Abstract
Introduction
Increasing physical activity (PA) reduces the risk of developing diabetes, highlighting the role of preventive medicine approaches. Changing lifestyle behaviours is difficult and is often predicated on the assumption that individuals are willing to change their lifestyles today to reduce the risk of developing disease years or even decades later. The self-monitoring technologies tested in this study will present PA feedback in real time, parallel with acute physiological data. Presenting the immediate health benefits of being more physically active may help enact change by observing the immediate consequences of that behaviour. The present study aims to assess user engagement with the self-monitoring technologies in individuals at moderate-to-high risk of developing type 2 diabetes.
Methods and analysis
45 individuals with a moderate-to-high risk, aged ≥40 years old and using a compatible smartphone, will be invited to take part in a 7-week protocol. Following 1 week of baseline measurements, participants will be randomised into one of three groups: group 1— glucose feedback followed by biobehavioural feedback (glucose plus PA); group 2—PA feedback followed by biobehavioural feedback; group 3—biobehavioural feedback. A PA monitor and a flash glucose monitor will be deployed during the intervention. Participants will wear both devices throughout the intervention but blinded to feedback depending on group allocation. The primary outcome is the level of participant engagement and will be assessed by device use and smartphone usage. Feasibility will be assessed by the practicality of the technology and screening for diabetes risk. Semistructured interviews will be conducted to explore participant experiences using the technologies.
Trial registration number
ISRCTN17545949. Registered on 15/05/2017.
Keywords: flash glucose monitoring, type 2 diabetes, physical activity, prevention, activity trackers, bio-behavioural feedback
Strengths and limitations of this study.
The study will present real-time biological and behavioural information to participants using wearable technologies; a novel concept which has not been used in physical activity research.
The study will be the first to deploy flash glucose monitors to people at risk of developing type 2 diabetes.
A validated survey will be used to identify individuals at moderate-to-high risk of developing type 2 diabetes, among which we expect a proportion to have pre-diabetes.
While the duration of the intervention (six continuous weeks) will permit the examination of change in engagement over time, the absence of additional follow-ups prevents the assessment of long-term use engagement and behaviour change maintenance.
Cost-effectiveness analysis will not be undertaken in this study.
Introduction
There is widespread concern regarding the increasing prevalence of non-communicable diseases such as type 2 diabetes.1 Type 2 diabetes currently imposes an annual cost of £23.7 bn through its associated complications2; however, this cost is likely to rise as it is projected to directly impact 592 million individuals worldwide by 2035.3 Another imposing challenge is the proportion of the population living with undiagnosed diabetes (current prevalence estimated at 45.8%)4; which is possibly, in part, attributable to its asymptomatic state prior to the presentation of complications. Regardless of diagnosis status, preventing the development of type 2 diabetes is an international priority moving forward.5 Pre-diabetes, categorised as either impaired fasting glucose or impaired glucose tolerance represents abnormal glucose homoeostasis and is placed between diabetes and normal regulation. Impaired fasting glucose has been defined as elevated fasting plasma glucose (100–126 mg/dL) while impaired glucose tolerance is characterised by an elevated 2-hour plasma glucose concentration (140–199 mg/dL) following intake of a 75 g glucose load.6 One in seven adults have impaired glucose regulation7 and, compared with individuals living with normal-circulating glucose levels, individual with pre-diabetes are 5 to 10 times more likely to develop type 2 diabetes8 with 5%–10% of people becoming diabetic annually.9 Diabetes is projected to be 1 of 10 leading causes of death worldwide10; thus, identification and prevention are crucial for early intervention. A lack of physical activity is considered one of the major risk factors for non-communicable diseases and is comparable to the ill-effects of obesity11 and smoking12 individually. Given that physical inactivity, where insufficient levels of physical activity are achieved, is attributed to an estimated 7% of type 2 diabetes cases,13 it is an important modifiable lifestyle behaviour to target. With the prevalence of impaired fasting glucose doubling in individuals at 40–59 years and remaining consistent beyond 60 years,14 targeting efforts towards specific age cohorts is crucial. Individuals with abnormal glucose homoeostasis are referred onto community-based lifestyle behaviour programmes such as The Healthier You: National Diabetes Prevention Programme (NDPP). Initiated in 2016, the programme aims to roll out nationally by 2020 as part of the National Health Service (NHS) Five Year Forward plan.15 The present study intends to implement a community screening approach, monitor participant retention and to investigate whether self-monitoring technologies providing feedback about physical activity and interstitial glucose levels play a role in the prevention pathway (which may be amenable to the NDPP framework).
With increasing recognition towards the integration of technology into usual care pathways (ie, emergence of NHS Digital), it is a crucial time to consider how technologies could contribute to the management of chronic diseases. Given recent consumer interest,16 wearable technologies permit people to self-monitor behaviour and health. Gardner and colleagues17 reviewed behavioural interventions and identified self-monitoring of behaviour as a particularly promising behaviour change technique. Similarly, continuous glucose monitoring technology has shown promise for longer term physiological outcomes (including glycated haemoglobin (HbA1c)),18 supporting the suggestion that more frequent engagement leads to better health outcomes.19 Self-monitoring of both behaviour and outcomes are listed within the taxonomy alongside 91 other ingredients (ie, feedback and goal setting) in behavioural interventions.20 As well as delivering key behaviour change techniques, self-monitoring technologies also support Control Theory.21 More specifically, people are presented with information about a present state via feedback (eg, 9000 steps) and are often provided a set goal to achieve (ie, 10 000 steps). Equipped with this information, people may make efforts to achieve the goal or desired outcome (ie, ≥10 000 steps) because they have been informed how they are performing relative to it. The majority of research to date has focused on the deployment of technologies to self-monitor movement behaviours22 or specific health markers23 in isolation. Although these approaches have shown to be beneficial to behaviour change in the short term, most user engagement is not sustained beyond 6 months.24 Despite research conducted on short-term improvements, it is not yet clear whether results are sustained with prolonged use.25 26 However, the rationale is that when provided with information about their current levels of activity, people may feel motivated to improve their behaviour.
