
Study population derived from matching UKALL2003 against the National Cancer Data Repository (NCDR) and English National Cancer Online Registration Environment (ENCORE).
Of the 110 patients on UKALL2003 not found on NCDR, 43 were identified on ENCORE. Thirty-one did not fulfil the study definition for the following reasons:
Twenty-six were diagnosed in 2011.
One diagnosed in 2003.
One aged 14 at time of diagnosis.
One treated in England but not a resident.
Two were provisional registrations that were not confirmed.
The 12 patients who fulfilled the study definition had been reported to the NCRAS after the NCDR was compiled. These patients were added to the 227 found on both the UKALL2003 and NCDR databases to make up the trial arm of this study.
Of the 67 patients on the UKALL2003 database not found on ENCORE, 60 were not resident in England confirmed by having a non-English address on NSTS and/or the treating hospital recorded on UKALL2003 as being outside England. The remaining seven patients, who did fulfil the study definition were excluded for a number of reasons, including not being reported to the registry by the treating hospital or insufficient details provided to allow a full registration and inaccurate personal details on the UKALL2003 database resulting in patients not being found on ENCORE.
Participation in UKALL2003
A total of 511 patients aged 15–24 years were included in the analyses, of whom 239 (47.5%) had participated in UKALL2003 (table 1a). The overall number of patients aged 20–24 years recruited (36 patients) was lower than patients aged 18–19 years (54 patients) and 15–17 years (149 patients). In total, 203 (84.9%) of 239 trial patients were aged 15–19 years. Trial participation improved over time, from 59.3% in 2004–2007 to 76.7% in 2008–2010 (p=0.007) in the group aged 15–17 years (the only group eligible for the trial for the entire study period) (table 1b). It is likely that the sequential changes in age eligibility criteria for UKALL2003 were partly responsible for low recruitment numbers in the older age groups. However, for the period 2008–2010, during which all 15–24 year olds were eligible for the study, participation remained higher in younger patients: 76.3% of 15–19 year olds compared with 45.1% of 20–24 year olds (p<0.001).
Table 1a.
Participation in UKALL2003 by age group an d year of diagnosis (A) All patients
| Year of diagnosis | 15–17 years | 18–19 years | 20–24 years | 15–24 years | ||||||||
| Proportion | Percentage | 95% CI | Proportion | Percentage | 95% CI | Proportion | Percentage | 95% CI | Proportion | Percentage | 95% CI | |
| 2004 | 19/34 | 55.9 | 39.5 to 71.1 | 0/6 | 0.0 | 0.0 to 39.0 | 0/25 | 0.0 | 0 to 13.3 | 19/65 | 29.2 | 19.6 to 41.2 |
| 2005 | 26/45 | 57.8 | 43.3 to 71.0 | 0/20 | 0.0 | 0 to 16.1 | 0/23 | 0.0 | 0 to 14.3 | 26/88 | 29.5 | 21.0 to 39.8 |
| 2006 | 18/29 | 62.1 | 44.0 to 77.3 | 9/26 | 34.6 | 19.4 to 53.8 | 0/19 | 0.0 | 0 to 29.9 | 27/74 | 36.5 | 26.4 to 47.9 |
| 2007 | 20/32 | 62.5 | 45.3 to 77.1 | 8/14 | 57.1 | 32.6 to 78.6 | 4/32 | 12.5 | 5.0 to 28.1 | 32/78 | 41.0 | 30.8 to 52.1 |
