Abstract
Background
Keloid therapy remains a great challenge for plastic surgeons, especially when the defect cannot be closed primarily, necessitating tissue transplantation. Here, we introduce a new treatment modality, called the sandwich therapy, for presternal keloids; the sandwich therapy incorporates preradiotherapy, superficial circumflex iliac artery perforator (SCIP) flap transplantation, and postradiotherapy.
Methods
From December 2012 to October 2013, 12 patients received the “sandwich therapy.” For the protocol, all patients went through 5 days of specific events: the precut procedure, preradiotherapy, resection and SCIP flap transplantation, donor site radiotherapy, and final presternal radiotherapy.
Results
All the flaps survived completely. No complication was observed during the perioperative period. With a mean follow-up of 12 months, only 1 case was reported with an incisional hypertrophic scar. In all patients, the main discomfort complaints were resolved postoperatively.
Conclusions
A low-tension or without-tension closure could be achieved with SCIP flap transplantation. The perioperative radiotherapy could further lower the risk of keloid recurrence. The sandwich therapy provides a new surgical approach to presternal keloid treatment.
Key Words: keloid, flap, therapy, surgery
The treatment regimens for keloids vary greatly; treatments include topical medicine, laser, steroid injection, surgery, radiation therapy and so on. Except for small lesions, none of these therapeutic options can work effectively and satisfactorily. Combination therapy is usually necessary to achieve more satisfactory outcomes. Surgical resection followed by radiation therapy has been proven to be an effective treatment modality for keloids.1
In the literature, numerous authors have reported successful experiences with surgical interventions for keloid therapy. However, most of them were dealing with small-sized lesions, in which the resultant defect could be closed primarily. For medium- or large-sized keloids, surgical intervention remains a great challenge. The skin graft technique is a relatively simple method and has been commonly used instead of direct closure. However, the disadvantage of the skin graft technique also remains obvious. The early state of the unvascularization of the skin graft precluded the immediate irradiation therapy that is necessary to guarantee the effectiveness of the keloid treatment. In a previous study, we reported a method utilizing preradiotherapy in skin graft surgery to circumvent the immediate irradiation issue.2 With a long-term follow up, we observed that some patients complained of the unsightly appearance and poor quality of the irradiated skin patch on the grafted area. Therefore, it was necessary to explore a flap-based reconstructive approach to improve the clinical outcomes. In the past 3 years, we developed a novel keloid treatment strategy, called “sandwich therapy,” in which we integrated preradiotherapy, microsurgical superficial circumflex iliac artery perforator (SCIP) flap reconstruction and postradiotherapy into a systematic approach for presternal keloid treatment.
PATIENTS AND METHODS
Indications
This study has obtained Institutional Review Board approval. We chose patients according to the following criteria:
The patients complained of a severe impairment of quality of life due to remittent infections or persistent uncomfortable feelings such as itchiness or pain;
The patients have tried conservative therapy before, but it failed;
The keloid was located on a presternal area;
The width of the keloid precluded primarily wound closure after excision;
The patients had no contraindication to irradiation therapy.
Treatment Protocol
The “sandwich therapy” in this study consists of 3 main parts: preradiotherapy, surgical excision and microsurgical reconstruction, and postradiotherapy treatment. Each patient experienced five days of specific therapeutic events, which established a tentative protocol for the “sandwich” therapy (Fig. 1).
FIGURE 1.
