Abstract
Adolescence is a time of continued brain maturation, particularly in limbic and cortical regions, which undoubtedly plays a role in the physiological and emotional changes. Prenatally stressed offspring rats were used to investigate the potential antidepressive-like effects of imperatorin (IMP) extracted from the root of radix angelica. After 4 weeks of treatment of IMP, behavioral tests (sucrose-preference test, forced-swimming test, and open-field test) were measured. 5-hydroxytryptamine (5-HT) concentration in the hippocampus and frontal cortex was measured using an enzyme-linked immunosorbent assay. Serotonin transporters (5-HTT) and 5-HT1A receptor (5-HT1AR) mRNA expression in the hippocampus and frontal cortex were also determined by real-time PCR. Administration with IMP (15 and 30 mg/kg/day, intragastrically) for 28 days markedly increased the percentage of sucrose (anhedonia), decreased the immobility time, and increased the number of total crossings, center crossings, rearing, and grooming in the male prenatally stressed offspring. Meanwhile, we found that 5-HT concentration in the hippocampus and frontal cortex was significantly increased in the IMP-treated group. Subsequently, we found significantly decreased 5-HTT and increased 5-HT1AR mRNA expressions in the hippocampus and frontal cortex after IMP treatment in the prenatally stressed male offspring. IMP showed antidepressive-like effects and increased 5-HT concentration in male prenatally stressed offspring, suggesting that IMP could be of therapeutic use in preventing depressive-like behavior in adolescence.
Keywords: 5-hydroxytryptamine, Angelica dahurica, imperatorin, offspring, prenatal stress
Introduction
Depression is a group of syndromes characterized by notable and persistent mood disorders, and is one of the most prevalent psychiatric disorders prevalent in humans, often beginning in adolescence and belonging to the category of ‘gloomy disease’ in Chinese medicine 1. It is a prototypical multifactorial disorder that profoundly affects individual emotions, thoughts, sense of self, behaviors, interpersonal relations, physical functioning, biological processes, work productivity, and overall life satisfaction. Therefore, the present research focuses on adolescent depression.
Mounting evidence suggests that acute and chronic stress induces changes in 5-hydroxytryptamine (5-HT) neurotransmission in the hippocampus and prefrontal cortex, thereby increasing the risk of psychiatric disorders, such as schizophrenia, attention deficit hyperactivity disorder, depression, anxiety, and autism 2. It has been well documented that prenatal stress (PS) impairs various aspects of brain functions including learning and memory, cognition, and emotion in both experimental animals and humans 3,4. Prenatally stressed offspring may show alterations in brain morphology and behavior 5,6, increased emotional reactivity, altered regulation of the hypothalamic–pituitary–adrenal (HPA) axis 7, and affected 5-HT system 8,9. 5-HT is a neuromodulator that regulates various brain functions including cognition and emotion through multiple receptors, and disorders of the 5-HT system are closely associated with various types of mental illness 10. Recent studies have shown that alterations in 5-HT signaling during brain development induce neurodevelopmental and neuropsychiatric disorders 11–13.
Depression is reported to be associated with multiple changes in central 5-HT and 5-HT receptors, including decreased tryptophan concentrations, impaired 5-HT synthesis or release, malfunctions at postsynaptic 5-HT receptors, and alterations in 5-HTT density 14. In some patients, increasing brain 5-HT function through blockade of the 5-HTT [selective serotonin reuptake inhibitor (SSRI) treatment] seems to be sufficient to promote clinical recovery, although preclinical studies show that this simple pharmacological action is accompanied by a complex program of molecular and cellular changes. The 5-HTT gene is one of the most intensively investigated genetic risk factors for depression 11. The current research assumes that the 5-HT is the neurotransmitter that is most associated with depression. Central and peripheral 5-HT levels decrease in depression patients. The 5-HT acts through the 5-HT receptor, which is closely related to depression. A decrease in 5-HT receptor expression levels in the cerebrum may be the mechanism responsible for depression. Not only does the content of 5-HT at hippocampus decrease but also the function and level of the 5-HT receptor are reduced during long-term stress state. In fact, facilitation of 5-HT signaling by 5-HT1A receptors in the hippocampus, elevated 5-HT levels, or SSRIs have been associated with antidepressive effects 15. An inverse relationship can be found in animals previously exposed to early life trauma such as PS, where later treatment with SSRIs may reverse the stress response 3,8.
