Abstract
Objectives:
To investigate the effect of a percutaneous radiofrequency (RF) heat lesion compared with a sham procedure, applied to the lateral branches of L5, S1, S2, S3, and S4 nerve roots.
Materials and Methods:
Sixty patients aged 18 years and above with a medical history and physical examination suggestive for sacroiliac joint pain and a reduction of 2 or more on a numerical rating scale (NRS, 0 to 10) after a sacroiliac joint test block were included in this study. Treatment group: percutaneous RF heat lesion at the lateral branches of S1, S2, S3, and S4 nerve roots and the posterior ramus dorsalis of L5; sham group: same procedure as the treatment group except for the RF heat lesion. Primary outcome measure: pain reduction (NRS). Secondary outcome measure: Global Perceived Effect.
Results:
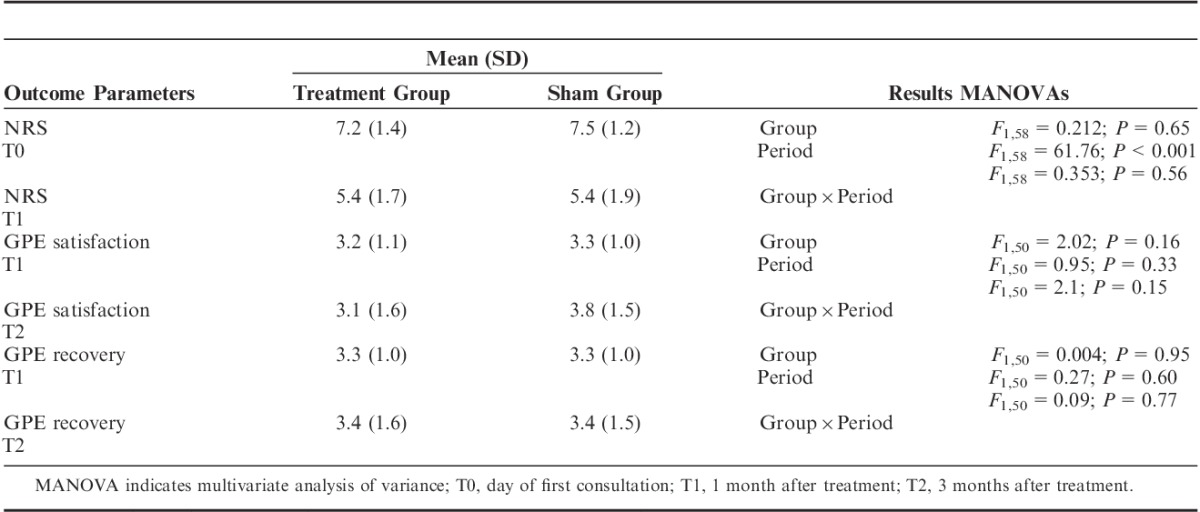
No statistically significant differences in pain level over time between the groups (Group×Period) (F1,58=0.353; P=0.56) nor within the treatment Group (F1,58=0.212; P=0.65) were found. The Period factor, however, yielded a significant difference (F1,58=61.67; P<0.001), that is, when pooled together the mean pain level of the patients was significantly reduced at T1 compared with T0. In the crossover group, 42.1% experienced a reduction in NRS of 2 or more at 1 month (P=0.65). No statistically significant difference in satisfaction over time between the groups was found (F1,50=2.1; P=0.15). The independent factors Group (F1,50=2.02; P=0.16) and Period (F1,50=0.95; P=0.33) also showed no statistically significant difference. The same applies to recovery: no statistically significant Group×Period effect (F1,51=0.09; P=0.77) was found, neither an effect of Group (F1,51=0.004; P=0.95) nor of Period (F1,51=0.27; P=0.60).
Discussion:
The hypothesis of no difference in pain reduction or in Global Perceived Effect between the treatment and sham group cannot be rejected.
Level of Evidence:
Level 1A.
Key words: sacroiliac joint, radiofrequency, RCT, sham, chronic pain
In patients with sacroiliac (SI) joint pain (constituting 10% to 38% of patients with chronic low back pain1–3), questions arise concerning the persons who might be more susceptible for these problems, how the diagnosis should be made, and what comprises optimal treatment. For diagnosing SI joint problems, besides a suggestive medical history and a physical examination,4–8 an intra-articular injection with local anesthetics is still being used. Every step has its limitations and the whole diagnostic cascade should lead toward sufficient evidence for treatment of the SI joint.