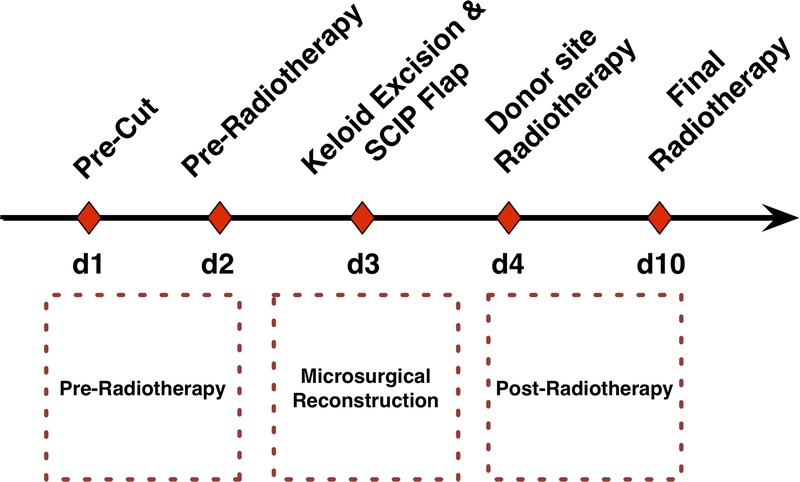
The sandwich therapeutic protocol for presternal keloids includes 3 main parts. (1) Before the reconstruction surgery, the patients received preradiation therapy, which necessitates a precut procedure on the first day and a 9-cGy dose of radiation on the perikeloid area on the second day. (2) The patient then underwent a keloid resection and SCIP flap transplantation on the third day. (3) The patient received the first donor site radiation therapy on the fourth day and the final radiation therapy for both the recipient and donor sites on the 10th day.
Day 1, Precut
On day 1, the patient received a keloid edge incision procedure. Under local anesthesia with or without intravenous sedation, an incision was designed just 1 to 2 mm outside of the keloid's perimeter. The circumferential incision was made into the healthy subcutaneous fat tissue, with a depth of 1 cm. The wound was then closed primarily with intradermal continuous sutures after meticulous coagulation and saline irrigation. This procedure aims at motivating the healing system and is followed by preradiotherapy.
Day 2, Preradiotherapy
The total irradiation dose to the presternal area of each patient was divided into 2 fractions, with 900 cGy each time. The first session of irradiation therapy was started within 24 hours after the precut procedure. The irradiated field included the whole keloid area, the precut incision, and the peripheral 1.5 cm marginal tissue.
Day 3, Keloid Excision and Groin Flap Transplantation
Keloid Excision and Recipient Vessel Exposure
Under general anesthesia, the keloid was excised along its previous marginal incision. Dissection proceeded on the supra fascia plane to remove the keloid en block, and no white fibroscar tissue was left on the wound bed. In circumstances when multiple presternal lesions were present, the adjacent minor defects were closed directly or by advancement flaps. Thus, 1 large congregated defect was ultimately produced. The area of the defect was always larger than that of the original keloid, as a result of contracture relieving. In all cases, the width of the defect could be reduced to no more than 9 cm with the help of an advancement flap and quilting sutures.3
The internal mammary artery perforators were then dissected carefully in the wound bed; usually, the upper intercostal space was preferred. In most cases, more than 2 sets of intercostal perforators were identified, and the large caliber ones were chosen as the recipient vessels (Fig. 3).
FIGURE 3.
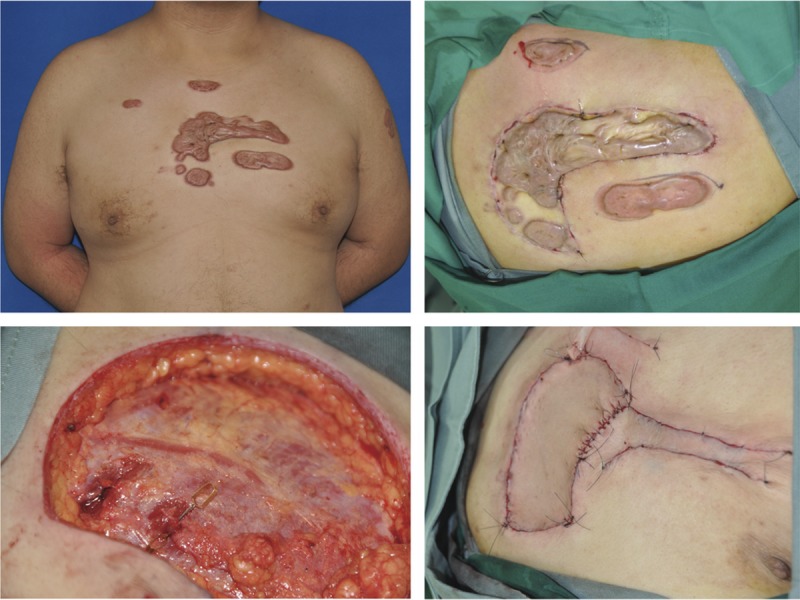
This patient had a tendency to develop numerous keloids in the presternal area and upper arm (Left above). He received the precut procedure on the first day, in which the biggest presternal keloid was incised peripherally, and intra-dermal sutures were used to close the wound (right above). On the third day, the keloid was removed surgically, and the second intercostal perforator was identified above the deep fascia (left below). A SCIP flap was transplanted for the coverage of the major per-sternal defect, while the minor defects were closed using advancement local flaps (right below).