| 2008 | 23/30 | 76.7 | 59.1 to 88.2 | 16/18 | 88.9 | 67.2 to 96.9 | 8/23 | 34.8 | 18.8 to 55.1 | 47/71 | 66.2 | 54.6 to 76.1 |
| 2009 | 24/31 | 77.4 | 60.2 to 88.6 | 13/20 | 65.0 | 43.3 to 81.9 | 10/26 | 38.5 | 22.4 to 57.5 | 47/77 | 61.0 | 49.9 to 71.2 |
| 2010 | 19/25 | 76.0 | 56.6 to 88.5 | 8/11 | 72.7 | 43.4 to 90.3 | 14/22 | 63.6 | 43.0 to 80.3 | 41/58 | 70.7 | 58.0 to 80.8 |
| 2004–2007 | 83/140 | 59.3 | 51.0 to 67.1 | 17/66 | 25.8 | 16.7 to 37.4 | 4/99 | 4.0 | 1.6 to 9.9 | 104/305 | 34.1 | 29.0 to 39.6 |
| 2008–2010 | 66/86 | 76.7 | 66.8 to 84.4 | 37/49 | 75.5 | 61.9 to 85.4 | 32/71 | 45.1 | 34.0 to 56.6 | 135/206 | 45.1 | 34.0 to 56.6 |
| 2004–2010 | 149/226 | 65.9 | 59.5 to 71.8 | 54/115 | 47.0 | 38.1 to 56.0 | 36/170 | 21.2 | 15.7 to 27.9 | 239/511 | 46.8 | 42.5 to 51.1 |
Recruitment to other clinical trials
Since the trial consent forms for ESPHALL and UKALLXII did not include explicit consent for data sharing with the national registries, it was not possible to access detailed information from these trial databases. However, the overall number of UK patients recruited to these trials within the study period included three patients aged 15–17 years recruited to ESPHALL and 100 patients aged 15–24 years recruited to UKALL XII. Although ESPHALL recruited only those with Philadelphia chromosome positive disease, UKALL XII recruited both Philadelphia chromosome positive and negative patients with ALL. Given that the lower age limit for UKALL XII for Philadelphia chromosome negative ALL was sequentially increased over time, the highest proportion of patients recruited to UKALL XII would be those with Philadelphia chromosome negative disease in the older age group.
Survival
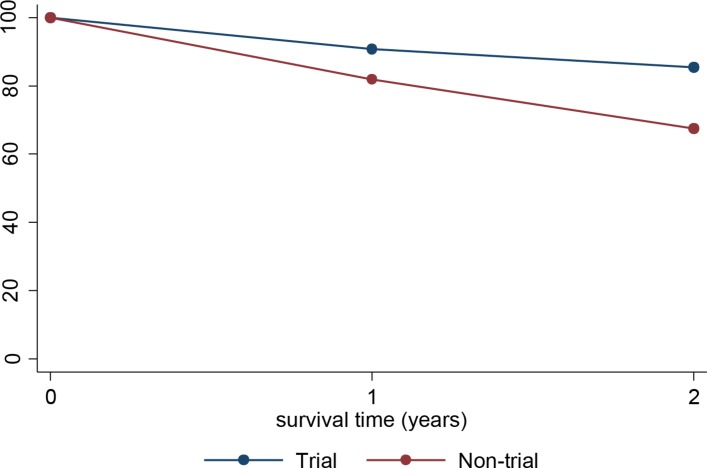
Five hundred and three patients were included in the survival analysis: 239 in the trial arm and 264 in the non-trial arm. Patients aged 15–24 years recruited to the UKALL2003 trial had 17.9% better 2-year survival (85.4% vs 67.5%, p<0.001), 8.9% better 1-year survival (90.8% vs 81.9%, p=0.004) and 11.6% better 2-year survival conditional on 1-year survival (94.1% vs 82.5%, p=0.001) compared with non-trial patients (table 2, figure 2). To determine whether the effects of trial recruitment on survival were measurable across the age cohort, we analysed 15–19 year olds and 20–24 year olds separately. The differences in survival at all time points remained significant in the 15–19 year old age group (table 2). Two-year and conditional 2-year survival showed a non-significant trend to better in trial patients than non-trial patients in the 20–24 year old cohort. The results for 2008–2010 (after closure of UKALLXII) are similar to those for the whole study period: 20–24 year olds in the trial had a 2-year survival 10.2% better than those not in the trial, but this did not reach statistical significance p=0.393.
Table 1b.
Trial participation over time in 15–17 year olds
| Year of diagnosis | Proportion | Percentage | 95% CI | p Value |
| 2004–2007 | 83/140 | 59.3 | 51.0 to 67.1 | 0.007 |
| 2008–2010 | 66/86 | 76.7 | 66.8 to 84.4 |
Figure 2.
Two-year relative survival by age group and trial status.
