Abstract
Purpose of review
The review summarizes recent epidemiological studies that examined the relationship between osteoporosis and sarcopenia to assess the impact of vitamin D status or supplementation on health outcomes related to these two medical conditions.
Recent findings
Osteoporosis and sarcopenia are major public health problems, but whether these two diseases should be considered alone or combined into a single condition is not clear. No consensual definition of osteosarcopenia is largely accepted. Most observational studies demonstrate some relationship between muscle and bone health. Vitamin D status is generally lower in study participants with bone or muscle wasting. Studies on the effects of vitamin D supplementation on muscle or bone health have provided conflicting results, likely because of the heterogeneity between studies. However, the most positive results were observed in study participants with low vitamin D status and in studies that avoided massive boluses of vitamin D.
Summary
More observational and interventional studies are needed to confirm the exact role of vitamin D in the pathophysiology and treatment of osteosarcopenia.
Keywords: 25-hydroxyvitamin D, osteoporosis, osteosarcopenia, vitamin D
INTRODUCTION
Bone and muscle are integrated organs with shared critical functions in structure, strength, and motion. Aging is accompanied by changes in body composition, such as a decrease in bone and muscle mass. Loss of bone and muscle with advancing age is a huge threat to independence in later life [1]. The two main diseases related to bone and muscle wasting are osteoporosis and sarcopenia, respectively. These diseases occur in relatively similar populations, and growing evidence from preclinical and clinical research supports a link between the conditions. This association has led to the concept of the bone–muscle unit. The key musculoskeletal pathways involved in the regulation of the bone–muscle unit were reviewed recently and were mainly focused on biomechanical loading, endocrine pathways, and activating receptor signalling [2▪▪]. Other recent reviews highlighted some in-vitro evidence of the bone–muscle interaction [3,4▪].
Vitamin D is a fat-soluble vitamin that plays a most important role in calcium and bone metabolism. Vitamin D metabolism is coordinated by the skin, the liver, and the kidney. Vitamin D is activated into 25-hydroxyvitamin D [25(OH)D] in the liver and then converted into 1,25-dihydroxyvitamin D, the active form of vitamin D, in the kidney. Changes in calcium absorption associated with vitamin D deficiency affect muscle and bone mass [5]. The age-related decline in vitamin D receptor expression and 1,25(OH)D activity affects proinflammatory cytokines in the skeletal muscle, and vitamin D deficiency enhances bone marrow adipogenesis and intramuscular adipose tissue, which reduces functionality in skeletal tissues [6]. In the muscle tissue, a regulatory effect of vitamin D on calcium flux, mineral homeostasis, and signalling pathways controlling protein anabolism has been reported [6].
The current review discusses and critically reviews the most recent epidemiological studies of osteosarcopenia and the potential impact of vitamin D on its pathogenesis and management. We include observational (i.e. cohort or case–control) and interventional (i.e. randomized and controlled) studies.
Box 1.