SCIP Flap Design and Elevation
A SCIP flap was designed according to the dimensions of presternal defects and dissected as previously reported.4–6 A 10-cm length exploration incision that was located 3 cm below the inguinal ligament was incised firstly to expose the superficial iliac circumflex vessels. The perforators of this vessel were clearly visualized and then dissected proximately. With a retrograde approach, the pedicle was skeletonized under the deep fascia to its origin point from the femoral triangle. After the pedicle dissection was accomplished, the flap was elevated suprafacially based on the perforators. Before pedicle division, the flap could be thinned with Metzenbaum scissors peripherally and microsurgical scissors centrally, with the perforators being carefully preserved. The flap was then detached after the preparation of recipient vessels.
Flap Inset
The flap was transferred to the presternal defect and positioned appropriately to facilitate microsurgical manipulation. The pedicle was then anastomosed end-to-end with the intercostal perforators with 9–0 prolene sutures. In most cases, the concomitant vein of the superficial iliac circumflex artery was used as the drainage outlet. In 2 cases, the superficial iliac circumflex vein was chosen for the anastomosis due to the relatively large lumen of the recipient vein. After the anastomosis was performed, the wound was meticulous coagulated and closed with three layers. The superficial fascia was closed with 3–0 interrupted absorbable sutures to minimize the wound tension. The skin was approximated continuously in the deep dermal and superficial dermal planes, with 4–0 nylon and 5–0 nylon sutures, respectively.
Donor Site Management
In this study, the donor site was closed primarily in all cases. No drain was left on the donor site. The wound was approximated in the same way as that of the presternal wound. The laxity of the donor site varies greatly between the sexes. In parous women, thanks to the significant abundance of the abdominal wall, the width of the flap could be designed up to 9 cm without causing too much tension on the wound. In male patients, however, it was always prudent to reduce the width of groin flap to no more than 7 cm, or otherwise the wound closure would be a challenging task.
Postoperative Care
The flaps were monitored in accordance with the conventional microsurgery protocol for 3 days. No heparin or any other anticoagulation agent was used after surgery. The patients were encouraged to mobilize on the first day after surgery to minimize the risks of venous thromboembolism.
Day 4, Donor Site Radiotherapy
In all keloid patients, the donor site received the same irradiation therapy as the recipient site. The patients were scheduled to have the first donor site radiotherapy on the first day postoperatively. The delivery dosage was 900 cGy at this time. The irradiated area included the incision and peripheral 1.5 cm margin.
Day 10, Final Radiotherapy
The patient received the last radiotherapy on the seventh day postoperatively. The donor site and the presternal area were all irradiated as the second session, with a dosage of 900 cGy for each site.