To determine whether the deaths were evenly spaced after diagnosis we next analysed the number of deaths in trial and non-trial patients by quarter-year from diagnosis. Table 3 shows the number and percentage of deaths by trial status for each quarter-year and year of follow-up. A total of 35 (14.6%) trial patients died in the 2 years following diagnosis compared with 86 (32.6%) of non-trial patients (p<0.001). The difference in the proportion of deaths between trial and non-trial patients was 9% (18.2%–9.2%; p=0.004); fewer deaths in trial patients during the first year and 11.6% (17.6%–6.0%; p<0.001) during the second. However, the largest number of deaths overall and the largest difference between trial and non-trial patients for any quarter was in the first 3 months after diagnosis: 6 (2.5%) trial patients died compared with 21 (8.0%) non-trial patients (p=0.007).
Table 2.
One and 2-year survival by age group and trial status
| Trial status | Age group | Number of patients | Deaths | Survival (%) | 95% CI | p Value |
| One-year survival | ||||||
| Trial | 15–19 | 203 | 17 | 91.7 | 86.9 to 94.7 | <0.001 |
| Non-trial | 134 | 30 | 77.6 | 69.6 to 83.8 | ||
| Trial | 20–24 | 36 | 5 | 86.2 | 69.8 to 94.0 | 0.969 |
| Non-trial | 130 | 18 | 86.2 | 79.0 to 91.1 | ||
| Trial | 15–24 | 239 | 22 | 90.8 | 86.4 to 93.9 | 0.004 |
| Non-trial | 264 | 48 | 81.9 | 76.6 to 86.0 | ||
| 2 year conditional on 1-year survival | ||||||
| Trial | 15–19 | 186 | 10 | 94.7 | 90.3 to 97.1 | 0.008 |
| Non-trial | 104 | 16 | 84.7 | 76.2 to 90.4 | ||
| Trial | 20–24 | 31 | 3 | 90.4 | 73.0 to 96.9 | 0.236 |
| Non-trial | 112 | 22 | 80.4 | 71.8 to 86.7 | ||
| Trial | 15–24 | 217 | 13 | 94.1 | 90.0 to 96.6 | 0.001 |
| Non-trial | 216 | 38 | 82.5 | 76.7 to 87.0 | ||
| Two-year survival | ||||||
| Trial | 15–19 | 203 | 27 | 86.8 | 81.3 to 90.7 | <0.001 |
| Non-trial | 134 | 46 | 65.7 | 57.0 to 73.1 | ||
| Trial | 20–24 | 36 | 8 | 77.9 | 60.5 to 88.3 | 0.381 |
| Non-trial | 130 | 40 | 69.3 | 60.6 to 76.5 | ||
| Trial | 15–24 | 239 | 35 | 85.4 | 80.3 to 89.3 | <0.001 |
| Non-trial | 264 | 86 | 67.5 | 61.5 to 72.8 | ||
Exploratory analysis of trial recruitment by place of care
The data available in national registry records were not sufficient to allow us to determine the reasons for non-recruitment of non-trial patients, how non-trial patients were treated or the training background of treating physicians (paediatric, TYA or adult). However, we were able to compare trial recruitment rates between patients for whom an enhanced TYAC cancer registration form had been submitted and those with no TYAC form. The trial recruitment rates were significantly different between the two groups: 73.5% of patients with a submitted TYAC form were recruited compared with 51.9% of those for whom a form was not submitted (p=0.001, table 4). The difference was highest among patients aged 20–24 years (61.5% trial recruitment among patients with associated TYAC forms versus 36.4% without). Patients for whom a TYAC form was submitted had a 2-year survival of 80.8% compared with 82.8% for those without a form (p=0.771).
Table 3.