With a view to sustaining the ‘honeymoon period’ of technology-bolstered behaviour change, a logical next step would be to deploy wearable technologies in combination. For example, studies investigating the acute effects of brief physical activity bouts or interruptions to prolonged sedentary behaviour on glucose levels in controlled settings have found reductions in postprandial glucose as a result of increased movement.27–30 As a result, the present study proposes that delivering behavioural and physiological feedback in parallel may be more persuasive rather than when delivered in isolation. This approach may offer a platform for people to self-educate themselves about the relationship between movement and acute health status (ie, walking after a meal leads to marked reductions in glucose levels); which may help sustain engagement with self-monitoring technologies. With ongoing developments, technologies such as flash glucose monitoring offer a wealth of information to users without the need for invasive fingerprick samples; offering a useful tool for individuals without diabetes (who are not accustomed to regular fingerprick blood samples).31 To date, an important limitation of the efforts to encourage people to be more physically active has been the assumption that we are willing to change our lifestyles today to reduce our risk of developing disease years or even decades later. Implementing specific behaviour change techniques such as self-monitoring, goal setting and feedback,20 wearable devices could empower individuals to manage their health through a change in behaviour by recognising movement patterns and observing influences on health. Building on previous findings which observed greater levels of brain activation in response to personalised glucose-related information (over behavioural information) (Whelan et al, submitted), the present study aims to examine the role of providing novel self-monitoring technologies presenting biobehavioural feedback in those living at moderate-to-high risk of type 2 diabetes.
Aims and objectives
Primary aim
The primary aim of this study is to investigate participant engagement using self-monitoring technologies for physical activity and interstitial glucose.
Secondary aims
The secondary aims of this study are to explore (1) the feasibility of the intervention trial at baseline, 1, 2, 3, 4, 5 and 6 weeks; (2) levels of physical activity and interstitial glucose levels at baseline, 1, 2, 3, 4, 5 and 6 weeks and (3) levels of technology readiness, health literacy, health status and attitudes towards one’s own health at baseline and post self-monitoring.
Methods and analysis
Study setting
Participants will be recruited from the community in Leicestershire, UK from May to November 2017. All appointments (three or four in total, depending on group allocation) will take place at the National Centre for Sport and Exercise Medicine at Loughborough University, UK.
Study design
The feasibility study protocol has been prepared in accordance with the Standard Protocol Items: Recommendations for Interventional Trials32 with reference to the Template for Intervention Description and Replication33 (see online supplementary file 1).
bmjopen-2017-018282supp001.pdf (153.6KB, pdf)
The study will aim to recruit 45 individuals with 15 participants randomly allocated to each of the three groups. No specific sample size has been calculated due to its feasibility status but study results will inform the sample size for a full-scale intervention.
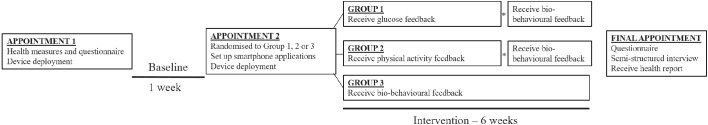
The Sensing Interstitial Glucose to Nudge Active Lifestyle study will last 7 weeks in total and is outlined in figure 1. Following baseline (1 week), participants will be randomised into one of three groups. Participants will be notified of their group allocation at the second appointment before starting the intervention period. Appointments will be arranged at the preceding appointment where possible. The study was registered on the International Standard Randomised Controlled Trial number (ISRCTN) Register (ISRCTN17545949) in May 2017.
Figure 1.
An illustration of the intervention design (*indicates a brief appointment at 4 weeks).
Randomisation
Participants will be block randomised using a 1:1:1 study allocation ratio, coordinated by a remote internet-based service (http://www.sealedenvelope.com/). Randomisation will be done by a member of the research group, independent to the present study. Baseline measures will be conducted prerandomisation. Participants will be notified of their group allocation at appointment 2. In the event of participants originating from the same household, identical group allocation will be employed to avoid any cross-contamination.
Inclusion criteria
Participants will be at least 40 years old, be at moderate-to-high risk of developing type 2 diabetes34 and use a compatible Android smartphone.
Compatible smartphones at the time of the study will be defined as having the following characteristics: an Android operating system of 4.0 or higher, Near Field Communication (NFC), a screen resolution of 480×800 to 1080×1920 and a screen size of 8.9–14.5 cm. Exceptions at the time of the study are the Samsung Galaxy 7, Samsung S8, Nexus 5X and Nexus 6P which cannot install the LibreLink application.
Exclusion criteria
Individuals with a clinical diagnosis of type 1 or type 2 diabetes, an HbA1c of ≥6.5% or have suspected/confirmed pregnancy will be excluded. Participants unable/unwilling to provide informed consent, cannot/unwilling to adhere to the study protocol or cannot read/write English will also be excluded.