Despite the prevalence and severe impact of depression, the efficacy of currently available antidepressants is often inconsistent and many of them exert undesirable side effects. With a growing number of herbal medicines being introduced to psychiatric practice, many of them have been chosen as alternative therapies for depression 16. Thus, developing safe and effective agents from traditional herbs may provide us with a good means to reduce the side effects as well as improve the efficacy. A growing number of newly discovered drugs were produced from simple plant and mineral sources. Many herbal drugs based on ethnomedicine were used traditionally in Chinese Medicine for psychiatric disorders such as depression, anxiety, schizophrenia, and autism 14,16. IMP [9-(3-methylbut-2-enyloxy)-7H-furo[3,2-g]chromen-7-one; Fig. 1a], a dietary furocoumarin, was wide spread and found not only in the medicinal plant such as Cnidium monnieri (L) cusson and Angelica dahurica (Fisch. ex Hoffm) Benth, et Hook. F 17, but also in popular culinary herbs such as parsley and fennel. IMP had been shown to enhance the protective activity of conventional antiepileptic drugs in seizures 18, improve cognitive impairment 19, and also have anticonvulsant 19 and sedative-hypnotic effects 20. Despite a growing understanding of the mechanisms by which IMP acts on mood disorders, the effects on depressive-like behavior in prenatally stressed male offspring have not been reported.
Fig. 1.
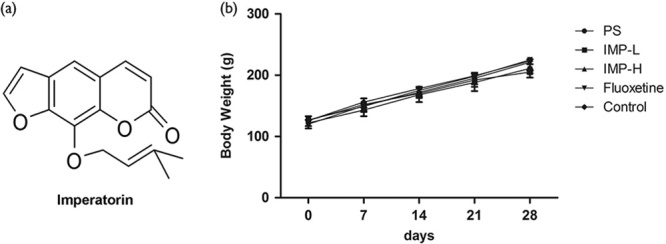
Chemical structure and effects of IMP on body weights in prenatally stressed offspring rats. (a) Chemical structure of IMP. (b) Effects of IMP on the body weights. PS group (●), IMP-L (■), IMP-H (▲), fluoxetine (▼), control (◆). *P<0.05 and **P<0.01 compared with the PS group, IMP, imperatorin; IMP-H, IMP administered at high concentrations; IMP-LPS, IMP administered at low concentrations; PS, prenatal stress.
The aim of the present study was to determine whether long-term IMP administration ameliorates depressive-like behavior in prenatally stressed offspring. Behavioral tests [sucrose-preference test, forced-swimming test (FST), and the open-field test (OFT)] were measured after 4 weeks of IMP treatment in prenatally stressed offspring rats. 5-HT concentration in the hippocampus and frontal cortex was evaluated 4 weeks after IMP administration. Then, to discover the possible mechanism, we also measured hippocampal and prefrontal cortex mRNA expressions of 5-HT and 5-HT1A receptors.
Methods
Animals and procedures
All procedures were carried out in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Institutional Animals Care and Use Committee at Xi’an Jiaotong University. All efforts were made to minimize the number of animals used and their suffering. Female Sprague-Dawley rats weighting 230–250 g and male Sprague-Dawley rats weighting 280–350 g were used. Animals were provided by the experimental animal center of Xi’an Jiaotong University Medical College. The rats were housed in an animal room with controlled temperature (22–28°C) and humidity (60%) on a 12-h-light/dark cycle (light on from 08:00 to 20:00) with free access to food and water. Virgin female rats were placed overnight with adult male rats (3 : 1) for mating. Vaginal smear was examined on the following morning (before 8:00). The day on which the smear was sperm positive was determined as embryonic day 0. Each pregnant rat was then housed separately.
The pregnant rats were separately exposed to restraint stress on days 14–20 of pregnancy three times daily for 45 min 21. To prevent habituation of animals to the daily procedure, restraint periods were randomly shifted within certain time periods (08:00–10:00, 11:00–13:00, and 15:00–17:00). The restraint device was a transparent plastic tube (6.8 cm in diameter) with air holes for breathing and closed end. The length could be adjusted to accommodate the size of the animals. The pregnant rats of the control were left undisturbed. On postnatal day 21, after all offspring were weaned, male and female pups were separated and housed four in each cage, respectively, until testing at 1 month of age. At the end of postnatal day 30, two male offspring rats from the same biological mother were selected randomly.
Administration of imperatorin in rats
IMP (purity >98% by high-performance liquid chromatography) from the supercritical fluid extraction of Angelica Dahurica was obtained from Shaanxi Huike Botanical Development Co. Ltd (Xi’an, China). IMP stock solutions were prepared in water containing 0.5% CMC-Na (m/v). In dose–response studies, IMP was administered at low concentrations of 15 mg/kg (IMP-L) and high concentrations of 30 mg/kg (IMP-H). The male prenatally unstressed offspring were treated with 0.5% CMC-Na used as the control group. All treatments or control were applied by intragastric administration for 4 weeks in male offspring rats at postnatal 30 days. Eight rats were used in each group.
After 4 weeks of administration of IMP, the depressive-like behavior in prenatally stressed offspring rats was assessed by the sucrose-preference test, the FST, and the OFT. After behavioral tests, the hippocampal and prefrontal cortex were removed and stored at −80°C until processing for enzyme-linked immunosorbent assay or mRNA extraction. The timeline of the present study is shown in Fig. 2.
Fig. 2.