Several types of treatment for trying to diminish SI joint pain are described in the literature, one of them is applying radiofrequency (RF) current to the nerves that provide the innervation.9,10 Several studies describe a success ratio between 64% and 80%.11–13 The application of RF current can be provided in several ways (pulsed or continuous, side of the lesion, number of lesions),3,10,14–17 the practicality of the application must always be considered. More recently evidence emerged about the use of cooled RF current in providing a significant and long-lasting pain relief.18–23
The Simplicity III probe (Neurotherm, Wilmington, MA) is a multielectrode RF probe that has a unique design that allows for positioning using a single percutaneous entry point. With this procedure the lateral branches of S1, S2, S3, and S4 are targeted at the same time (a L5 dorsal root ramus RF lesioning is performed separately). There are no randomized-controlled trials (RCTs) available concerning the use of this device in diminishing SI joint pain. In this randomized sham-controlled double-blind multicenter clinical trial (Current Controlled Trials ISRCTN45914408) the percutaneous RF treatment of SI joint pain with this probe was evaluated and compared with a sham procedure. A crossover was provided for the sham-operated group after 3 months if no significant pain relief was obtained.
MATERIALS AND METHODS
Study Design
We conducted a randomized sham-controlled double-blind multicenter clinical trial in patients with SI joint pain for >3 months. The medical ethics committee from Erasmus University Medical Centre approved the protocol. Written informed consent was obtained from all participants.
Participants
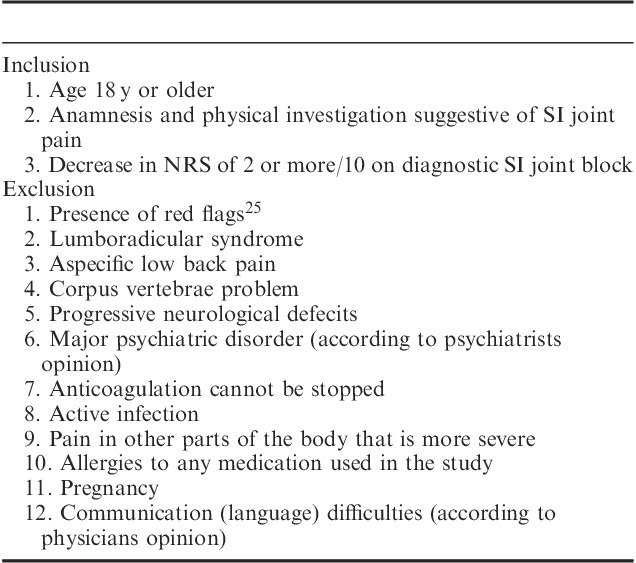
Suitable patients for the study were recruited from a population of patients referred to the multidisciplinary pain centers of 2 general hospitals with reports of ongoing low back pain for >3 months. Conservative care (rest, analgesics, and physiotherapy) had failed to improve their burden. These patients were managed according to the flowchart presented in Figure 1. When a SI joint problem was suspected, details of medical history, physical examination, and—if necessary—additional tests4–7 leading, either wholly or in part, to the diagnosis of SI joint pain can be found in Table 1, patients met the inclusion and exclusion criteria24 (Table 2), and if the test SI joint injection with local anesthetics was positive (decrease in numerical rating scale [NRS] of 2 or more on a 0 to 10 point scale26), the patient was eligible for the RCT. Each patient received a general brochure containing information concerning scientific research involving human subjects (Ministry of Health, Welfare and Sports27) and a brochure (including the questionnaires) explaining the complete procedure. After giving written informed consent patients were enrolled in the study.
FIGURE 1.
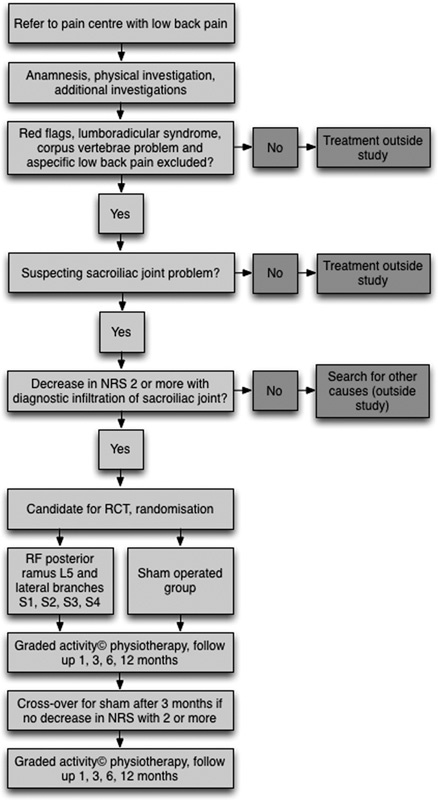
Study flowchart. NRS indicates numerical rating scale; RCT, randomized-controlled trial; RF, radiofrequency.