Recruitment procedure
Participants will be recruited at community sites through the distribution of posters and leaflets in community organisations and local businesses based in Leicestershire, UK. Individuals will also be recruited through an existing Movement Insights Lab participant database. All individuals will be directed to complete a brief survey to determine level of risk for type 2 diabetes. Participant information sheets will be provided (copies available on request). The questions will be presented via an online survey platform (Qualtrics, Provo, Utah, USA) and will relate to gender, age, ethnic background, familial history of diabetes, waist circumference, body mass index (BMI) and blood pressure. The validated survey34 has been used in studies applying risk score algorithms on primary care electronic data.35 Waist circumference will be replaced with clothing size and fit following guidance offered by Battram and colleagues.36 Moderate-to-high risk individuals will be contacted by the research team to take part in the study. Ineligible individuals (ie, low risk, increased risk or a moderate/high risk, but are not aged at least 40 years old nor use an Android smartphone) will be directed to Diabetes UK ‘Type 2 diabetes: What to do if you’re at risk’ information booklets (available at: https://www.diabetes.org.uk/Global/professionals/KYR%20Booklet.pdf).
Study procedure
First appointment and baseline
An outline of the study procedure is presented in figure 1. Appointment one will involve informed consent, health measures (height, weight, percentage body fat, waist circumference, blood pressure, HbA1c, grip strength, quadriceps strength and aerobic fitness; full methodological details are provided in the measures section below) and a brief demographics questionnaire. Participants will complete a physical activity readiness questionnaire37 before completing the aerobic fitness assessment for screening purposes. Participants will be fitted with a waist-worn accelerometer and a wrist-worn activity tracker. Neither device will provide feedback to the participant during the seven consecutive days of wear. Participants will be asked to install two mobile applications onto a personal Android smartphone. Both smartphone applications will sit idle on the smartphone for the duration of baseline. Participants will be asked to sync the activity tracker via the smartphone application; switching on Wi-Fi and Bluetooth simultaneously at least once every 5 days for ≥1 hour to ensure the sync occurs.
Second appointment and intervention
One week later (following baseline), participants will attend appointment 2 where they will be informed of their group allocation. Participants will be asked to complete a brief questionnaire to continue wearing the activity tracker during the intervention (settings may or may not be adjusted) and to return the accelerometer. A glucose sensor will be deployed to each participant to measure interstitial glucose levels. Participants will be provided with additional supplies of glucose sensors to last for the four (groups 1 and 2) or six weeks (group 3) of the intervention. Accounts for both the activity tracker and glucose sensor will be connected to Diasend (Diasend, Chicago, Ilinois, USA). An overview of the three groups is provided below.
Group 1 (glucose feedback followed by biobehavioural feedback)
Real-time interstitial glucose feedback will be presented to participants for 4 weeks via the LibreLink application (Abbott Diabetes Care, Alameda, California, USA). Participants will install the LibreLink mobile application (Abbott Diabetes Care, Alameda, California, USA) onto a personal Android smartphone to interact with the Freestyle Libre via NFC for measurement of interstitial glucose. The glucose monitor has a lifespan that restricts wear to 14 consecutive days. The application will remind participants to scan every 7 hours and to remove/replace after 14 days. The LibreLink application will continuously display the number of days left.
Group 2 (physical activity feedback followed by biobehavioural feedback)
Real-time physical activity feedback will be presented for 4 weeks via the Fitbit application. In contrast to group 1, participants will not have the LibreLink application installed and so will not have access to glucose feedback. Participants will be informed that the glucose sensor is functional (recording data) and participants will be asked to remove and replace the expired sensor with another sensor after 14 days.
Device unmasking for groups 1 and 2 after 4 weeks
At the end of the first 4 weeks of the intervention, participants in groups 1 and 2 will attend a brief appointment (up to 1 hour in duration). For group 1, the researcher will adjust settings to reveal physical activity feedback via the Fitbit application and device. For group 2, the researcher will install the LibreLink application to reveal glucose feedback. All participants will be able to access biobehavioural feedback for the remaining 2 weeks of the intervention.
Group 3 (biobehavioural feedback)
Participants in group 3 will receive biobehavioural feedback for the full 6 weeks via the two independent LibreLink and Fitbit applications. Participants will install the LibreLink mobile application onto a personal Android smartphone to interact with the Freestyle Libre to measure interstitial glucose. The application will remind participants to scan every 7 hours and to remove/replace the sensor after 14 days.
Final appointment
All participants (groups 1, 2 and 3) will be asked to attend the final appointment at the end of the intervention where they will complete a questionnaire (identical to appointment 2, apart from the revised Diabetes Knowledge Test) and a semistructured interview. All participants will also receive a personalised health report containing results from the health measures conducted at appointment one.
Device masking
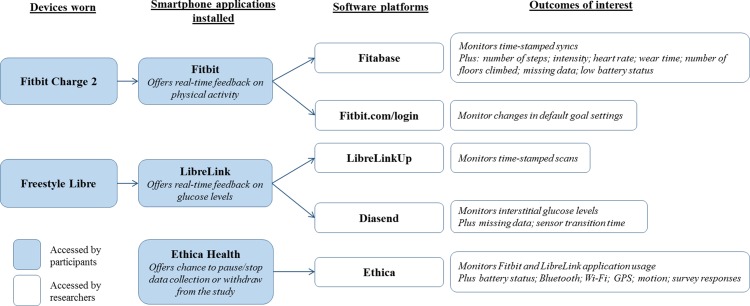
All email accounts and password combinations will be manually generated and managed by the research team to prevent use of identifiable information. During baseline wear, the activity tracker will be physically masked using black tape applied to the screen; leaving only time and date viewable. Participants will be asked not to tamper with the screen; however, if they do manipulate the masking, it should be readily apparent to the research team. Settings on the application will also be adjusted to remove physical activity metrics from the device screen and notifications fully restricted on their phone and activity tracker. However, participants will not be locked out of the application due to the requirement to sync the device. Time spent on the Fitbit application will be inspected using Ethica Data (Kitchener, Ontario, Canada) to identify potential unauthorised use. The activity tracker will also be set to all-day sync to minimise data loss with data automatically transferred (Wi-Fi and Bluetooth must both be simultaneously switched on). When required to prevent access to glucose feedback, participants will wear the glucose sensors for 14-day period as normal but will not be asked to install the LibreLink application nor scan the sensor (ie, no data will be collected). This will standardise wear across all three groups.