Number and percentage of patients aged 15–24 who died in the 2 years following diagnosis, by 3-month period, year and trial status
| Year | Quarter | In trial | Not in trial | p Value | ||||||
| Number of patients at start of period | Number of deaths | % Died | 95% CI | Number of patients at start of period | Number of deaths | % Died | 95% CI | |||
| First | 239 | 6 | 2.5 | 1.2 to 5.4 | 264 | 21 | 8.0 | 5.3 to 11.9 | 0.007 | |
| Second | 233 | 4 | 1.7 | 0.7 to 4.3 | 243 | 5 | 2.1 | 0.9 to 4.7 | 0.785 | |
| Third | 229 | 5 | 2.2 | 0.9 to 5.0 | 238 | 11 | 4.6 | 2.6 to 8.1 | 0.148 | |
| Fourth | 224 | 7 | 3.1 | 1.5 to 6.3 | 227 | 11 | 4.8 | 2.7 to 8.5 | 0.351 | |
| First | – | 239 | 22 | 9.2 | 6.2 to 13.5 | 264 | 48 | 18.2 | 14.0 to 23.3 | 0.004 |
| Fifth | 217 | 2 | 0.9 | 0.3 to 3.3 | 216 | 11 | 5.1 | 2.9 to 8.9 | 0.011 | |
| Sixth | 215 | 4 | 1.9 | 0.7 to 4.7 | 205 | 10 | 4.9 | 2.7 to 8.7 | 0.0851 | |
| Seventh | 211 | 3 | 1.4 | 0.5 to 4.1 | 195 | 8 | 4.1 | 2.1 to 7.9 | 0.0965 | |
| Eighth | 208 | 4 | 1.9 | 0.8 to 4.8 | 187 | 9 | 4.8 | 2.6 to 8.9 | 0.108 | |
| Second | – | 217 | 13 | 6.0 | 3.5 to 10.0 | 216 | 38 | 17.6 | 13.1 to 23.2 | <0.001 |
| First and second | – | 239 | 35 | 14.6 | 10.7 to 19.7 | 264 | 86 | 32.6 | 27.2 to 38.4 | <0.001 |
Table 4.
Number and percentage of patients in trial by whether a TYAC form was received by age group in 2009–2010
| Age | Proportion of patients with a TYAC form who were in trial | Proportion of patients without a TYAC form who were in trial | p Value | ||||
| Proportion | Percentage | 95% CI | Proportion | Percentage | 95% CI | ||
| 15–19 | 45/57 | 78.9 | 66.7 to 87.5 | 19/30 | 63.3 | 45.5 to 78.1 | 0.117 |
| 20–24 | 16/26 | 61.5 | 42.5 to 77.6 | 8/22 | 36.4 | 19.7 to 57.0 | 0.082 |
| 15–24 | 61/83 | 73.5 | 63.1 to 81.8 | 27/52 | 51.9 | 38.7 to 64.9 | 0.011 |
Discussion
By combining trial and national cancer registry data, we have demonstrated a significant survival advantage to participation in the most recently completed, large prospective clinical trial for TYA patients with ALL, which is measurable at a population level. This is the first study to have demonstrated such a survival advantage in any cancer type in the TYA age group. The difference in survival was highly significant, with a 17.9% superior survival at 2 years in trial patients compared with non-trial patients. The risk of mortality at 2 years in those treated outside of the trial was twice that of those on UKALL2003 (32.6% compared with 14.6%, p<0.001) and was most striking during the first 3 months after diagnosis (8.0% compared with 2.5%, p=0.007).
This retrospective analysis of the impact of clinical trial recruitment on survival was possible because the consent process for UKALL2003 included explicit consent for the trial data to be shared with regional and national cancer registries and because the UK has full population coverage for cancer registration. This study, however, did show a number of limitations of the cancer registration system for England during the period 2004–2010 including (1) accuracy of diagnosis: 20 patients on UKALL2003 with a diagnosis other than ALL on NCDR and (2) timeliness: 12 patients on ENCORE but not on NCDR. In addition, the main source of diagnostic data for cancer registries during the study period was histopathology laboratories, leading to difficulties in obtaining high quality data on patients with ALL. There were no data available for the well-established prognostic variables in ALL, thus precluding comparison of the frequency of different risk groups between those treated on or off trial. Data were collected by regional registries and then compiled into a national database, which took considerable time. PHE has made many improvements since taking over the NCRAS a few years ago including (1) Moving to a single, completely integrated national registry, (2) setting up systems that report in real time from a wide variety of sources, such as MDTs, leading to more timely and accurate data, (3) reviewing access to specialist haematological diagnostic datasets to improve the data quality of haematological cancers and (4) producing an analysis database that is constantly kept up to date.
We also recognise a number of potential confounding variables in this study. Potential confounders which could increase the observed difference between those on trial compared with those treated off trial include centre effect, selection bias and use of a superior protocol and those reducing the difference include recruitment to other ALL clinical trials.