TABLE 1.
Details About Medical History, Physical Examination, and Additional Tests in Patients Leading, Either Wholly or in Part, to the Diagnosis of Sacroiliac Joint Pain4–7

TABLE 2.
Inclusion and Exclusion Criteria24 for Patients With SI Joint Pain Eligible for RCT
Study Interventions
Test SI joint injection: the injection was performed under fluoroscopy with a 10-cm Sluijter-Mehta Kit (SMK) needle (Cotop via Neurotherm). The patient lies in the prone position on the operating table with a pillow under the pelvis. From the anteroposterior view, the c-arm is rotated contralaterally until the medial cortical line of the posterior articulation is in focus. Local anesthesia with 1 mL lidocaine 2% was given for skin infiltration. Needle insertion is 1 to 2 cm cranially from the lower border of the SI joint at the level of the zone of maximal radiographic translucency. Introduction of the needle into the SI joint is characterized by a change in resistance. On a lateral view, the needle tip should appear anterior to the dorsal border of the sacrum. The SI joint was injected with a total of 3 mL lidocaine 2%.
RF heat lesion of the ramus dorsalis of L5 and lateral branches of S1, S2, S3, and S4 with a RF probe with 3 independent active electrodes versus sham: when patients were candidates for the trial they were randomized in 2 study groups.
Treatment group: monitoring according to American Society of Anesthesiologists House of Delegates Standards for Basic Anesthetic Monitoring.28 Continuous intravenous propofol, target-controlled infusion (0.5 μg/mL), and remifentanyl (0.05 μg/kg/min). Continuous oxygen 15 L/min (nonrebreather mask and bag). The patient lies in the prone position on the operating table with a pillow under the pelvis. Skin infiltration with 1 mL lidocaine 2% per level. The skin entry point for the RF probe with 3 independent active electrodes is identified at the ipsilateral, lateral, inferior border of the sacrum, and 1 cm lateral of and below the S4 foramen. Infiltration over the course of the RF probe with 3 independent active electrodes with 10 mL lidocaine 2%, staying lateral to the sacral foramen, in contact with the sacrum, and medial to the SI joint. Inserting and advancing RF probe, maintaining continuous contact with the sacrum on a cephalad and slightly lateral line, staying lateral to the sacral foramen, medial to the SI joint, and ventral to the ilium, until contact with the sacral ala prevents further advancement. Percutaneous RF heat lesion (85°C, each step 90 s, total of 5 steps) with a RF lesion generator (NT2000; Neurotherm) at the lateral branches of S1, S2, S3, and S4 nerve roots. Percutaneous RF heat lesion (85°C for 90 s, same lesion generator) of the L5 dorsal root primary ramus with a 10-cm SMK needle, placed to lie in contact with the S1 superior articular process just slightly above the groove formed between the superior articular process and sacral ala; then advanced with needle position confirmed using fluoroscopy (anteroposterior and lateral view) and motor stimulation (2 Hz and at least 1 V).
Sham-operated group: same procedure as in treatment group except for the RF heat lesions.
A crossover was provided for the sham-operated group after 3 months if no significant pain relief was obtained.
Outcomes
The main study parameter was pain reduction NRS25,29–32). The 0 to 10 verbal NRS-11 is a tool that enjoys widespread clinical use due to its ease of administration. When using the NRS-11 patients are asked to rate their pain on a scale from 0 to 10, where 0 represents “no pain” and 10 represents “the worst pain possible,” using whole numbers (11 integers including zero). Often the value of “4” is used to confirm clinical nursing judgment as to the need for further intervention or documentation that the patient’s goals for analgesia have been achieved.