Data management and storage procedures
All data collected will be anonymised by assigning a participant identification. Accounts with the three applications (Fitbit, LibreLink and Ethica Health) will be setup using study-specific (‘dummy’) email addresses and passwords (accessible only to the research team) to minimise use of personalised information. All data will be stored securely on the Loughborough University server, as password-protected, encrypted documents and original paperwork kept in locked storage. No directly personally identifiable information will be collected through these platforms. Global positioning system (GPS) will be collected via Ethica Data which could theoretically be ‘reverse-engineered’ to reidentify individuals; however, all participants will be explicitly informed about all information monitored as part of the study. For individuals who do not wish to have their location services monitored, we will set up a ‘reduced access’ version of Ethica Data (application usage, screen state and survey responses only).
Primary outcomes
User engagement: Quantitative
Time spent on the official free Fitbit and LibreLink applications will be quantified using Ethica Data as well as time-stamped data relating to when the smartphone screen was turned on and off. In combination, these two data sources will reveal the proportion of time that the devices’ applications were used in relation to total smartphone use. These data will be recorded at either a day level (eg, aggregate time) or event level (eg, record of each time an application was opened) depending on the Android smartphone model. How often and how much time spent on the two applications compared with other applications on participants’ smartphones will also be quantified. Number of times the activity tracker syncs (occurs when the application is opened, assumed to see feedback about physical activity) and scans of the glucose sensor (occurs when the participant scans and to see feedback about interstitial glucose levels) will also be recorded. Compulsory engagement will be participants having to sync the activity tracker at least once every 5 days and scan the glucose sensors at least once every 7 hours. The number of syncs and scans recorded over and above compulsory engagement will reflect optional engagement. Identifying when and how often syncs and scans happen and how these patterns change over the course of the intervention (from week 1 to 6) will indicate engagement with the technology. We will also identify if participants change the goal settings relating to steps, floors climbed and active minutes on the Fitbit application. These settings will be checked daily between the hours of 18:00-19:00 by the research team and changes will be flagged with details of the original and new setting logged. In addition, assessing whether participants responded to prompts offered by the activity tracker will also be conducted (ie, did participants achieve 250 steps/hour? See Behaviour Change Techniques section for further detail).
Remote monitoring of participant glucose and physical activity will be completed using Diasend and Fitabase (Small Steps Labs LLC, San Diego, California, USA), respectively. Diasend will connect with the Freestyle Libre via the LibreLink application and data will be recorded and accessed through this software. Additional data sources to be monitored by Ethica Data include battery status (ie, smartphone plugged in? Charging?), Bluetooth and Wi-Fi (turned on or off). Quantifying these data sources will provide valuable insight into participant behaviour (eg, Do participants only use Wi-Fi and Bluetooth for the purpose of our intervention? Are participants charging it more often in the intervention compared with baseline?). Ethica Data will also monitor location (GPS), motion (pedometer, accelerometer, gravity, gyroscope, linear acceleration, magnetic field, orientation) and survey responses. These digital streams will monitor smartphone usage and will provide detailed data on human behaviour during a free-living, naturalistic setting. In total, 14 data sources will be monitored. In the event a participant raises concerns relating to the number and/or type of data sources being monitored, a restricted coverage option of only three data sources (application usage, screen state and survey responses) will be offered.
User engagement: qualitative
For participants who complete the 6-week intervention, a semistructured interview will be completed (20–40 min) during the final appointment at the National Centre for Sport and Exercise Medicine, Loughborough University, UK. The interview will aim to identify potential barriers and facilitators to using self-monitoring technologies. In particular, how participants experience receiving feedback relating to physical activity and interstitial glucose levels. These interviews will explore individual experiences using the device(s) and mobile application(s), adherence to syncing (Fitbit) and scanning (Freestyle Libre), wearing multiple devices and the perceived effect of viewing feedback on actual behaviour. In addition, participants will be asked about future intentions to continue wearing self-monitoring devices and identify any recommended changes for future study designs.
If a participant decides to withdraw from the study at any time prior to the final appointment, they will be able to leave the study via (1) the Ethica Health application on their personal smartphone (aligning with a dynamic consenting process38) or by (2) contacting the research team via telephone or email. Participants that decide to withdraw via Ethica Health will be directed to complete a brief exit survey on the application. The research team will contact all participants for an optional exit interview (5–10 min) via telephone. This will be recorded using Tapeacall (http://www.tapeacall.com/) and will explore reasons for not completing the study.
Secondary outcomes
Feasibility
Guidelines used to assess the feasibility of this study were informed by Bowen and colleagues.39 Both qualitative and quantitative data will be collected to assess feasibility of deploying novel self-monitoring technologies in parallel. In total, we will assess intervention feasibility as outlined in table 1.
Table 1.
An overview of the feasibility components to be assessed
| Feasibility component | Data source (indicator of feasibility) |
| Practicality of technology/intervention |
|
| Acceptability of technology/intervention |
|
*Full coverage: application usage, screen state, Bluetooth, Wi-Fi, GPS, pedometer, accelerometer, gravity, gyroscope, linear acceleration, magnetic field, orientation, battery and survey responses.
†Restricted coverage: application usage, screen state and survey responses.