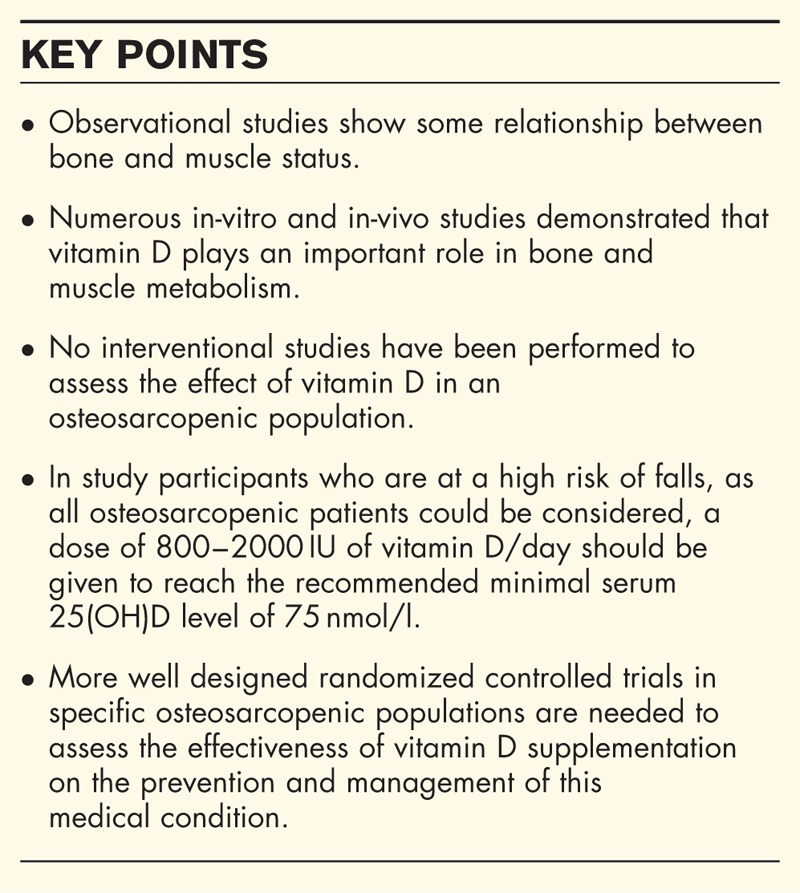
no caption available
OSTEOPOROSIS, SARCOPENIA, OR OSTEOSARCOPENIA?
Osteoporosis and sarcopenia are major public health problems [7–9,10▪]. Osteoporosis is defined as a systemic skeletal disease that is characterized by low bone mass and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture. Osteoporotic fractures are a major cause of morbidity. These injuries are associated with increased mortality and generate costs in excess of 37 billion Euros for patient management in Europe [11]. Sarcopenia is operationally defined as the loss of muscle mass and muscle function, and a recent meta-analysis-associated sarcopenia with a higher rate of mortality [pooled odds ratio (OR) of 3.596 (95% (confidence interval) CI 2.96–4.37)] and functional decline [pooled OR of six studies 3.03 (95% CI 1.80–5.12)] [12]. A higher rate of falls and a higher incidence of hospitalizations were also noted. Recent simulations estimated that the number of individuals with sarcopenia will rise from 19 740 527 in 2016 to 32 338 990 in 2045 (a 63.8% increase), which corresponds to the overall prevalence rates in the elderly of 20.2 and 22.3% for 2016 and 2045, respectively [13]. The impact of both diseases on quality of life is substantial [14,15].
Whether osteoporosis and sarcopenia should be considered alone or combined into a single condition is not clear. Combining the loss of bone mass and muscle strength into a single diagnosis called ‘sarcoosteopenia’ was previously suggested based on the abundant literature documenting the positive relationship of muscle and bone mass, which is partially because of muscle-induced skeletal strain [16]. Other terms, such as sarcoosteoporosis or osteosarcopenia, have also been suggested, but no consensus has been reached. Anyway, the operational definition of the combination of osteoporosis and sarcopenia is not clear, which resulted in heterogeneity in all epidemiological-related studies.
RELATIONSHIP BETWEEN OSTEOPOROSIS AND SARCOPENIA
Muscle–bone interactions have been exhaustively investigated in preclinical and clinical studies over the last decade. However, recent data are of particular interest to better understand the relationship between bone and muscle health. Some of these studies characterized the phenotype of osteosarcopenic patients [17,18]. The first study examined a cohort of 680 study participants from Australia and demonstrated that osteosarcopenic patients (defined as low bone mineral density, low muscle strength, and low muscle mass) were older and mostly women, were at a high risk for depression and malnutrition, had a BMI lower than 25, and exhibited a higher prevalence of peptic disease, inflammatory arthritis, maternal hip fracture, history of atraumatic fracture, and impaired mobility [17]. The second study was a small cross-sectional study of 68 prefrail German adults that reported that osteosarcopenic participants (defined as low bone mineral density and low muscle mass) exhibited significantly reduced hand grip strength, increased chair rising and sit-to-stand power times, and significantly increased bone turnover markers [18].