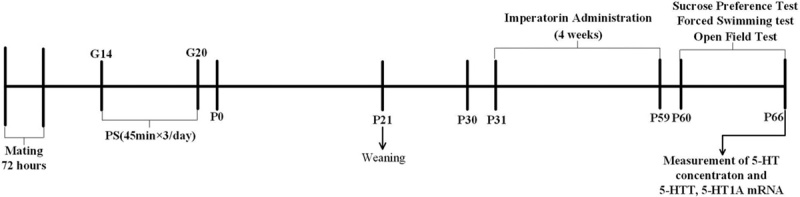
Timeline showing a summary of the experimental design. G, gestational age; 5-HT, 5-hydroxytryptamine; P, postnatal age (days); PS, prenatal stress.
Sucrose-preference test
After 4 weeks of administration of IMP, sucrose preference was assessed by a modified version of the sucrose-preference test 22 in the male prenatally stressed offspring. The sucrose consumption tests were performed using a two-bottle test, with rats having free access to both water and a sucrose solution. Animals were first trained to consume water in the two bottles and water consumption was measured for 24 h. The next day, sucrose consumption tests began: a bottle filled with a 2% sucrose solution as replaced with a bottle of water for 4 h. Bottles were counterbalanced across the left and the right sides of the feeding compartment, and alternated in position from test to test. Sucrose preference (%) was calculated as follows:
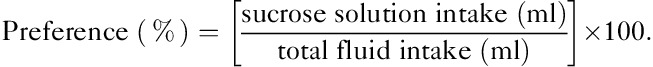 |
Forced-swimming test
After 4 weeks of administration of IMP, FST was assessed in the male PS offspring. The forced-swimming test was performed according to previous reports 22. Rats were forced to swim in a cylindrical tank (50 cm in height; 20 cm in diameter) filled with water (23–25°C) to a depth of 40 cm for 8 min. All movements of the rat in 8-min swim session were recorded by a video camera. The immobility time (floating in water without active movements of forepaws) was measured manually by a trained rater blinded to the status of rats. The immobility time percentage was calculated as follows:
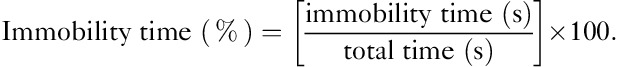 |
During the study, water in the tank was changed for each rat.
Open-field test
After 4 weeks of administration of IMP, OFT was assessed in the male PS offspring. During a 5-min observation period, rats were exposed to an open field (150×150×49.5 cm3; black acrylic walls, green floor) divided into a 5×5 grid of equally sized squares using white tape. The central region of the box (3×3=9 squares) was subdivided into a large center and a small center of eight and one squares, respectively. The test started by placing the rat in the same side of the small center. During the test, the time spent in the center was recorded. In addition, the frequency of the following behaviors was quantified: total crossing counts (the number of total squares crossed with the four paws), center crossing counts (the number of center squares crossed with the four paws), rearing counts (standing on the hind legs, with or without contact with the sides of the arena), and grooming counts (using the paws or tongue to clean/scratch body). The open field was cleaned with a 5% ethanol solution between each test.
Enzyme-linked immunosorbent assay
After the behavioral test, rats were decapitated after anesthesia and whole brains were quickly removed, the prefrontal cortex and hippocampus were isolated, frozen in liquid nitrogen, and stored at −80°C for posterior biochemical analysis. The levels of 5-HT in the prefrontal cortex and hippocampus were measured using a paired antibody quantitative enzyme-linked immunosorbent assay kit according to the manufacturer’s (ADL Inc., Massachusetts, USA) instructions. Dispensed antigen standards and samples were added to each well of 96-well plates precoated with primary antibodies. After adding biotin conjugate reagent and enzyme conjugate reagent to each well, the plates were incubated at 37°C for 30 min. Then, the plates were rinsed five times with distilled water and measured using a microtiter plate reader (Perkin-Elmer, Waltham, Massachusetts USA). Data were expressed as ng/g protein.
RNA isolation and real-time PCR
The real-time RT-PCR was used to detect for 5-HTT and 5-HT1AR. Total mRNA was isolated from tissues using the trizol method and quantified by nanodrop spectrophotometry (Thermo Scientific, Massachusetts, USA). First-strand cDNA was synthesized from 1 μg of each mRNA sample with a random primer and a reverse transcription kit (Takara, Shiga, Japan). PCR reactions were carried out using rat-specific primers for 5-HTT forward (5′-GGCGGAGATGAGGAATGAAG-3′) and reverse (5′-AAGAAGATGATGGCAAAGAACG-3′); 5-HT1AR forward (5′-CCACGGCTACACCATCTAC-3′) and reverse (5′-TGACAGTCTTGCGGATTCG-3′). To normalize the 5-HTT and 5-HT1AR data for quantitative analysis, we performed RT-PCR on the same cDNA samples using GAPDH primers forward (5′-ATGGTGAAGGTCGGTGTGAACG-3′) and reverse (5′-CGCTCCTGGAAGATGGTGATGG-3′). SYBR Green real-time PCR Master Mix (Takara) was used for real-time PCR to detect the abundance of PCR products among samples. Amplification conditions were as follows: denaturation for all pairs of primers was 94°C 30 s; annealing for 5-HTT, 60°C 30 s, 5-HT1AR, 56°C 30 s; and elongation for 5-HTT and 5-HT1AR, 72°C 20 s. Forty cycles were performed. The relative expression of 5-HTT and 5-HT1AR mRNA was calculated using the 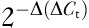 comparative method, with 5-HTT and 5-HT1AR gene normalized against the internal endogenous reference GAPDH gene for the same sample.