The secondary study parameter was Global Perceived Effect (GPE).33–35 The type of rating of perceived effect is a “transition scale” or GPE scale. The GPE scale asks the patient to rate, on a numerical scale, how much their condition has improved or deteriorated at some predefined timepoint. The GPE has several qualities that make it an appealing tool for use in clinical practice and research; being a single question, it is easy and quick to administer and the results are seemingly simple to interpret. Such scales have been recommended for use as a core outcome measure for chronic pain trials and been advocated to increase the relevance of information from clinical trials to clinical practice.33
Follow-up
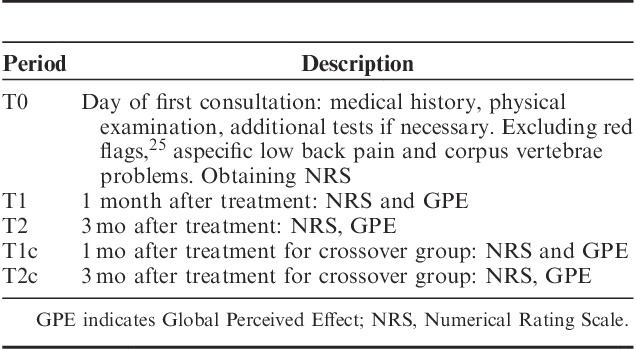
The results of the crossover group were analyzed separately, and compared with those who received the actual treatment in the first case. Time periods for follow-up are presented in Table 3. Both groups received graded activity36,37 physiotherapy, which constitutes an individual, submaximal, gradually increased exercise program, with an operant-conditioning behavioral approach, based on the results of the tests and the demands of the patient’s work.
TABLE 3.
Time Periods for Follow-up
Statistical Considerations
Difference in patients’ sex between the experimental groups was analyzed using the Fisher exact test. Difference in age was analyzed using the independent samples Mann-Whitney U test; and the difference in body mass index using the independent samples t test. The data on the NRS-11, GPE (subscales “Satisfaction” and “Recovery”) were analyzed by means of multivariate analysis of variance (MANOVA) for repeated measurements using as independent variables, Group (treatment and sham) and Time (in case of the NRS-11 Period T0-T1, in case of the GPE subscales Period T1-T2 as independent variables).
For the skewed distributed variables we nevertheless decided to use MANOVA for repeated measurements analysis of variance. We did so, because, although the MANOVA test requires that each dependent variable entered into the analysis be normally distributed it can still be used in case of skewly distributed dependent variable(s). The Monte Carlo experiments have shown that for sample size 3 or 5 it is still possible to analyze leptokurtic, rectangular, J-shaped, moderately, and markedly skewed distributions. These experiments demonstrated that the empirically determined rejection region of the F-distribution would be no larger than α=0.08 when the usual 5% rejection is used.38
The percentage of patients requesting crossover and subsequently reporting a significant pain relief was analyzed using the One-Sample Binomial Test (reference probability, 0.5). Only patients in the sham group could switch to the intervention.
The sample size was computed using the NRS-11 as the primary outcome parameter. A statistically detectable and clinically relevant within/between interaction effect size (f(V)) of 0.2 on this scale was chosen. The power of the study (1−β) was chosen to be 0.8, an allocation ratio of 1:1, and the 2-sided level of significance (α) to be 0.05. The required a priori total sample size computed by this method is 60.
Data were analyzed using SPSS for Windows, version 22 (International Business Machines [IBM] Corporation, Software Group, NY). The primary comparison was done at T1.
Blinding
On the basis of the required sample size calculation, 60 envelopes (30 “treatment group” and 30 “sham group”) were prepared, sealed, mixed, and placed together in a box. Patients chose an envelope randomly. Patients and their pain physicians were completely unaware of the content of the envelope during any stage (or T2 in case of sham procedure without reduction in NRS of 2 or more) of the investigation. The pain research nurse was the only one aware of the contents and performed the treatment accordingly. Regarding the RF lesion generator, all sound indicators were turned off and the generator itself was visually hidden from the patient by means of a linen cloth, hung between 2 metal infusion poles. The pain physician left the operating theater when the actual treatment (RF current or sham) took place. The same time period was taken for an actual—or a sham treatment.
RESULTS
Patients were included and treated between February 2012 and June 2014. Of 79 eligible patients (1 patient entered the study without a written informed consent) a total of 19 patients resigned due to various reasons: no significant pain reduction after diagnostic block (9), no more pain after diagnostic block (2), afraid of unemployment (1), not enough time (1), shortly after signing the informed consent form, no reason specified (1), second opinion (1), cumbersome sedation (1), chronic pain turned bearable (1), fear of needles (1), and without reporting a cause (1).