Any potential benefit to participation of young people in clinical trials may be derived from the treating centre rather than trial participation itself (centre effect). Centres offering clinical trials are often larger, academic institutions, seeing a higher number of patients with a specific disease, a larger clinical research infrastructure and a more resourced workforce, all of which may contribute to a more favourable outcome.16 Over the last 10 years, there has been a gradual reconfiguration of services providing care for young people aged 16–24 years with cancer in the UK; this includes the development of Principal Treatment Centres, designation of other hospitals offering TYA services closer to the patients’ homes and an overview of the holistic care of all patients provided by the TYA multidisciplinary team (MDT). Between 2004 and 2010, these pathways were not fully established, but the submission of TYAC enhanced cancer registration forms have been used as a surrogate indicator that an individual patient received treatment according to regionally agreed and commissioned TYA pathways under the supervision of the TYA PTC. It is therefore interesting to note that a significantly higher proportion of patients recruited onto UKALL2003 had been registered centrally via the TYAC notification system than non-trial patients (73.5% with a TYAC form compared with 51.9% without, p=0.001). This was particularly marked in the older age group. However, the finding that 2-year survival was not higher in those patients with a submitted TYAC form does not support the argument that the better survival for those in the trial was mainly due to having been managed at larger centres.
While selection bias, in which the highest risk patients (eg, those with a very high white count, renal or hepatic impairment or large mediastinal mass at presentation) could not participate in the trial, may be a confounder it is unlikely to have had a significant impact on our results as (1) UKALL2003 was a population-based trial for which all children and young people with ALL were eligible, irrespective of severity or risk group at presentation and (2) it also permitted recruitment within 7 days of commencement of chemotherapy. Patients with Philadelphia chromosome positive (Ph +ve) ALL were ineligible for UKALL2003, but Ph +ve patients were included in the non-trial arm, as NCDR did not include details of Ph chromosome status. The presence of the Philadelphia chromosome in ALL confers a poorer prognosis25 and could therefore increase the survival difference observed in this study. However, we feel that this is unlikely to have been a significant confounding variable since the prevalence of Ph +ve ALL in UKALL2003 overall was only 1.8% and the observed difference between groups was non-significant in the older patients despite the frequency of the Philadelphia chromosome increasing with increasing age.
During the study period, TYA patients with Ph +ve disease were eligible for the ESPHALL or UKALLXII (until 2006) trials, depending on age, and older patients with Ph −ve disease were eligible for recruitment onto UKALLXII (lower age limit 18th birthday from 2004, 20th birthday from 2006 and 25th birthday from 2007). While we do not know the proportions of non-UKALL2003 patients recruited to these trials, if trial participation in itself confers a survival advantage, recruitment of patients to these other clinical trials would be expected to reduce observed differences between our two study groups, reduce any residual confounding from the exclusion of Ph +ve patients from UKALL2003 and may have also contributed to the non-significant difference in survival between trial and non-trial patients aged 20–24 years. This is further supported by the observation that only three UK patients aged 15–17 years were recruited to ESPHALL (Ph +ve ALL only), but 100 patients aged 16–24 years were recruited to UKALLXII (Ph +ve and –ve ALL). The relative contribution of treatment protocol on outcome differences was impossible to assess in this study, since the registry dataset did not include which specific regimen was used.
Recruitment to clinical trials in TYA patients with cancer is poor due to a range of factors including lack of an available trial, heterogenous referral pathways, differences in treating centres as well as lack of desire to participate by this age group.4 9 13 26 Overall, approximately 50% of TYA patients in England with ALL participated in UKALL2003. Although this figure is lower than the proportion of younger children who participated in the trial, it compares favourably to other cancer trials reported in this age group; in the UK between 2005 and 2010, only 850 of 2860 patients aged 15–19 years (30%)and 562 of 4011 patients aged 20–24 years (14%) were recruited to a clinical trial.4 Encouragingly, recruitment to UKALL2003 improved over time. In part, this was expected in the group aged 18–24 years, given the sequential increase in age eligibility criteria over time. However, even in the patients aged 15–17 years who were eligible for participation throughout the study period, participation increased from 59.3% in 2004–2007 to 76.7% in 2008–2010 (p=0.007). Despite this, there were still a substantial number of young people who did not participate, particularly among 20–24 year olds, where participation was only 45.1%. Fern et al propose that there are five factors (five ‘A’s’) important in improving TYA cancer trial recruitment; available, accessible, aware, appropriate and acceptable. The relatively high level of recruitment of TYA patients with ALL to UKALL2003 and its improvement over time probably reflects an increasing awareness (as a result of newsletters and the trial coordinators presenting at a series of national meetings) and acceptability among paediatric and adult haematologists that UKALL2003 offered an effective (ie, appropriate) treatment strategy, with increasing availability and accessibility as the age range was increased and a greater number of adult centres opened the trial.