GPS, global positioning system
Physical activity levels
ActiGraph
In an effort to determine the physical activity levels of the participants relative to general population, participants will be asked to wear an ActiGraph wGT3X-BT (ActiGraph, Pensacola, Florida, USA) accelerometer for 7 days during waking hours and to remove for any water-based activities (eg, showering and swimming). The waist-worn (ie, over the right hip, midclavicular line) ActiGraph will quantify time spent sedentary, in light and moderate-to-vigorous physical activity (MVPA) as well as daily step counts and will function as a data logger (ie, no feedback provided). ActiGraph accelerometers have been validated40 41 and extensively deployed42–44 to measure physical activity under free-living conditions. Data from the ActiGraph will be collected at 100 Hz and integrated into 60 s epochs using ActiLife (ActiGraph, Pensacola, Florida, USA) and processed using Kinesoft (Kinesoft, Loughborough, UK). Non-wear will be defined as 60 min of consecutive zeros (allowing for up to 2 min of interruptions) with a minimum wear of 600 waking minutes used to define a valid day.43 A minimum of four valid days will be used to define a valid file with sedentary time classified as <100 cpm, light activity as 100-2019 cpm and MVPA as >=2020 cpm.43
Fitbit
The Fitbit Charge 2 (Fitbit, San Francisco, California, USA) will be worn on the wrist associated with the non-dominant arm and, while being sweat, rain and splash proof, participants will be asked to remove the device for water-based activities. The Fitbit records intensity (ie, minutes spent lightly active, fairly active and very active) in addition to heart rate and step count. Heart rate will be assessed using Fitbit’s proprietary PurePulse optical heart rate technology. To examine changes in physical activity over the study duration, participants will be requested to wear the device for the full 7 weeks and data will be analysed in 60 s epochs following export from Fitabase. Previous models of the Fitbit have been validated for step count.45 A waking protocol will be implemented with non-wear defined as a loss of a heart rate signal. Participants will be requested to sync the Fitbit at least once every 5 days (rather than the company recommendations of 7 days) to minimise data loss. Syncs beyond 7 days will result in day level data rather than minute level data. These syncs will either occur automatically (ie, without the application open) or will be user driven (ie, with the application open) depending on how the all-day sync is set and heart rate will be set to automatic (only record heart rate when device is worn).
Interstitial glucose levels
Freestyle Libre
The minimally invasive Freestyle Libre flash glucose monitor (Abbott Diabetes Care, Alameda, California, USA) will be covered with Tegaderm (3M Health Care, St Paul, Minnesotta, USA) to help maintain position and adhesion during the 14-day sensor life span. Three strips of Tegaderm will be provided to participants per sensor to allow for replacement when the Tegaderm becomes dirty. Participants will be asked to wear the device continuously without removal for water-based activities. The Freestyle Libre demonstrates consistent accuracy throughout the 14 days with a mean absolute relative difference of 11.4% compared with capillary blood glucose, a lag time of 4.5–4.8 min and is not impacted by physical characteristics including age, BMI and HbA1c.31 Participants will be requested to scan the glucose monitor at least once every 7 hours (rather than the company recommendations of 8 hours) to minimise data loss. If participants experience skin irritation on the non-dominant arm in the region of application, participants will be advised to switch to their dominant arm. Interstitial glucose data will be downloaded in 15 min epochs using Diasend, an online platform connected to the LibreLink application. Participant accounts will be linked to Diasend from the point of LibreLink application installation. Figure 2 illustrates how the numerous components connect to achieve the primary and secondary aims.
Figure 2.
A schematic of how the wearable technologies, mobile applications and software connect.
Levels of technology readiness, health status and attitude
All questionnaires will be completed electronically using an online platform for immediate data entry (http://www.onlinesurveys.ac.uk/; Bristol, UK). At appointment 2, quality of life will be assessed via the 26-item EuroQoL 5 Dimensions 5 Levels,46 technology readiness via the 16-item Technology Readiness Index V. 2.0,47 health literacy via the eight-item eHealth Literacy Scale,48 diabetes knowledge via the 20-item revised diabetes knowledge test49 and general attitude towards developing diabetes via the eight-item general attitudes section of the Risk Perception Survey for Developing Diabetes.50
Other measures
Participant characteristics
Self-reported age, sex, ethnic background, employment, household income, postcode (to provide an Index of Multiple Deprivation score) and education will be recorded.