The flowchart of the progress through the phases of the RCT is presented in Figure 2. The demographic data of the treatment and sham groups are presented in Table 4. There was no statistically significant differences between the parameters of the groups.
FIGURE 2.
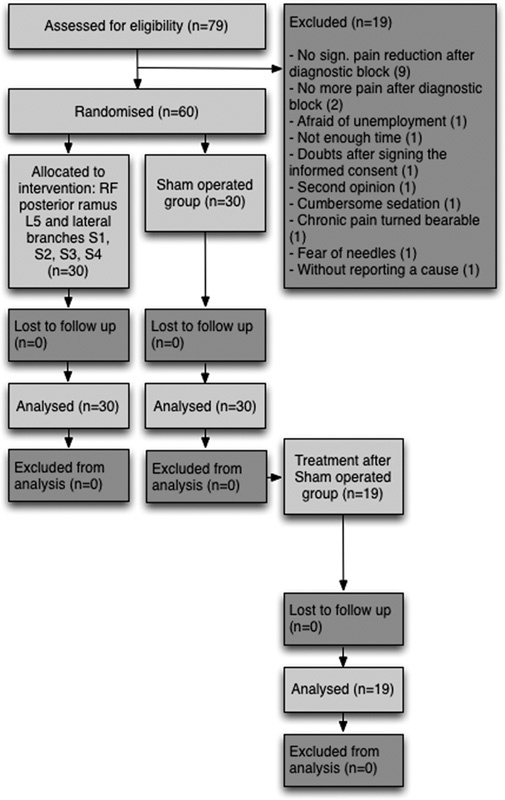
Flow diagram of the progress through the phases of the randomized-controlled trial. RF indicates radiofrequency.
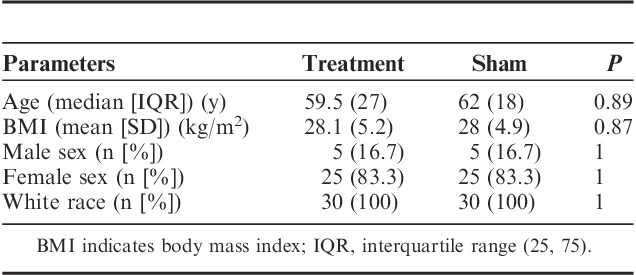
TABLE 4.
Demographic Data of the Treatment and Sham Groups
No statistically significant difference in pain level over time between the groups (Group×Period) (F1,58=0.353; P=0.56) nor in the factor Group (F1,58=0.212; P=0.65) was found. The Period factor, however, yielded a significant difference (F1,58=61.67; P<0.001), that is, when pooled together the mean pain level of the patients was significantly reduced at T1 compared with T0 (Fig. 3). In the crossover group, 8 of 19 patients experienced a reduction in NRS of 2 or more at 1 month crossover (P=0.65).
FIGURE 3.
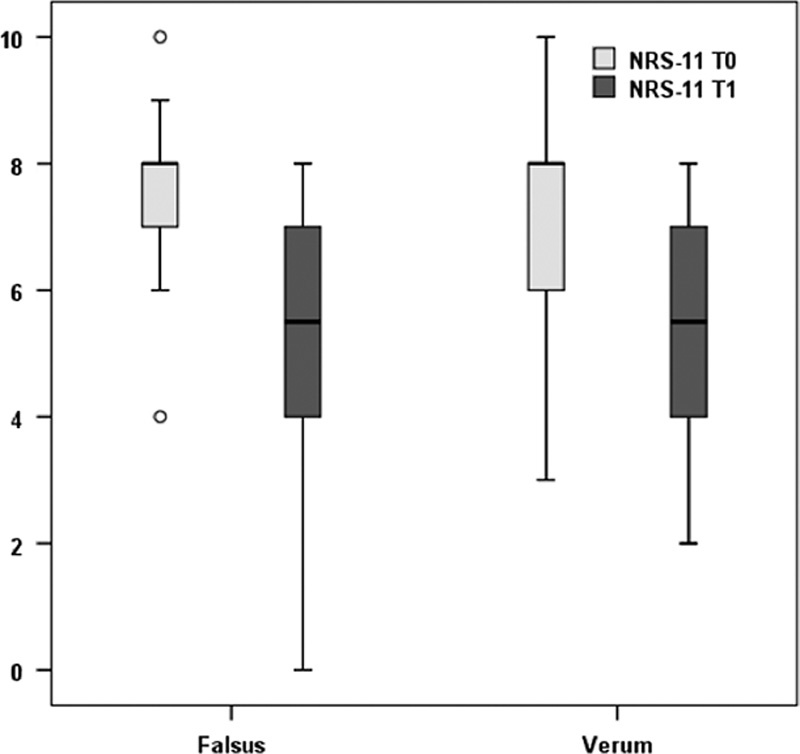
Boxplot of numerical rating scale (NRS)-11 scores by group and by moment of measurement (verum=treatment group; falsus=sham group).