In conclusion, we report a specific survival advantage to participation in the UKALL2003 trial for TYA patients with ALL. This is the first study to have demonstrated a survival advantage of trial participation in TYA patients with any cancer and suggests a benefit to an individual patient in taking part. These data highlight the importance of national and international efforts to (1) improve recruitment of TYA patients with cancer to clinical trials, (2) configure TYA cancer services to provide young people with access to appropriate trials and the opportunity to participate and (3) the need to continue to collect accurate treatment and outcome data at a population level to allow evaluation of treatment in patients who have not accessed clinical trials. In England, these duties are the responsibilities, respectively, of the National Cancer Research Institute, NHS England and PHE, working together towards the goal of best patient outcomes. The data we present suggest that significant improvements in the delivery of appropriate cancer treatment are still much needed for this vulnerable young population.
Supplementary Material
Acknowledgments
We would like to thank the patients, families and clinicians who participated in UKALL2003. Also thanks to the r
egional and institutional data managers.
Footnotes
Contributors: The study was designed by RH, SS, MK, AM, RF, CS and MMC. RH, CR and AV designed, recruited and analysed data for the UKALL2003 study. The statistical analysis of this study was performed by SS, MK, AM and MMC. All authors contributed to the interpretation of data and preparation of the manuscript and approve this final version.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: There are no additional unpublished data from this study.
References
- 1. O’Hara C, Moran A, Whelan JS, et al. . Trends in survival for teenagers and young adults with cancer in the UK 1992-2006. Eur J Cancer 2015;51:2039–48. 10.1016/j.ejca.2015.06.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meadows AT. Pediatric cancer survivors: past history and future challenges. Curr Probl Cancer 2003;27:112–26. 10.1016/S0147-0272(03)00025-4 [DOI] [PubMed] [Google Scholar]
- 3. Barr RD. Adolescents, young adults, and cancer – the international challenge. Cancer 2011;117:2245–9. 10.1002/cncr.26052 [DOI] [PubMed] [Google Scholar]
- 4. Fern LA, Lewandowski JA, Coxon KM, et al. . Available, accessible, aware, appropriate, and acceptable: a strategy to improve participation of teenagers and young adults in cancer trials. Lancet Oncol 2014;15:e341–e350. 10.1016/S1470-2045(14)70113-5 [DOI] [PubMed] [Google Scholar]
- 5. Burke ME, Albritton K, Marina N. Challenges in the recruitment of adolescents and young adults to cancer clinical trials. Cancer 2007;110:2385–93. 10.1002/cncr.23060 [DOI] [PubMed] [Google Scholar]
- 6. Bleyer WA, Tejeda H, Murphy SB, et al. . National cancer clinical trials: children have equal access; adolescents do not. J Adolesc Health 1997;21:366–73. 10.1016/S1054-139X(97)00110-9 [DOI] [PubMed] [Google Scholar]
- 7. Krailo MD, Bernstein L, Sullivan-Halley J, et al. . Patterns of enrollment on cooperative group studies. An analysis of trends from the Los Angeles County Cancer Surveillance Program. Cancer 1993;71:3325–30. [DOI] [PubMed] [Google Scholar]
- 8. Fern L, Davies S, Eden T, et al. . Rates of inclusion of teenagers and young adults in England into National Cancer Research Network clinical trials: report from the National Cancer Research Institute (NCRI) Teenage and Young Adult Clinical Studies Development Group. Br J Cancer 2008;99:1967–74. 10.1038/sj.bjc.6604751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tai E, Buchanan N, Westervelt L, et al. . Treatment setting, clinical trial enrollment, and subsequent outcomes among adolescents with cancer: a literature review. Pediatrics 2014;133:S91–S97. 