comparative method, with 5-HTT and 5-HT1AR gene normalized against the internal endogenous reference GAPDH gene for the same sample.
Statistical analysis
The values are presented as means±SEM. Differences between groups were compared using the one-way analysis of variance combined with the use of GraphPad Prism software 5.0. (GraphPad Software Inc., La Jolla, USA) P less than 0.05 was considered statistically significant.
Results
Effects of imperatorin on body weight in prenatally stressed offspring
Postnatal body weights of the male offspring are shown in Fig. 1b. Both male offspring treated with IMP or fluoxetine did not present any significant changes in body weights on 0, 7, 14, 21, and 28 days.
Effects of imperatorin on the sucrose-preference test in prenatally stressed offspring
After PS treatment, the percentage of sucrose consumed with the sucrose concentration of 2% in the male offspring was significantly reduced compared with the control group (P<0.05) (Fig. 3), whereas administration with IMP-L, IMP-H, or fluoxetine for 28 days markedly increased the percentage of sucrose in the male offspring (P<0.05) (Fig. 3).
Fig. 3.
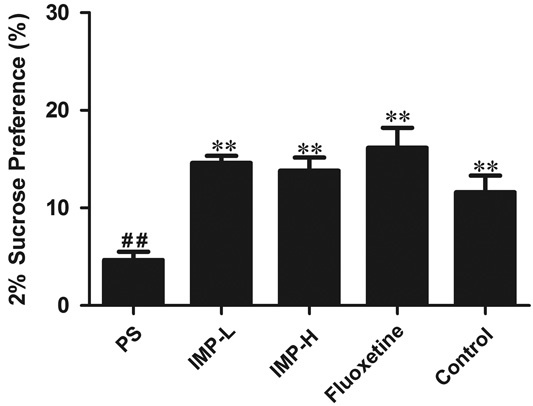
Effects of IMP on the sucrose-preference test of prenatally stressed offspring rats. The percentage of sucrose consumed at sucrose concentrations of 2%. Values represent means±SEM (n=10). *P<0.05 and **P<0.01 compared with the PS group, ##P<0.01 compared with the control group. IMP, imperatorin; IMP-H, IMP administered at high concentrations; IMP-LPS, IMP administered at low concentrations; PS, prenatal stress.
Effects of imperatorin on the forced-swimming test in prenatally stressed offspring
The result of FST is presented in Fig. 4. After PS treatment, immobility time (P<0.05, Fig. 4a) and the immobility time percentage (P<0.05, Fig. 4b) in the male offspring were significantly increased compared with the control group, respectively (P<0.05), whereas administration with IMP-L, IMP-H, or fluoxetine for 28 days markedly decreased the immobility time (P<0.05, Fig. 4a) and the immobility time percentage (P<0.05, Fig. 4b) in the male offspring (P<0.05).
Fig. 4.
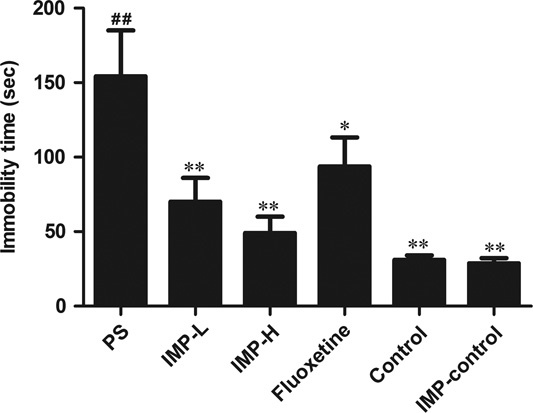
Effects of IMP on the forced-swimming test of prenatally stressed offspring rats. Immobility time in the forced-swimming test. Values represent means±SEM (n=10). *P<0.05 and **P<0.01 compared with the PS group, ##P<0.01 compared with the control group. IMP, imperatorin; IMP-H, IMP administered at high concentrations; IMP-LPS, IMP administered at low concentrations; PS, prenatal stress.