Health, physical functioning and fitness
HbA1c will be assessed at the first appointment using a point-of-care system, (Afinion AS100 Analyser, Alere, Waltham, Massachusetts). Results will be processed immediately following collection. Participants receiving a result ≥6.5% will be ineligible, readings of 5.7%–6.4% classified as with pre-diabetes51 and readings of <5.7% classified as euglycaemic. A measure of height will be conducted using a Seca stadiometer (Seca, Hamburg, Germany) and weight and body fat percentage will be measured using Tanita scales (Tokyo, Japan). Two measures of waist circumference will be taken at the midpoint between the lowest rib and top of the iliac crest; if the difference exceeds 1 cm, the two measurements will be repeated.52 Three measures of blood pressure will be recorded using an Omron digital monitor (Omron, Kyoto, Japan) with the first measure taken after the participant has remained seated for 10 min. Grip strength will be assessed using a handheld Takei dynamometer (Takei Scientific Instruments, Tokyo, Japan) while standing with hands positioned down each side. Quadriceps strength will be assessed using the DAVID G200 knee extension machine (David Health Solutions, Helsinki, Finland). Aerobic fitness will be assessed using the modified Canadian Aerobic Fitness Test (mCAFT).53 The mCAFT is a submaximal step-test protocol with participants instructed to complete ≥1 3-min stages of stepping at a speed dictated by an audio track. Heart rate will be monitored throughout with the stepping stages continued until heart rate ≥85% of age-predicted maximal heart rate. Participants’ scores for aerobic fitness will be defined according to the following formula: 10*[17.2 + (1.29×oxygen cost at the final stage) - (0.09×weight in kg) - (0.18×age in years)].53
Behaviour change techniques
Prior to starting the intervention, the researcher will implement the default settings for levels of physical activity (behaviour change technique (BCT) 1.1: goal setting (behaviour)) (ie, 10 000 steps and 10 floors climbed) and glucose (BCT 1.3: goal setting (outcome)) (ie, 4.0–5.9 mmol/L). Participants will be fully informed that they can freely change the goals set for physical activity as preferred (ie, should the default value be too easy/difficult) via the Fitbit application. However, participants will be advised to not make any changes via the LibreLink application for the target glucose range. Attainment of a goal will be assessed as either complete or incomplete. Participants will be asked to sync the Fitbit (at least once every 5 days) and scan the Freestyle Libre (at least once every 7 hours) if they are in the respective group to receive feedback from these devices. This action has a dual purpose; to minimise data loss and to encourage continued engagement with the technologies. Participants will also receive haptic feedback (BCT 7.1: prompts/cues; ie, a gentle vibration) as a reminder to move by the Fitbit 10 min prior to the end of each hour (default 09:00-18:00) if 250 steps have not been taken. The reminder to move prompt aims to encourage interruptions in prolonged sedentary bouts as is recommended by the UK Physical Activity Guidelines.54 In relation to the other behaviour change techniques, participants will be able to monitor physical activity levels using the Fitbit Charge 2 (BCT: 2.3: self-monitoring of behaviour) and glucose levels using the Freestyle Libre (BCT: 2.4: self-monitoring of outcome(s) of behaviour) which is a minimally invasive device that presents feedback about glucose (BCT: biofeedback).
Quantitative data analysis
Analysis of primary outcomes
Ethica Data is a fee-for-service platform that will be used to provide time-stamped data relating to application usage. This is an application installed on participants’ phones and sits idle during the study period. The number of scans and syncs will be unobtrusively assessed using the free LibreLinkUp mobile application (Abbott Diabetes Care, Alameda, California, USA) and Fitabase, respectively. Fitabase is a fee-for-service platform that permits access to download 60 s epoch Fitbit data (ie, levels of physical activity) and remote monitoring of Fitbit devices (eg, battery level and time since last sync event) via Bluetooth and Wi-Fi. Identification of moments where participants have decided to change the goal settings will be completed by accessing the online Fitbit account. The researchers will remotely access participants’ accounts daily between 18:00 and 19:00 to note goal settings; recording the date and live/current settings for all metrics (eg, step count) to help identify any changes.
Analysis of secondary outcomes
To assess eligibility, uptake and retention, we will manually record how many individuals complete the screening survey, how many meet our inclusion criteria and of these how many decide to enrol. In addition, the screening survey will also identify recruitment sources. Identifying non-usage attrition and dropout attribution is crucial to assess the feasibility of an intervention as they are both important but distinct constructs.55 Non-usage attrition, where participants have disengaged from the intervention but have not dropped out, will be defined as participants who attend appointment 2 but do not sync the Fitbit or scan the Freestyle Libre. Dropout attrition will be defined as participants who explicitly withdraw from the study via Ethica Health or direct contact with the research team. The number of participants who enrol into the full coverage (all 14 data sources monitored) or restricted coverage (only three data sources monitored) for Ethica Data will also be recorded. Diasend is a fee-for-service platform that permits access to download 15 min epoch data from the Freestyle Libre and remote monitoring of multiple LibreLink accounts. Descriptive statistics of the sample will be conducted. In addition, two-way repeated measures analyses of covariances (ANCOVAs) will be conducted to assess changes in engagement (dependent) according to group (independent) having adjusted for participant characteristics. Similarly, two-way repeated measures ANCOVAs will be conducted to assess changes in physical activity (dependent) according to group (independent) having adjusted for baseline physical activity, Fitbit wear time and participant characteristics. All data will be analysed using SPSS Version 24.0).
Qualitative data analysis
All interviews will be audiorecorded (with informed consent), transcribed verbatim and analysed using thematic analysis. This will involve standard thematic data analysis procedures; identifying emerging patterns in the interview.56 Transcripts will be analysed using constant comparison with initial free coding and emergent themes interrogated.57 The interview schedule and coding schedule will be modified to follow new leads until new themes no longer emerge. The analysis will create a coding frame that ‘fits’ the data.57 Transcripts will be uploaded into NVivo qualitative data analysis software (QSR International).
Dissemination
This work will inform a full-scale randomised-controlled trial (RCT) by enabling a sample size calculation. The full-scale RCT will primarily aim to investigate changes in physical activity and interstitial glucose levels in individuals randomised into the three groups. Overall, the findings seek to encourage the implementation of technologies into usual preclinical care pathways; in particular, how engaging with self-monitoring technologies (providing biobehavioural feedback) may positively influence rates of uptake, adherence, retention and behaviour change.
We will publicise study findings online, present them at international conferences relating to diabetes, physical activity and digital health and publish via a peer-reviewed journal.
Acknowledgements
The authors would like to acknowledge the support received from Alison Stanley and Rebecca Pritchard and the Patient and Public Involvement Group (NIHR Leicester Biomedical Research Centre-Lifestyle) for contributing to the study design and providing feedback on the participant-facing documents. The authors would also like to acknowledge Francesca Denton (School of Sport, Exercise and Health Sciences, Loughborough University) for her enthusiasm and willingness to support data collection. The primary author would like to thank Professor Paul Morgan for his contribution to the wider supervision team. This activity will be conducted under the auspices of the National Centre for Sport and Exercise Medicine (NCSEM), a collaboration between several universities, NHS trusts and sporting and public bodies. The views expressed are those of the authors and not necessarily those of NCSEM England or the partners involved.