No statistically significant difference in satisfaction over time between the groups (Group×Period) was found (F1,50=2.1; P=0.15). The independent Group (F1,50=2.02; P=0.16) and Period factors (F1,50=0.95; P=0.33) also showed no statistically significant difference (8 missing cases on T2). The same applies to recovery: no statistically significant Group×Period effect (F1,51=0.09; P=0.77) was found, neither an effect of Group (F1,51=0.004; P=0.95) nor of Period (F1,51=0.27; P=0.60) (7 missing cases on T2) (Table 5).
TABLE 5.
Numerical Rating Scale (NRS) and Global Perceived Effect (GPE) Scales of the Treatment and Sham Groups
During the trial we noted 1 unexpected and unsuspected serious adverse event, due to a fall from the stairs during the follow-up period.
DISCUSSION
In this RCT the proportion of patients who reported significant pain relief (NRS≥2) after the sham procedure was even higher (but not statistically significant) than those after the actual treatment. In the crossover group (3 mo after the sham procedure) the number of people that demonstrated a statistically significant reduction after the RF treatment was 42.1%, which equals the number of positive results (43.3%) from the primary treatment group.
The number of positive SI joint test blocks was 86.1% (62/72 blocks), which is higher than expected when considering the available literature.1,2,8 Possible reasons could be (the combination of) multidisciplinary assessment, rating of the decrease in NRS as a result of the test injection with local anesthetics according to Ostelo et al26 (positive test injection with local anesthetics when a decrease in NRS of 2 or more on a 0 to 10 point scale is obtained) instead of a decrease of 50% in NRS, using only local anesthetics instead of corticosteroids and the probability that, based on the diagnostic cascade used, the patients did not have SI joint pain. The false-positive rate of a single, uncontrolled, SI joint injection with local anesthetics is around 20%,2 but can be as high as 54%.1 The local anesthetic diffuses out of the joint in 61% of cases, becoming an intra-articular as well as extra-articular injection.12
The presence of pain distal to the knee in patients with SI joint pain is described but not often found and SI joint denervation often will not relieve this type of pain when present. Instead of using “sciatica (often S1)” as inclusion criterion it would have been better to use “pain predominantly below L5.” As stated the whole diagnostic cascade should be taken into account and not a single item. Another limitation of this study is the fact that we used only 1 diagnostic test block instead of using a double diagnostic test block. Having considered the daily practice in pain management, this sham RCT was completed with 1 diagnostic test block.
Regarding the internal validity of this study: (1) Because of the anatomy of the sacrum, we sometimes did not reach the S4 branch with the RF probe with 3 independent active electrodes, performing a L5 to S3 RF procedure. How much does the S4 branch attribute to SI joint pain? The size of the lesion by the RF probe with 3 independent active electrodes might be smaller than the one from the cooled RF treatment variant39 but, again, what is the (exact) influence of that? (2) Age was non-normally (bimodally) distributed (Fig. 3); this might reflect differences in disease type, encompassing different structures (anatomic changes, disorders of the capsuloligamentous structures, and diastasis from pregnancy and childbirth and disorders from the vascular plexus or complex neural network), and operative procedures.1,5 (3) Pain scores were measured during follow-up at specific time periods (Table 3). Using average pain scores over certain time periods (ie, past month), based on pain diaries might have led to a different result. (4) All patients received graded activity36,37 physiotherapy, but not at a single center; as a consequence gaining evidence of equal quality of physiotherapy accompaniment was difficult and we therefore do not know whether—and if so to which extend—this factor has confounded the treatment outcome.
On the basis of this RCT the hypothesis of no difference in pain reduction or in GPE between the treatment and sham group cannot be rejected (level of evidence 1A40).