Other studies assessed the interconnection between muscle and bone wasting in cross-sectional studies. The skeletal muscle mass index in 216 Japanese women with recent vertebral fracture was lower, and the prevalence of sarcopenia was higher than that of 1608 controls, even after adjusting for age, which suggests that sarcopenia is a risk factor for vertebral fractures [19]. Another study of 17 891 study participants of African-American, Whites, and Chinese ethnicities demonstrated that study participants with sarcopenia (defined by a low muscle mass) were two times more likely to have osteopenia/osteoporosis compared with normal study participants (OR = 2.04; 95% CI = 1.61, 2.60) [20]. Similarly, study participants with sarcopenia (low muscle mass and low grip strength) were approximately two times more likely to exhibit osteopenia/osteoporosis than were normal study participants (OR = 1.87; 95% CI = 1.09, 3.20). These results were consistent with the preliminary data from the SarcoPhAge study, which demonstrated more osteoporotic (based on bone mineral density assessment) women among sarcopenic study participants (based on muscle mass and muscle strength or physical function) than among nonsarcopenic study participants [21]. A significantly lower appendicular lean mass index was also observed in osteoporotic women compared with nonosteoporotic women (P = 0.025). Lower muscle strength was also noted in osteoporotic study participants (P = 0.023).
Few perspective studies have been published recently. One study followed a cohort of 750 women aged 50–94 years for one decade [22▪]. By the end of follow-up, there were 190 deaths. After controlling for potential confounding variables, low bone mineral density was significantly associated with a higher risk of mortality, and the low appendicular lean mass had borderline significance. Another study that followed 1099 study participants aged over 60 years found that osteoporosis (based on bone mineral density value) was a significant predictive factor for sarcopenia occurrence (defined as low muscle mass and low muscle strength or low physical performance) in the next 4 years [23▪▪]. The associated risk, expressed as the OR, was 2.99 (95% CI 1.46–6.12; P = 0.003).
These recent results confirm an interaction between muscle and bone. However, caution must be kept in mind when interpreting these results because the definitions of muscle or bone wasting were highly heterogeneous between studies and the design of some studies did not capture the causality of the relationship.
POTENTIAL EFFECT OF VITAMIN D ON BONE AND MUSCLE HEALTH
There are many reasons to consider how vitamin D may have a potential beneficial effect on muscle and bone, as recently reviewed by various experts and scientific societies [6,24–28]. To the best of our knowledge, very few studies were designed to assess vitamin D status or the effects of vitamin D supplementation in osteosarcopenic patients. However, the interaction between muscle and bone led to the hypothesis that an improvement in one organ may be beneficial to the other. Therefore, it is important to review the most recent studies of the relationship between vitamin D and muscle or bone health.
Observational studies consistently demonstrate a lower level of 25(OH)D, which is the metabolite measured for evaluating the vitamin D status of an individual [29], in osteoporotic patients (defined by low bone mineral density or the presence of fracture) compared with nonosteoporotic study participants. Far fewer studies are available in sarcopenic study participants. However, three recent studies are of particular interest for muscle and bone issues. The first study was performed on US women over 65 years of age residing in long-term care and demonstrated that initially deficient women defined as 25(OH)D less than 50 nmol/l exhibited a greater decline in physical function at 12 and 24 months, a larger increase in cognitive deficits at 12 months and more falls compared with women sufficient in vitamin D at baseline, despite daily supplementation of 800 IU of vitamin D in all participants [30]. The second study investigated the relationship of serum 25(OH)D levels with physical activity and muscle fatigue in 85 healthy older adults aged 65 years or over [31]. The results demonstrated that the 25(OH)D levels were significantly and independently associated with self-reported muscle fatigue scores and some biomarkers considered by the authors as markers of muscle fatigue (e.g. troponin I and lactate dehydrogenase). The last study assessed the association between vitamin D status and the incidence of sarcopenia in 1705 men aged 70 years and over [32▪▪]. Men with 25(OH)D levels in the lowest quartiles (<40 nmol/l) exhibited significant associations with an increased odds of incident sarcopenia compared with men having 25(OH)D levels in the highest quartiles (>68.9 nmol/l) over 5 years with an OR of 2.53 (95% CI 1.14, 5.64) in an adjusted analysis. The results of these recent observational studies confirm the potential interaction between vitamin D and muscle and bone health.