Supplementary Material
Footnotes
Contributors: All authors have contributed to the design of the work, acquisition and analysis plan. MW, AK, MO, LS and DE were involved in the development of the intervention and design of the trial. They have also been involved in drafting the work or revising it critically for important intellectual content.
Funding: This work will be funded in part by philanthropic support received from the late Dr the Honourable David Saul. This work has also been supported in part by the Higher Education Institution Challenge for Patient Supported Quality Improvement and Education in Health and Social Care (funded by the East Midlands Academic Health Science Network) for the involvement of members of the public in research and by Loughborough University School of Sport, Exercise and Health Science for research facilitation funds. The funders had no role in the design, analysis or writing of this article.
Competing interests: None declared.
Ethics approval: Loughborough University Ethics Advisory Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional unpublished data are still being collected and analysed and are only available to members of the study team.
References
- 1. World Health Organization. Global status report on non communicable diseases, 2014. [Google Scholar]
- 2. Hex N, Bartlett C, Wright D, et al. Estimating the current and future costs of type-1 and type-2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med 2012;29:855–62. 10.1111/j.1464-5491.2012.03698.x [DOI] [PubMed] [Google Scholar]
- 3. Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137–49. 10.1016/j.diabres.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 4. Beagley J, Guariguata L, Weil C, et al. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract 2014;103:150–60. 10.1016/j.diabres.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 5. Barry E, Roberts S, Oke J, et al. Efficacy and effectiveness of screen and treat policies in prevention of type 2 diabetes: systematic review and meta-analysis of screening tests and interventions. BMJ 2017;356:i6538 10.1136/bmj.i6538 [DOI] [PubMed] [Google Scholar]
- 6. Genuth S, Alberti KG, Bennett P, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–7. [DOI] [PubMed] [Google Scholar]
- 7. Diabetes UK. Position Statement: early identification of people with type 2 diabetes London Diabetes UK, 2006. [Google Scholar]
- 8. Santaguida PL, Balion C, Hunt D, et al. Diagnosis, prognosis, and treatment of impaired glucose tolerance and impaired fasting glucose. Evid Rep Technol Assess 2005:1-11. [PMC free article] [PubMed] [Google Scholar]
- 9. Forouhi NG, Luan J, Hennings S, et al. Incidence of Type 2 diabetes in England and its association with baseline impaired fasting glucose: the Ely study 1990-2000. Diabet Med 2007;24:200–7. 10.1111/j.1464-5491.2007.02068.x [DOI] [PubMed] [Google Scholar]
- 10. Tabák AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279–90. 10.1016/S0140-6736(12)60283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 2005;352:1138–45. 10.1056/NEJMsr043743 [DOI] [PubMed] [Google Scholar]
- 12. National Research Council and Committee on Population. Explaining divergent levels of longevity in high-income countries: National Academies Press, 2011. [PubMed] [Google Scholar]
- 13. Lee IM, Shiroma EJ, Lobelo F, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–29. 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–94. 10.2337/dc08-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. NHS England. Five year Forw view, 2016. [Google Scholar]
- 16. Ferguson T, Rowlands AV, Olds T, et al. The validity of consumer-level, activity monitors in healthy adults worn in free-living conditions: a cross-sectional study. Int J Behav Nutr Phys Act 2015;12:42 10.1186/s12966-015-0201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gardner B, Smith L, Lorencatto F, et al. How to reduce sitting time? A review of behaviour change strategies used in sedentary behaviour reduction interventions among adults. Health Psychol Rev 2016;10:89–112. 10.1080/17437199.2015.1082146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vigersky RA, Fonda SJ, Chellappa M, et al. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012;35:32–8. 10.2337/dc11-1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fonda SJ, Lewis DG, Vigersky RA. Minding the gaps in continuous glucose monitoring: a method to repair gaps to achieve more accurate glucometrics. J Diabetes Sci Technol 2013;7:88–92. 10.1177/193229681300700110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81–95. 10.1007/s12160-013-9486-6 [DOI] [PubMed] [Google Scholar]
- 21. Carver CS, Scheier MF. Control theory: a useful conceptual framework for personality-social, clinical, and health psychology. Psychol Bull 1982;92:111–35. 10.1037/0033-2909.92.1.111 [DOI] [PubMed] [Google Scholar]
- 22. Cadmus-Bertram LA, Marcus BH, Patterson RE, et al. Randomized trial of a fitbit-based physical activity intervention for women. Am J Prev Med 2015;49:414–8. 10.1016/j.amepre.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polonsky WH, Fisher L. Self-monitoring of blood glucose in noninsulin-using type 2 diabetic patients: right answer, but wrong question: self-monitoring of blood glucose can be clinically valuable for noninsulin users. Diabetes Care 2013;36:179–82. 10.2337/dc12-0731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ledger D, McCaffrey D, Partners E. Inside Wearables: How the Science of Human Behavior Change O!ers the Secret to Long-Term Engagement, 2014. http://endeavourpartners.net/assets/Endeavour-Partners-Wearables-White-Paper-20141.pdf.