Footnotes
Ethical approval has been granted by the Medical Ethics Committee (Medisch Ethische Toetsings Commissie) (METC) Erasmus MC, Rotterdam, The Netherlands on February 7, 2012 (multicenter approval on July 10, 2012), reference number MEC-2011-244.
The authors declare no conflict of interest.
REFERENCES
- 1.Sizer PS, Jr, Phelps V, Thompsen K. Disorders of the sacroiliac joint. Pain Pract. 2002;2:17–34. [DOI] [PubMed] [Google Scholar]
- 2.Hansen HC, McKenzie-Brown AM, Cohen SP, et al. Sacroiliac joint interventions: a systematic review. Pain Physician. 2007;10:165–184. [PubMed] [Google Scholar]
- 3.Cohen SP, Chen Y, Neufeld NJ. Sacroiliac joint pain: a comprehensive review of epidemiology, diagnosis and treatment. Expert Rev Neurother. 2013;13:99–116. [DOI] [PubMed] [Google Scholar]
- 4.Laslett M, Williams M. The reliability of selected pain provocation tests for sacroiliac joint pathology. Spine. 1994;19:1243–1249. [DOI] [PubMed] [Google Scholar]
- 5.Dreyfuss P, Michaelsen M, Pauza K, et al. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine. 1996;21:2594–2602. [DOI] [PubMed] [Google Scholar]
- 6.Laslett M, Aprill CN, McDonald B, et al. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10:207–218. [DOI] [PubMed] [Google Scholar]
- 7.Robinson HS, Brox JI, Robinson R, et al. The reliability of selected motion- and pain provocation tests for the sacroiliac joint. Man Ther. 2007;12:72–79. [DOI] [PubMed] [Google Scholar]
- 8.Berthelot JM, Labat JJ, Le Goff B, et al. Provocative sacroiliac joint maneuvers and sacroiliac joint block are unreliable for diagnosing sacroiliac joint pain. Joint Bone Spine. 2006;73:17–23. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SP. Epidemics, evolution, and sacroiliac joint pain. Reg Anesth Pain Med. 2007;32:3–6. [DOI] [PubMed] [Google Scholar]
- 10.Cohen SP, Hurley RW, Buckenmaier CC, 3rd, et al. Randomized, placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen SP, Abdi S. Lateral branch blocks as a treatment for sacroiliac joint pain: a pilot study. Reg Anesth Pain Med. 2003;28:113–119. [DOI] [PubMed] [Google Scholar]
- 12.Yin W, Willard F, Carreiro J, et al. Sensory stimulation-guided sacroiliac joint radiofrequency neurotomy: technique based on neuroanatomy of the dorsal sacral plexus. Spine. 2003;28:2419–2425. [DOI] [PubMed] [Google Scholar]
- 13.Gevargez A, Groenemeyer D, Schirp S, et al. CT-guided percutaneous radiofrequency denervation of the sacroiliac joint. Eur Radiol. 2002;12:1360–1365. [DOI] [PubMed] [Google Scholar]
- 14.Ferrante FM, King LF, Roche EA, et al. Radiofrequency sacroiliac joint denervation for sacroiliac syndrome. Reg Anesth Pain Med. 2001;26:137–142. [DOI] [PubMed] [Google Scholar]
- 15.Vallejo R, Benyamin RM, Kramer J, et al. Pulsed radiofrequency denervation for the treatment of sacroiliac joint syndrome. Pain Med. 2006;7:429–434. [DOI] [PubMed] [Google Scholar]
- 16.Burnham RS, Yasui Y. An alternate method of radiofrequency neurotomy of the sacroiliac joint: a pilot study of the effect on pain, function, and satisfaction. Reg Anesth Pain Med. 2007;32:12–19. [DOI] [PubMed] [Google Scholar]
- 17.Cosman ER, Jr, Gonzalez CD. Bipolar radiofrequency lesion geometry: implications for palisade treatment of sacroiliac joint pain. Pain Pract. 2011;11:3–22. [DOI] [PubMed] [Google Scholar]
- 18.Karaman H, Kavak GO, Tüfek A, et al. Cooled radiofrequency application for treatment of sacroiliac joint pain. Acta Neurochir (Wien). 2011;153:1461–1468. [DOI] [PubMed] [Google Scholar]
- 19.Patel N, Gross A, Brown L, et al. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med. 2012;13:383–398. [DOI] [PubMed] [Google Scholar]
- 20.Hansen H, Manchikanti L, Simopoulos TT, et al. A systematic evaluation of the therapeutic effectiveness of sacroiliac joint interventions. Pain Physician. 2012;15:E247–E278. [PubMed] [Google Scholar]
- 21.Stelzer W, Aiglesberger M, Stelzer D, et al. Use of cooled radiofrequency lateral branch neurotomy for the treatment of sacroiliac joint-mediated low back pain: a large case series. Pain Med. 2013;14:29–35. [DOI] [PubMed] [Google Scholar]
- 22.Ho KY, Hadi MA, Pasutharnchat K, et al. Cooled radiofrequency denervation for treatment of sacroiliac joint pain: two-year results from 20 cases. J Pain Res. 2013;6:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng J, Pope JE, Dalton JE, et al. Comparative outcomes of cooled versus traditional radiofrequency ablation of the lateral branches for sacroiliac joint pain. Clin J Pain. 2013;29:132–137. [DOI] [PubMed] [Google Scholar]
- 24.Accident Rehabilitation and Compensation Insurance Corporation of New Zealand and the National Health Committee. New Zealand Low Back Pain Guide. Wellington. 1997.