Effects of imperatorin on the open-field test in prenatally stressed offspring
PS significantly decreased the number of total crossings (P<0.05), center crossings (P<0.05), rearing (P<0.05), and grooming (P<0.05) in the male offspring compared with their control group respectively, shown in Fig. 5. After treatment with IMP-L, IMP-H, or fluoxetine for 28 days, the number of total crossings, center crossings, rearing, and grooming were significantly increased in the male offspring compared with the PS group, respectively (P<0.05, Fig. 5). There was no significant difference between the IMP-H group and the control group in the total number of line crosses (P<0.05, Fig. 5a).
Fig. 5.
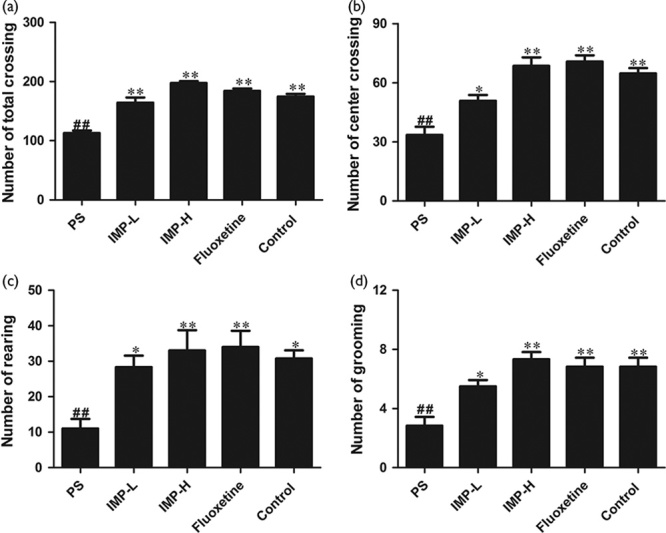
Effects of IMP on the open-field test of prenatally stressed offspring rats. (a) The number of total crossings. (b) The number of center crossings. (c) The number of rearings. (d) The number of grooming. Values represent means±SEM (n=10). *P<0.05 and **P<0.01 compared with the PS group, ##P<0.01 compared with the control group. IMP, imperatorin; IMP-H, IMP administered at high concentrations; IMP-LPS, IMP administered at low concentrations; PS, prenatal stress.
Effects of imperatorin on 5-hydroxytryptamine concentration in the hippocampus and the frontal cortex of prenatally stressed offspring
The effects of IMP treatment on the 5-HT concentration are summarized in Fig. 6a and b. In the hippocampus and prefrontal cortex, PS significantly reduced 5-HT levels compared with the control group (P<0.01). IMP treatment for 4 weeks could reverse the effects of PS offspring rats significantly in hippocampus and prefrontal cortex tissues (P<0.01). A similar tendency of results was found in the groups that received fluoxetine treatment.
Fig. 6.
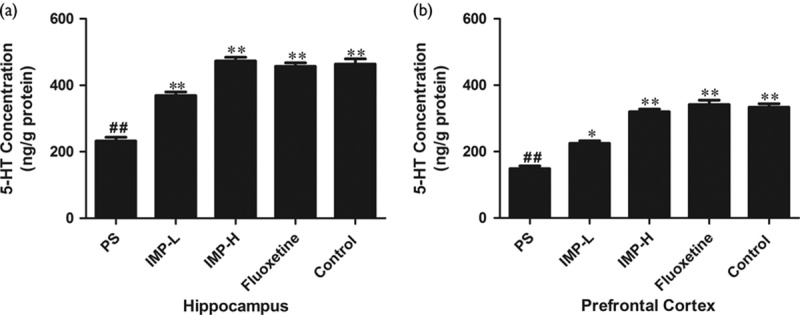
Effects of IMP on the 5-HT concentration in the hippocampus and frontal cortex of prenatally stressed offspring rats. (a) 5-HT concentration in the hippocampus. (b) 5-HT concentration in the frontal cortex. Values represent means±SEM (n=10). *P<0.05 and **P<0.01 compared with the PS group, ##P<0.01 compared with the control group. 5-HT, 5-hydroxytryptamine; IMP, imperatorin; IMP-H, IMP administered at high concentrations; IMP-LPS, IMP administered at low concentrations; PS, prenatal stress.
Effects of imperatorin on 5-HTT and 5-HT1AR mRNA expression in the hippocampus and the frontal cortex of prenatally stressed offspring
To investigate the influence of IMP on the 5-HT system in the PS offspring rats, we analyzed 5-HTT and 5-HT1AR mRNA expression in both the hippocampus and the frontal cortex, which is critical for 5-HT trafficking. 5-HT1AR mRNA expression was significantly reduced by PS compared with the control group (P<0.01, Fig. 7). 5-HTT mRNA expression was significantly increased by PS. After treatment with IMP for 4 weeks, the 5-HTT were markedly decreased and 5-HT1AR mRNA expressions were markedly increased in the hippocampus and frontal cortex in the offspring rats compared with the PS group (P<0.05, Fig. 7).
Fig. 7.