RESULTS
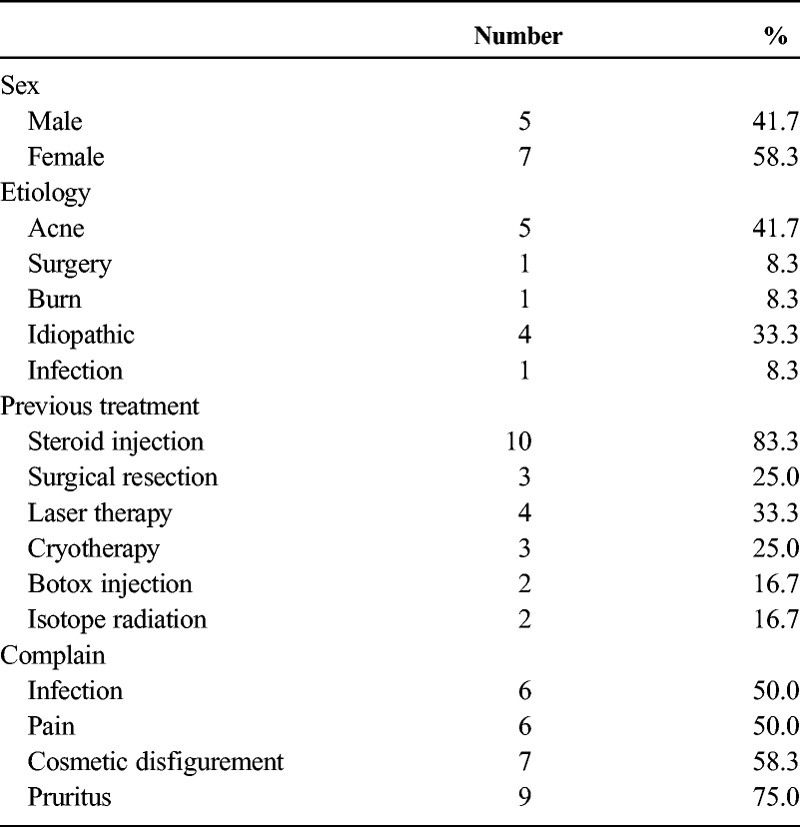
From December 2012 to October 2013, 12 patients received the “sandwich therapy” in our department. The demographic information is shown in Table 1. The patients included 5 men and 7 women with a mean age of 31.2 years (range, 19 to 52 years). The causes of these keloids varied. Most patients reported no prior surgery or trauma in the affected area. All patients had tried conservative treatments before, with an intralesion steroid injection being the most commonly used therapy applied in 10 (83%) patients. All patients presented with obvious symptoms directly related to the keloid. Six patients (50%) suffered from intermittent local infection. Nine patients (75%) complained of pruritus, and half of these patients endured chronic pain.
TABLE 1.
Patient Characteristics
All keloids were located between the second and the seventh rib in the presternal area. The mean size of the keloid was 130 cm2 (range, 64 to 190 cm2). The most commonly used recipient vessel was the third intercostal perforators of the internal mammary vessel, followed by the second intercostal counterpart (Fig. 2).
FIGURE 2.
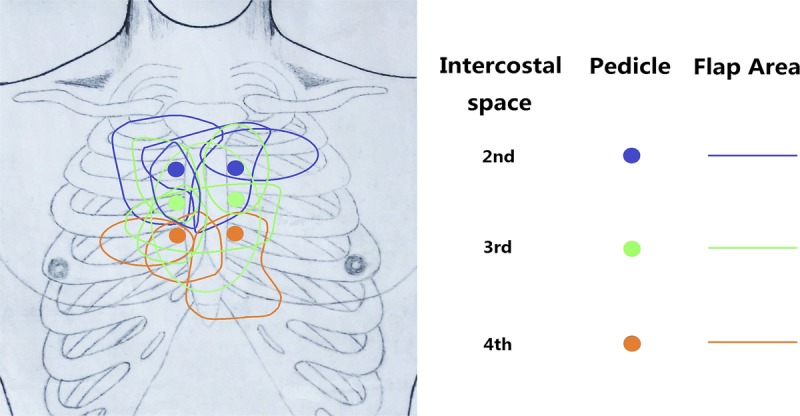
After keloid resection, the recipient vessels were explored and identified in the second, third and fourth intercostal spaces. The SCIP flaps were transplanted and anastomized with the appropriate vessels regarding the inset position and vessel caliber. The sketch shows the dimensions of the SCIP flaps and their intercostal perforator pedicles.
The microsurgical reconstructions were successful in all these patients. The postsurgery recovery courses were uneventful. No microvascular crisis was observed. All flaps survived completely. The patients were routinely discharged from the hospital on the third day postoperatively (Fig. 3).
With a mean follow up of 14 months (range, from 9 months to 24 months), there was no keloid recurrence in these patients (Figs. 4–6). Only 1 case presented with a mild incisional hypertrophic scar. In all patients, the preoperative discomfort complaints were reported as resolved. However, postoperative hyperpigmentation was observed as a common phenomenon in 9 cases, especially in the early half year. At the end of this study, there are 4 cases still presenting with hyperpigmentation in the irradiated area.
FIGURE 4.