The effects of vitamin D supplementation on fractures, falls, and muscle function were investigated in a large number of randomized controlled trials. Various meta-analyses summarized the effects observed in these trials. These meta-analyses show that vitamin D should be added to calcium supplementation to significantly reduce the incidence of fracture [33–35]. Even if the heterogeneity in doses of the vitamin D and calcium is high, it is generally accepted that at least 800 IU/day should be added to a minimum of 500 mg/day of calcium supplementation to have an effect on fractures. The results are globally similar for the effect on falls, but recent studies have clearly shown that very large doses of vitamin D (i.e. 300 000 or 500 000 IU) significantly increased the number of falls, thus making the interpretation of meta-analyses including these trials quite convoluted [36▪,37]. The two recent meta-analyses that assessed the effect of vitamin D supplementation on muscle strength provided conflicting results [38,39]. One meta-analysis focused on community-dwelling older persons and included 15 studies that demonstrated no improvement in muscle strength after the administration of vitamin D with or without calcium supplements [38]. The other study included 30 randomized controlled trials of 5615 individuals (no age restriction was included in this meta-analysis) and showed that vitamin D supplementation had a small positive impact on muscle strength but no effect on muscle mass or muscle power [39]. This meta-analysis was of particular interest because the sensitivity analyses showed, in sensitivity analyses, that supplementation of people who presented a 25(OH)D level less than 30 nmol/l resulted in a significantly greater improvement in muscle strength compared with people who presented a 25(OH)D level at least 30 nmol/l (P = 0.02). Higher effects were also found for people who demonstrated an increased 25(OH)D concentration of at least 25 nmol/l within the study duration. However, it should be pointed out that in these two meta-analyses, a huge heterogeneity was observed regarding vitamin D metabolites, doses, and mode of administration. Consequently, more well designed experimental studies are needed to confirm the exact place of vitamin D supplementation in the management of concomitant bone and muscle disorders.
More recent studies, which were not included in these meta-analyses, provided some interesting information. The first study in 130 sedentary men aged 65–90 years with 25(OH)D levels less than 75 nmol/l and Short Physical Performance Battery test (SPPB) scores of 9 or less showed that daily vitamin D (4000 IU) for 9 months did not improve the SPPB scores or gait speed [40▪]. However, the SPPB is only a surrogate marker of muscle function, and it lacks sensitivity for measuring clinical change making the interpretation of the results more convoluted. Another study demonstrated that daily 800 IU and twice weekly 20 000 IU of vitamin D had no effect on muscular strength, balance, or quality of life in 297 postmenopausal women with osteopenia or osteoporosis compared with daily 800 IU alone [41▪]. However, it should be pointed that most of the participants in this study exhibited adequate vitamin D status (i.e. over 50 nmol/l), and 30% exhibited baseline levels more than 75 nmol/l, which supports the results of previous meta-analyses that found no effect of vitamin D in study participants with vitamin D status over 30 nmol/l. Post hoc analyses of the PROVIDE study have showed results of particular interest [42▪]. The PROVIDE study randomized sarcopenic older adults to a vitamin D and leucine-enriched whey protein supplement (20 g whey protein, 3 g total leucine, 9 g carbohydrates, 3 g fat, 800 IU vitamin D, and a mixture of vitamins, minerals, and fibres, twice a day) or isocaloric control and showed that study participants who received the vitamin D and leucine-enriched whey protein medical nutrition drink gained more appendicular muscle mass and improved lower extremity function, as assessed using the chair stand test, compared with controls. The post hoc study demonstrated that participants with higher baseline 25(OH)D concentrations (>50 nmol/l) and dietary protein intake (>1.0 g/kg/day) had, independently of other determinants, greater gains in appendicular muscle mass, skeletal muscle index, and relative appendicular muscle mass in response to the nutritional intervention. The authors hypothesized that vitamin D could act synergistically with leucine and insulin to stimulate muscle protein synthesis, likely through sensitizing the anabolic pathways induced by insulin and leucine. They concluded that the current cutoffs in the recommendations for vitamin D (50 nmol/l) and protein intake (1.0–1.2 g/kg/day) should be considered the ‘minimum’ for adults with sarcopenia to respond adequately to nutritional strategies aimed at attenuating muscle loss.