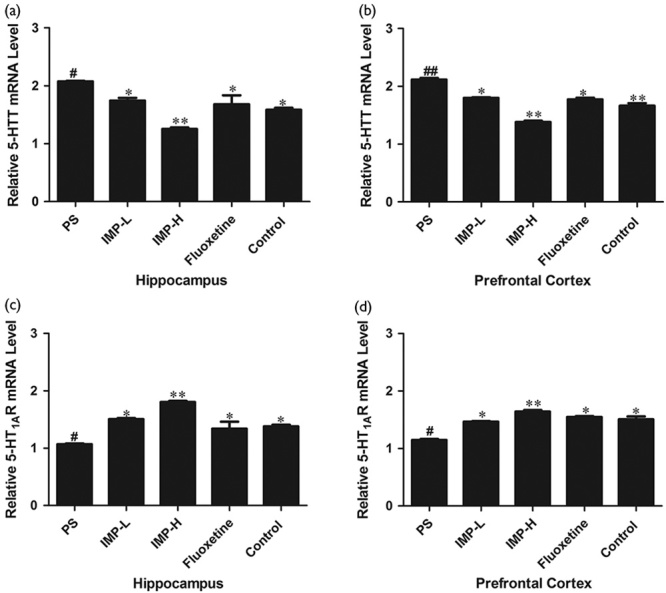
Effect of IMP on the 5-HTT, 5-HT1AR mRNA expression in the hippocampus and frontal cortex of prenatally stressed offspring rats. Results of (a) 5-HTT mRNA in the hippocampus, (b) 5-HTT mRNA in the frontal cortex, (c) 5-HT1AR mRNA in the hippocampus, (d) 5-HT1AR mRNA in the frontal cortex were quantified by real-time PCR. Values represent means±SEM (n=3). *P<0.05 and **P<0.01 compared with the PS group, #P<0.05 and ##P<0.01 compared with the control group. IMP, imperatorin; IMP-H, IMP administered at high concentrations; IMP-LPS, IMP administered at low concentrations; PS, prenatal stress.
Discussion
The chronic PS model is currently the most widely used model of depression, making mother animals receive prenatal long-term mild stress to simulate various negative events faced by people in their daily life 23. In the present study, the pregnant rats were separately exposed to restraint stress on days 14–20 of pregnancy three times daily for 45 min, the body weights of the male offspring did not change compared with the control group, but the intake of 2% sucrose decreased in the depression model groups, showing that these rats had clinical symptoms of anhedoni and thus had depression. Chronic treatment of male PS offspring rats with IMP for 4 weeks abolished the depressive-like behavior, decreased 5-HTT, and increased 5-HT1AR mRNA expression in the hippocampus and the frontal cortex. This may depend on changes in 5-HT levels because PS alter 5-HT neurons and brain 5-HT concentration in the offspring 24. It was also shown that the effects of PS on mRNA expression of 5-HT receptors were recovered by IMP. Therefore, the adjustment of the 5-HT system may be one of the mechanisms of IMP for antidepressant effects.
Environmental factors during prenatal altered the mRNA expression of 5-HT receptors in the frontal cortex and hippocampus of male offspring 25. Many research have shown that 5-HT was related to depression and this was mediated through different and common 5-HT receptor subtypes. Grippo et al. 24 showed chronic mild stress-induced behavioral and physiological changes, and may alter 5-HT1AR function. An increase in the 5-HT level can increase the neurogenesis in the hippocampus, thus causing an improvement in depression, causing a change in depressive behavior to normal status 11. In the present study, it was found that administration of IMP increased the mRNA of 5-HT1AR in the hippocampus and prefrontal cortex to the level of control offspring rats. This indicates that IMP has the potential to improve 5-HT deficiency and affect the 5-HT system in depressive rats.
Considerable evidence from preclinical laboratory studies indicates that PS affects the hormonal and behavioral development of offspring 26. The effects of PS in rodents and nonhuman primates on HPA reactivity to stress, morphological changes in the brain, motor behavior, and learning are determined. PS has been found to alter baseline and stress-induced responsivity of the HPA axis and levels and distribution of regulatory neurotransmitters, such as norepinepherine, dopamine, acetylcholine, and 5-HT, and to modify key limbic structures. A previous study show that 5-HT in the central nervous system plays a key role in the regulation of mood, appetite, sleep, and memory 27. 5-HT function in numerous neuropsychiatric disorders, such as depression, schizophrenia, and 5-HT receptor subtypes in this context, may lead to improved drug development and therapeutic approaches 28. It has been shown that adult depression level was regulated positively by the 5-HT1A receptor during the postnatal period. In the present study, the depression level was elevated by prenatal stress, chronic treatment of male PS offspring rats with IMP for 4 weeks abolished the depressive-like behavior and moderated the 5-HT system, and may show a potential therapeutic use for depression. IMP has been used in traditional Chinese medicine for their anti-inflammatory, antitumoral, anticonvulsant, vasodilatory, and antihypertensive properties 17,29–31. More directly, IMP was wide spread and found not only in the radix angelicae but also in popular culinary herbs such as parsley and fennel. Thus, IMP, which is wide spread in Chinese medicinal herb and popular culinary herbs, is a very good choice for the treatment of depressive-like behavior in adolescence.