- 25. Barwais FA, Cuddihy TF, Tomson LM. Physical activity, sedentary behavior and total wellness changes among sedentary adults: a 4-week randomized controlled trial. Health Qual Life Outcomes 2013;11:183 10.1186/1477-7525-11-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tudor-Locke C, Lutes L. Why Do Pedometers Work? Sports Medicine 2009;39:981–93. 10.2165/11319600-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 27. Peddie MC, Bone JL, Rehrer NJ, et al. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am J Clin Nutr 2013;98:358–66. 10.3945/ajcn.112.051763 [DOI] [PubMed] [Google Scholar]
- 28. DiPietro L, Gribok A, Stevens MS, et al. Three 15-min bouts of moderate postmeal walking significantly improves 24-h glycemic control in older people at risk for impaired glucose tolerance. Diabetes Care 2013;36:3262–8. 10.2337/dc13-0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reynolds AN, Mann JI, Williams S, et al. Advice to walk after meals is more effective for lowering postprandial glycaemia in type 2 diabetes mellitus than advice that does not specify timing: a randomised crossover study. Diabetologia 2016;59:2572–8. 10.1007/s00125-016-4085-2 [DOI] [PubMed] [Google Scholar]
- 30. Dunstan DW, Kingwell BA, Larsen R, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 2012;35:976–83. 10.2337/dc11-1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bailey T, Bode BW, Christiansen MP, et al. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther 2015;17:787–94. 10.1089/dia.2014.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 34. Gray LJ, Taub NA, Khunti K, et al. The leicester risk assessment score for detecting undiagnosed type 2 diabetes and impaired glucose regulation for use in a multiethnic UK setting. Diabet Med 2010;27:887–95. 10.1111/j.1464-5491.2010.03037.x [DOI] [PubMed] [Google Scholar]
- 35. Gray LJ, Davies MJ, Hiles S, et al. Detection of impaired glucose regulation and/or type 2 diabetes mellitus, using primary care electronic data, in a multiethnic UK community setting. Diabetologia 2012;55:959–66. 10.1007/s00125-011-2432-x [DOI] [PubMed] [Google Scholar]
- 36. Battram DS, Beynon C, He M. The reliability and validity of using clothing size as a proxy for waist circumference measurement in adults. Appl Physiol Nutr Metab 2011;36:183–90. 10.1139/h11-001 [DOI] [PubMed] [Google Scholar]
- 37. Warburton DER, Jamnik VK, Bredin SSD, et al. The physical activity readiness questionnaire (PAR-Q) and electronic physical activity readiness medical examination (ePARmed-X). Heal Fit J Can 2011;4:3–23. [Google Scholar]
- 38. Teare HJA, Morrison M, Whitley EA, et al. Towards ‘Engagement 2.0’: Insights from a study of dynamic consent with biobank participants. Digit Health 2015;1:205520761560564 10.1177/2055207615605644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med 2009;36:452–7. 10.1016/j.amepre.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Plasqui G, Westerterp KR. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity 2007;15:2371–9. 10.1038/oby.2007.281 [DOI] [PubMed] [Google Scholar]
- 41. MELANSON EL, Freedson PS. Validity of the Computer Science and Applications, Inc. (CCA) activity monitor. Medicine & Science in Sports & Exercise 1995;27:934–40. 10.1249/00005768-199506000-00021 [DOI] [PubMed] [Google Scholar]
- 42. Hagströmer M, Oja P, Sjöström M. Physical activity and inactivity in an adult population assessed by accelerometry. Med Sci Sports Exerc 2007;39:1502–8. 10.1249/mss.0b013e3180a76de5 [DOI] [PubMed] [Google Scholar]
- 43. Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181–8. 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 44. Chaudhury M, Esliger DW. Health survey for England: physical activity and fitness. Obesity 2008;1:59–88 http://www.hscic.gov.uk/catalogue/PUB00430/heal-surv-phys-acti-fitn-eng-2008-rep-v2.pdf. [Google Scholar]
- 45. Lee JM, Kim Y, Welk GJ. Validity of consumer-based physical activity monitors. Med Sci Sports Exerc 2014;46:1840–8. 10.1249/MSS.0000000000000287 [DOI] [PubMed] [Google Scholar]
- 46. Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parasuraman A, Colby CL. An updated and streamlined technology readiness index. Journal of Service Research 2015;18:59–74. 10.1177/1094670514539730 [DOI] [Google Scholar]
- 48. Norman CD, Skinner HA. eHealth Literacy: essential skills for consumer health in a networked world. J Med Internet Res 2006;8:e9 10.2196/jmir.8.2.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Collins GS, Mughal S, Barnett AH, et al. Modification and validation of the revised diabetes knowledge scale. Diabet Med 2011;28:306–10. 10.1111/j.1464-5491.2010.03190.x [DOI] [PubMed] [Google Scholar]
- 50. Walker EA, Mertz CK, Kalten MR, et al. Risk perception for developing diabetes: comparative risk judgments of physicians. Diabetes Care 2003;26:2543–8. 10.2337/diacare.26.9.2543 [DOI] [PubMed] [Google Scholar]
- 51. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl 1):S81–90. [DOI] [PubMed] [Google Scholar]
- 52. World Health Organization. Waist circumference and waist-hip ratio: Report of a WHO expert consultation. Geneva 2011. 8 11. [Google Scholar]
- 53. Health Appraisal F. P. H.-R., & Strategy C CSEP. Ontario Can Soc Exerc Physiol The Canadian physical activity, fitness and lifestyle approach, 2004. [Google Scholar]
- 54. UK Department of Health. Physical Activity Guidelines for Adults 2011. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/213740/dh_128145.pdf. [Google Scholar]
- 55. Alkhaldi G, Hamilton FL, Lau R, et al. The effectiveness of prompts to promote engagement with digital interventions: a systematic review. J Med Internet Res 2016;18:e6 10.2196/jmir.4790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 57. Glaser BG. The constant comparative method of qualitative analysis. Soc Probl 1965;12:436–45. 10.2307/798843 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-018282supp001.pdf (153.6KB, pdf)