Given the heterogeneity in the design of observational and interventional studies, there are still some controversies about the exact role of vitamin D supplementation in musculoskeletal health. However, experts generally agree that a minimum level of 50 nmol/l of 25(OH)D must be reached in the general elderly population and that 75 nmol/l should be the target in fragile study participants who are at elevated risk for falls and fractures [24,43]. A dose of 800–2000 IU of vitamin D/day should be given, under the supervision of a physician, to reach this last target.
CONCLUSION
More studies confirmed the interconnection between bone and muscle, which reemphasizes the importance of the bone–muscle unit. However, huge heterogeneity, primarily in the definition of muscle or bone disorders in the various published epidemiological studies, complicates the interpretation and clinical implications of these studies. Vitamin D plays a role in muscle and bone metabolism, and it has been widely investigated in observational and interventional studies. The conflicting results of these studies are primarily the result of the huge heterogeneity in study design. However, interventional studies performed using more physiological doses of vitamin D (i.e. avoiding very large, nonphysiological doses) or in study participants with low vitamin D status have provided more beneficial effects on muscle and bone health compared with studies that included more study participants with optimal vitamin D status.
Acknowledgements
None.
Financial support and sponsorship
O.B. received research grants and/or consulting fees from IBSA, Merck Sharp & Dohme, Novartis, Nutraveris, Pfizer, Rottapharm, Servier, SMB, Theramex, Bayer and Genevrier. E.C. received consulting and/or lecture fees from Amgen, SMB, IDS, DiaSorin, Abbott Diagnostics, Fujirebio, Roche Diagnostics, and Sanofi. J.Y.R. received research grants and/or consulting fees from Servier, Novartis, Negma, Lilly, Wyeth, Amgen, GlaxoSmithKline, Roche, Merckle, NycomedTakeda, NPS, IBSA-Genevrier, Theramex, UCB, Asahi Kasei, Endocyte, Merck Sharp and Dohme, Rottapharm, Teijin, Teva, Analis, NovoNordisk, Ebewee Pharma, Zodiac, Will Pharma, Meda, and Bristol Myers Squibb.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011; 305:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2▪▪.Laurent MR, Dubois V, Claessens F, et al. Muscle-bone interactions: from experimental models to the clinic? A critical update. Mol Cell Endocrinol 2016; 432:14–36. [DOI] [PubMed] [Google Scholar]; The study reviews key musculoskeletal molecular pathways involved in regulation of the bone–muscle unit.
- 3.Tagliaferri C, Wittrant Y, Davicco MJ, et al. Muscle and bone, two interconnected tissues. Ageing Res Rev 2015; 21:55–70. [DOI] [PubMed] [Google Scholar]
- 4▪.Reginster JY, Beaudart C, Buckinx F, Bruyere O. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care 2016; 19:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]; The article reviews recently published evidence for common pathways explaining bone and muscle wasting in normal ageing and pathological conditions.