10.1542/peds.2014-0122C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parsons HM, Harlan LC, Seibel NL, et al. . Clinical trial participation and time to treatment among adolescents and young adults with cancer: does age at diagnosis or insurance make a difference? J Clin Oncol 2011;29:4045–53. 10.1200/JCO.2011.36.2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Downs-Canner S, Shaw PH. A comparison of clinical trial enrollment between adolescent and young adult (AYA) oncology patients treated at affiliated adult and pediatric oncology centers. J Pediatr Hematol Oncol 2009;31:927–9. 10.1097/MPH.0b013e3181b91180 [DOI] [PubMed] [Google Scholar]
- 12. Mitchell AE, Scarcella DL, Rigutto GL, et al. . Cancer in adolescents and young adults: treatment and outcome in Victoria. Med J Aust 2004;180:59–62. [DOI] [PubMed] [Google Scholar]
- 13. Grigsby TJ, Kent EE, Montoya MJ, et al. . Attitudes toward cancer clinical trial participation in young adults with a history of cancer and a healthy college student sample: a preliminary investigation. J Adolesc Young Adult Oncol 2014;3:20–7. 10.1089/jayao.2013.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shaw PH, Ritchey AK. Different rates of clinical trial enrollment between adolescents and young adults aged 15 to 22 years old and children under 15 years old with cancer at a children’s hospital. J Pediatr Hematol Oncol 2007;29:811–4. 10.1097/MPH.0b013e31815814f3 [DOI] [PubMed] [Google Scholar]
- 15. Stiller CA, Eatock EM. Patterns of care and survival for children with acute lymphoblastic leukaemia diagnosed between 1980 and 1994. Arch Dis Child 1999;81:202–8. 10.1136/adc.81.3.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferrari A, Bleyer A. Participation of adolescents with cancer in clinical trials. Cancer Treat Rev 2007;33:603–8. 10.1016/j.ctrv.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 17. Kumar A, Soares H, Wells R, et al. . Are experimental treatments for cancer in children superior to established treatments? Observational study of randomised controlled trials by the Children’s Oncology Group. BMJ 2005;331:1295 10.1136/bmj.38628.561123.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peppercorn JM, Weeks JC, Cook EF, et al. . Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet 2004;363:263–70. 10.1016/S0140-6736(03)15383-4 [DOI] [PubMed] [Google Scholar]
- 19. Vora A, Goulden N, Mitchell C, et al. . Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol 2014;15:809–18. 10.1016/S1470-2045(14)70243-8 [DOI] [PubMed] [Google Scholar]
- 20. Vora A, Goulden N, Wade R, et al. . Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol 2013;14:199–209. 10.1016/S1470-2045(12)70600-9 [DOI] [PubMed] [Google Scholar]
- 21. Hough R, Rowntree C, Goulden N, et al. . Efficacy and toxicity of a paediatric protocol in teenagers and young adults with Philadelphia chromosome negative acute lymphoblastic leukaemia: results from UKALL 2003. Br J Haematol 2016;172:439–51. 10.1111/bjh.13847 [DOI] [PubMed] [Google Scholar]
- 22. Dickman PW, Lambert PC, Coviello E, et al. . Estimating net survival in population-based cancer studies. Int J Cancer 2013;133:519–21. 10.1002/ijc.28041 [DOI] [PubMed] [Google Scholar]
- 23. Dickman PW, Sloggett A, Hills M, et al. . Regression models for relative survival. Stat Med 2004;23:51–64. 10.1002/sim.1597 [DOI] [PubMed] [Google Scholar]
- 24. Observatories APHO 1. APHO. Technical Briefing 3, Commonly Used Public Health Statistics and their Confidence Intervals. 2008.
- 25. Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med 2006;354:166–78. 10.1056/NEJMra052603 [DOI] [PubMed] [Google Scholar]
- 26. Hendricks-Ferguson VL, Cherven BO, Burns DS, et al. . Recruitment strategies and rates of a multi-site behavioral intervention for adolescents and young adults with cancer. J Pediatr Health Care 2013;27:434–42. 10.1016/j.pedhc.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.