- 25.Breivik EK, Bjornsson GA, Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clin J Pain. 2000;16:22–28. [DOI] [PubMed] [Google Scholar]
- 26.Ostelo RWJG, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain—towards international consensus regarding minimal important change. Spine. 2008;33:90–94. [DOI] [PubMed] [Google Scholar]
- 27.Ministry of Health, Welfare and Sports. Medisch-Wetenschappelijk Onderzoek [Medical and scientific research]. Available at: http://www.rijksoverheid.nl/documenten-en-publicaties/brochures/2008/10/02/medisch-wetenschappelijk-onderzoek.html. Accessed August 2010.
- 28.American Society of Anesthesiologists House of Delegates. Standards for basic anesthetic monitoring. Available at: http://www.asahq.org/. Accessed June 2014.
- 29.Grotle M, Brox JI, Vollestad NK. Concurrent comparison of responsiveness in pain and functional status measurement used for patients with low back pain. Spine. 2004;29:E492–E501. [DOI] [PubMed] [Google Scholar]
- 30.Van der Roer N, Ostelo RW, Bekkering GE. Minimal clinically important change for pain intensity, functional status, and general health status in patients with nonspecific low back pain. Spine. 2006;31:578–582. [DOI] [PubMed] [Google Scholar]
- 31.Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on a 11-point numerical pain rating scale. Pain. 2001;94:149–158. [DOI] [PubMed] [Google Scholar]
- 32.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331–1334. [DOI] [PubMed] [Google Scholar]
- 33.Kamper SJ, Ostelo RWJG, Knol DL, et al. Global Perceived Effect scales provided reliable assessments of health transition in people with musculoskeletal disorders, but ratings are strongly influenced by current status. J Clin Epidemiol. 2010;63:760–766. [DOI] [PubMed] [Google Scholar]
- 34.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- 35.Fischer D, Stewart AL, Bloch DA, et al. Capturing the patient’s view of change as a clinical outcome measure. J Am Med Assoc. 1999;282:1157–1162. [DOI] [PubMed] [Google Scholar]
- 36.Lindstrom I, Ohlund C, Eek C, et al. The effect of graded activity on patients with subacute low back pain: a randomized prospective clinical study with an operant-conditioning behavioral approach. Phys Ther. 1992;72:279–293. [DOI] [PubMed] [Google Scholar]
- 37.Staal JB, Hlobil H, Twisk JW, et al. Graded activity for low back pain in occupational health care: a randomized, controlled trial. Ann Intern Med. 2004;140:77–84. [DOI] [PubMed] [Google Scholar]
- 38.Keppel G. Design and Analysis A Researchers Handbook. Englewood Cliffs, NJ: Prentice Hall Inc.; 1973. [Google Scholar]
- 39.Gupta A. Radiofrequency ablation techniques for chronic sacroiliac joint pain. Pain Med News. 2010;6:1–8. [Google Scholar]
- 40.Guyatt G, Gutterman D, Baurmann MH, et al. Grading strength of recommendations and quality of evidence in clinical guidelines. Chest. 2006;129:174–181. [DOI] [PubMed] [Google Scholar]