- 5.Gunton JE, Girgis CM, Baldock PA, Lips P. Bone muscle interactions and vitamin D. Bone 2015; 80:89–94. [DOI] [PubMed] [Google Scholar]
- 6.Sanders KM, Scott D, Ebeling PR. Vitamin D deficiency and its role in muscle-bone interactions in the elderly. Curr Osteoporos Rep 2014; 12:74–81. [DOI] [PubMed] [Google Scholar]
- 7.Edwards MH, Dennison EM, Aihie Sayer A, et al. Osteoporosis and sarcopenia in older age. Bone 2015; 80:126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gielen E, Bergmann P, Bruyere O, et al. Osteoporosis in frail patients: a consensus paper of the Belgian bone club. Calcif Tissue Int 2017; 101:111–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strandberg TE, Michel JP, Maggi S. Healthy ageing requires a triple strategy. Aging Clin Exp Res 2016; 28:369–370. [DOI] [PubMed] [Google Scholar]
- 10▪.Beaudart C, Rizzoli R, Bruyere O, et al. Sarcopenia: burden and challenges for public health. Arch Public Health 2014; 72:45. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first systematic review assessing all potential health outcomes of sarcopenia.
- 11.Kanis JA, Cooper C, Rizzoli R, et al. European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO expert consensus meeting. Osteoporos Int 2017; 28:2023–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaudart C, Zaaria M, Pasleau F, et al. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS One 2017; 12:e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ethgen O, Beaudart C, Buckinx F, et al. The future prevalence of sarcopenia in Europe: a claim for public health action. Calcif Tissue Int 2017; 100:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaudart C, Biver E, Reginster JY, et al. Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia. J Cachexia Sarcopenia Muscle 2017; 8:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Si L, Winzenberg TM, de Graaff B, Palmer AJ. A systematic review and meta-analysis of utility-based quality of life for osteoporosis-related conditions. Osteoporos Int 2014; 25:1987–1997. [DOI] [PubMed] [Google Scholar]
- 16.Binkley N, Buehring B. Beyond FRAX: it's time to consider ‘sarco-osteopenia’. J Clin Densitom 2009; 12:413–416. [DOI] [PubMed] [Google Scholar]
- 17.Huo YR, Suriyaarachchi P, Gomez F, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc 2015; 16:290–295. [DOI] [PubMed] [Google Scholar]
- 18.Drey M, Sieber CC, Bertsch T, et al. FiAT intervention group. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging Clin Exp Res 2016; 28:895–899. [DOI] [PubMed] [Google Scholar]
- 19.Hida T, Shimokata H, Sakai Y, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J 2016; 25:3424–3431. [DOI] [PubMed] [Google Scholar]
- 20.He H, Liu Y, Tian Q, et al. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int 2016; 27:473–482. [DOI] [PubMed] [Google Scholar]
- 21.Locquet M, Beaudart C, Reginster JY, et al. Prevalence of concomitant bone and muscle wasting in elderly women from the SarcoPhAge cohort: preliminary results. J Frailty Aging 2017; 6:18–23. [DOI] [PubMed] [Google Scholar]
- 22▪.Pasco JA, Mohebbi M, Holloway KL, et al. Musculoskeletal decline and mortality: prospective data from the Geelong osteoporosis study. J Cachexia Sarcopenia Muscle 2017; 8:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]; The study examines the relationship between musculoskeletal deterioration and all-cause mortality in women followed over a decade.
- 23▪▪.Yoshimura N, Muraki S, Oka H, et al. Is osteoporosis a predictor for future sarcopenia or vice versa? Four-year observations between the second and third ROAD study surveys. Osteoporos Int 2017; 28:189–199. [DOI] [PubMed] [Google Scholar]; An interesting 4-year follow-up study that assesses the prevalence, incidence, and relationships between sarcopenia and osteoporosis.