The removal of 5-HT in the synaptic cleft is primarily through specific protein in the nerve terminal, named the 5-HT transporter or the serotonin transporter, 5-HTT 12, as fluoxetine is a SSRIs. IMP improved the behavior; our results emphasized that there were significantly lower levels of 5-HT in the PS group than the control group, and IMP could ameliorate the 5-HT level deficiency in hippocampus and prefrontal cortex tissues by stress, and showed marked serotonergic activation in brain. Prenatal stress induced 5-HTT mRNA in the hippocampus and prefrontal cortex. Statistical analysis indicated statistically significant decrease in 5-HTT mRNA in the hippocampus and the prefrontal cortex after IMP treatment for 4 weeks. On the basis of these findings, it is tempting to speculate that the antidepressant effects of IMP were by upregulation of the level of 5-HT along with promotion of reuptake in prenatally stressed offspring rats. It has been reported that IMP can inhibit nicotine-induced anxiety-related and memory-related response 32,33, facilitate glutamate release 34, and improve aticonvulsant effects in seizure 35. In our study, IMP not only abolished the depressive-like behavior but also increased 5-HT concentration, 5-HT1AA mRNA and decreased 5-HTT in the hippocampus and prefrontal cortex in PS offspring, showing the antidepressive effect. The distinctly stimulatory effect on the 5-HT level of IMP is caused by suppression of 5-HT reuptake and eventual increase in the content of 5-HT in the brain. On the basis of the results from the present study, it is suggested that IMP could be of therapeutic use in preventing depressive-like behavior in adolescence.
Acknowledgements
This work was supported by the Natural Science Foundation of China and Shaanxi (Grant nos 31600822, 15JS107, 2016JQ8059, and 14NW14) and the Student’s Platform for Innovation and Entrepreneurship Training Program of China and Northwest University (Grant no. 201610697049 and 2016182).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Yanjun Cao and Jiahui Liu contributed equally to the writing of this article.
References
- 1.Hankin BL. Adolescent depression: description, causes, and interventions. Epilepsy Behav 2006; 8:102–114. [DOI] [PubMed] [Google Scholar]
- 2.Delany FM, Byrne ML, Whittle S, Simmons JG, Olsson C, Mundy LK, et al. Depression, immune function, and early adrenarche in children. Psychoneuroendocrinology 2016; 63:228–234. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock M. The long-term behavioral consequences of prenatal stress. Neurosci Biobehav Rev 2008; 32:1073–1086. [DOI] [PubMed] [Google Scholar]
- 4.Fumagalli F, Molteni R, Racagni G, Riva MA. Stress during development: Impact on neuroplasticity and relevance to psychopathology. Prog Neurobiol 2007; 81:197–217. [DOI] [PubMed] [Google Scholar]
- 5.Jia N, Yang K, Sun Q, Cai Q, Li H, Cheng D, et al. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Dev Neurobiol 2009; 70:114–125. [DOI] [PubMed] [Google Scholar]
- 6.Bock J, Wainstock T, Braun K, Segal M. Stress in utero: prenatal programming of brain plasticity and cognition. Biol Psychiatry 2015; 78:315–326. [DOI] [PubMed] [Google Scholar]
- 7.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res 2007; 1156:152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: a psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology 2015; 62:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akatsu S, Ishikawa C, Takemura K, Ohtani A, Shiga T. Effects of prenatal stress and neonatal handling on anxiety, spatial learning and serotonergic system of male offspring mice. Neurosci Res 2015; 101:15–23. [DOI] [PubMed] [Google Scholar]
- 10.Sharp T, Cowen PJ. 5-HT and depression: is the glass half-full? Curr Opin Pharmacol 2011; 11:45–51. [DOI] [PubMed] [Google Scholar]
- 11.Lesch K-P, Waider J. Serotonin in the modulation of neural plasticity and networks: implications for neurodevelopmental disorders. Neuron 2012; 76:175–191. [DOI] [PubMed] [Google Scholar]
- 12.Ansorge MS, Hen R, Gingrich JA. Neurodevelopmental origins of depressive disorders. Curr Opin Pharmacol 2007; 7:8–17. [DOI] [PubMed] [Google Scholar]
- 13.Weinstock M. Alterations induced by gestational stress in brain morphology and behavior of the offspring. Prog Neurobiol 2001; 65:427–451. [DOI] [PubMed] [Google Scholar]
- 14.Yuan R, Lin Y. Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Ther 2000; 86:191–198. [DOI] [PubMed] [Google Scholar]
- 15.Filip M, Bader M. Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep 2009; 61:761–777. [DOI] [PubMed] [Google Scholar]