- 24.Rizzoli R, Stevenson JC, Bauer JM, et al. ESCEO Task Force. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas 2014; 79:122–132. [DOI] [PubMed] [Google Scholar]
- 25.Bruyere O, Cavalier E, Souberbielle JC, et al. Effects of vitamin D in the elderly population: current status and perspectives. Arch Public Health 2014; 72:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int 2015; 2015:953241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaudart C, Dawson A, Shaw SC, et al. IOF-ESCEO Sarcopenia Working Group. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 2017; 28:1817–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruyere O, Cavalier E, Buckinx F, Reginster JY. Relevance of vitamin D in the pathogenesis and therapy of frailty. Curr Opin Clin Nutr Metab Care 2017; 20:26–29. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann M, Farrell CL, Pusceddu I, et al. Assessment of vitamin D status: a changing landscape. Clin Chem Lab Med 2017; 55:3–26. [DOI] [PubMed] [Google Scholar]
- 30.Kotlarczyk MP, Perera S, Ferchak MA, et al. Vitamin D deficiency is associated with functional decline and falls in frail elderly women despite supplementation. Osteoporos Int 2017; 28:1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Eisa ES, Alghadir AH, Gabr SA. Correlation between vitamin D levels and muscle fatigue risk factors based on physical activity in healthy older adults. Clin Interv Aging 2016; 11:513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪▪.Hirani V, Cumming RG, Naganathan V, et al. Longitudinal associations between vitamin D metabolites and sarcopenia in older Australian men: the concord health and aging in men project. J Gerontol A Biol Sci Med Sci 2017; doi: 10.1093/gerona/glx086. [DOI] [PubMed] [Google Scholar]; One of the few epidemiological studies investigating the association between vitamin D status and incidence of sarcopenia.
- 33.Avenell A, Mak JC, O’Connell D. Vitamin D and vitamin D analogues for preventing fractures in postmenopausal women and older men. Cochrane Database Syst Rev 2014; 14 4:CD000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avenell A, Bolland MJ, Grey A, Reid IR. Further major uncorrected errors in National Osteoporosis Foundation meta-analyses of calcium and vitamin D supplementation in fracture prevention. Osteoporos Int 2017; 28:733–734. [DOI] [PubMed] [Google Scholar]
- 35.Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 2016; 27:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36▪.Gallagher JC. Vitamin D and falls: the dosage conundrum. Nat Rev Endocrinol 2016; 12:680–684. [DOI] [PubMed] [Google Scholar]; An interesting point of view of the importance of the dosage of vitamin D in relation to falls.
- 37.Bolland MJ, Grey A, Gamble GD, Reid IR. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol 2014; 2:573–580. [DOI] [PubMed] [Google Scholar]
- 38.Rosendahl-Riise H, Spielau U, Ranhoff AH, et al. Vitamin D supplementation and its influence on muscle strength and mobility in community-dwelling older persons: a systematic review and meta-analysis. J Hum Nutr Diet 2017; 30:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaudart C, Buckinx F, Rabenda V, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2014; 99:4336–4345. [DOI] [PubMed] [Google Scholar]
- 40▪.Levis S, Gomez-Marin O. Vitamin D and physical function in sedentary older men. J Am Geriatr Soc 2017; 65:323–331. [DOI] [PubMed] [Google Scholar]; A study assessing the effect of 4000 IU daily of vitamin D on physical function.
- 41▪.Grimnes G, Emaus N, Cashman KD, Jorde R. The effect of high-dose vitamin D supplementation on muscular function and quality of life in postmenopausal women: a randomized controlled trial. Clin Endocrinol (Oxf) 2017; 87:20–28. [DOI] [PubMed] [Google Scholar]; The effect of 20 000 IU of vitamin D twice a week on muscle strength, balance, and quality of life.
- 42▪.Verlaan S, Maier AB, Bauer JM, et al. Sufficient levels of 25-hydroxyvitamin D and protein intake required to increase muscle mass in sarcopenic older adults: the PROVIDE study. Clin Nutr 2017; pii: S0261–5614(17)30010-9. doi: 10.1016/j.clnu.2017.01.005. [DOI] [PubMed] [Google Scholar]; A study highlighting the importance of vitamin D status in response to nutritional intervention.
- 43.Rizzoli R, Boonen S, Brandi ML, et al. Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin 2013; 29:305–313. [DOI] [PubMed] [Google Scholar]