- 16.Zhao H, Wan X, Chen J-X. A mini review of traditional Chinese medicine for the treatment of depression in China. Am J Chin Med 2009; 37:207–213. [DOI] [PubMed] [Google Scholar]
- 17.Cao YJ, He X, Wang N, He LC. Effects of imperatorin, the active component from Radix Angelicae (Baizhi), on the blood pressure and oxidative stress in 2K,1C hypertensive rats. Phytomedicine 2013; 20:1048–1054. [DOI] [PubMed] [Google Scholar]
- 18.Luszczki JJ, Wojda E, Andres-Mach M, Cisowski W, Glensk M, Glowniak K, et al. Anticonvulsant and acute neurotoxic effects of imperatorin, osthole and valproate in the maximal electroshock seizure and chimney tests in mice: a comparative study. Epilepsy Res 2009; 85:293–299. [DOI] [PubMed] [Google Scholar]
- 19.Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, et al. Effects of imperatorin on scopolamine-induced cognitive impairment and oxidative stress in mice. Psychopharmacology (Berl) 2015; 232:931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi MM, Piao JH, Xu XL, Zhu L, Yang L, Lin FL, et al. Chinese medicines with sedativeehypnotic effects and their active components. Sleep Med Rev 2016; 29:108–118. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z, Li X, Chen W, Zhao Y, Li H, Cai Q, et al. Prenatal stress causes gender-dependent neuronal loss and oxidative in rat hippocampus. J Neurosci Res 2004; 78:837–844. [DOI] [PubMed] [Google Scholar]
- 22.Sun H, Guan L, Zhu Z, Li H. Reduced levels of NR1 and NR2A with depression-like behavior in different brain regions in prenatally stressed juvenile offspring. PloS One 2013; 8:e81775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjaer SL, Wegener G, Rosenberg R, Lund SP, Hougaard KS. Prenatal and adult stress interplay – behavioral implications. Brain Res 2010; 1320:106–113. [DOI] [PubMed] [Google Scholar]
- 24.Grippo AJ, Sullivan NR, Damjanoska KJ, Crane JW, Carrasco GA, Shi J, et al. Chronic mild stress induces behavioral and physiological changes, and may alter serotonin 1A receptor function, in male and cycling female rats. Psychopharmacology (Berl) 2005; 179:769–780. [DOI] [PubMed] [Google Scholar]
- 25.Miyagawa K, Tsuji M, Ishii D, Takeda K, Takeda H. Prenatal stress induces vulnerability to stress together with the disruption of central serotonin neurons in mice. Behav Brain Res 2014; 277:228–236. [DOI] [PubMed] [Google Scholar]
- 26.Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci Biobehav Rev 2002; 26:457–470. [DOI] [PubMed] [Google Scholar]
- 27.Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behav Brain Res 2008; 195:198–213. [DOI] [PubMed] [Google Scholar]
- 28.Hayes DJ, Greenshaw AJ. 5-HT receptors and reward-related behaviour: a review. Neurosci Biobehav Rev 2011; 35:1419–1449. [DOI] [PubMed] [Google Scholar]
- 29.Łuszczki JJ, Andres-Mach M, Gleńsk M, Skalicka-Woźniak K. Anticonvulsant effects of four linear furanocoumarins, bergapten, imperatorin, oxypeucedanin, and xanthotoxin, in the mouse maximal electroshock-induced seizure model: a comparative study. Pharmacol Rep 2010; 62:1231–1236. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Zhang Y, Wang N, He L. Antioxidant effect of imperatorin from Angelica dahurica in hypertension via inhibiting NADPH oxidase activation and MAPK pathway. J Am Soc Hypertens 2014; 8:527–536. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Wang QL, Zhan YZ, Duan HJ, Cao YJ, He LC. Role of store-operated calcium entry in imperatorin-induced vasodilatation of rat small mesenteric artery. Eur J Pharmacol 2010; 647:126–131. [DOI] [PubMed] [Google Scholar]
- 32.Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I, et al. Effects of imperatorin on nicotine-induced anxiety- and memory-related responses and oxidative stress in mice. Physiol Behav 2013; 122:46–55. [DOI] [PubMed] [Google Scholar]
- 33.Budzynska B, Kruk-Slomka M, Skalicka-Wozniak K, Biala G, Glowniak K. The effects of imperatorin on anxiety and memory-related behavior in male Swiss mice. Exp Clin Psychopharmacol 2012; 20:325. [DOI] [PubMed] [Google Scholar]
- 34.Wang SJ, Lin TY, Lu CW, Huang WJ. Osthole and imperatorin, the active constituents of Cnidium monnieri (L.) Cusson, facilitate glutamate release from rat hippocampal nerve terminals. Neurochem Int 2008; 53:416–423. [DOI] [PubMed] [Google Scholar]
- 35.Luszczki JJ, Glowniak K, Czuczwar SJ. Time–course and dose–response relationships of imperatorin in the mouse maximal electroshock seizure threshold model. Neurosci Res 2007; 59:18–22. [DOI] [PubMed] [Google Scholar]