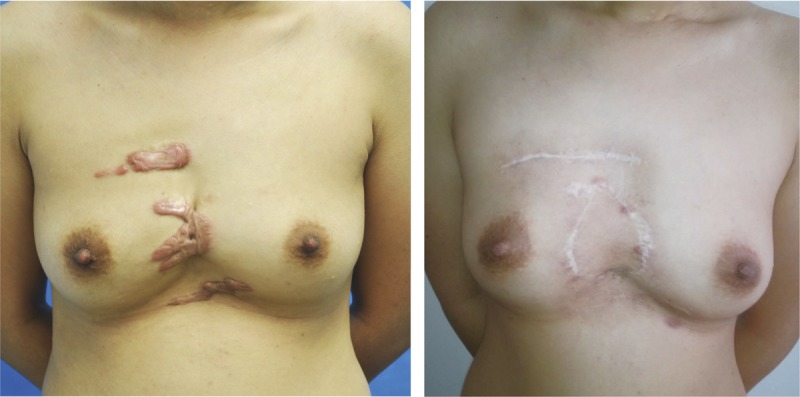
A 26-year-old woman presented with per-sternal keloids and contractures. She received sandwich therapy, and a SCIP flap was transferred microsurgically to release the contracture between the breasts. With a follow-up of 2 years, the patient showed no signs of keloid recurrence.
FIGURE 6.
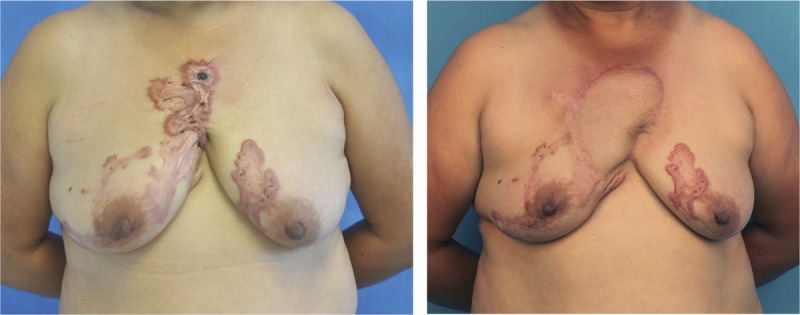
This is a 42-year-old woman with pruritus and infection in a per-sternal keloid. After the precut procedure and preradiation therapy, the keloid was resected en bloc, and a SCIP flap was utilized to provide a stable coverage of the defect. She was shown at 9 months later after the postradiation therapy, and there was no sign of keloid recurrence.
FIGURE 5.
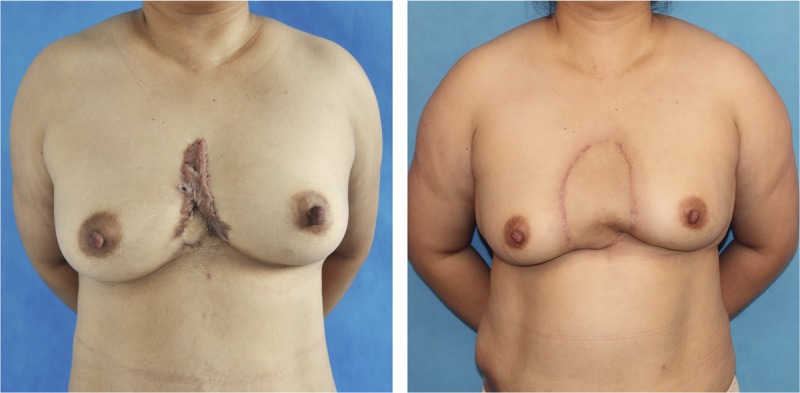
A 56-year-old lady complained of intermittent discomfort of itching and pain in a presternal keloid. She received standard Sandwich therapy, and a large SCIP flap was used to provide a minimal tension wound closure. She was shown at 12 months postoperatively with a satisfactory clinical outcome.
DISCUSSION
Although conservative therapy has been currently recommended as the first line treatment for keloids, surgical intervention remains an indispensable option, especially for those with a history of failed therapy. In the literature, surgical excision combined with postoperative radiotherapy has been proven an effective treatment modality for keloids.7,8 In this study, we established a unique therapy protocol for presternal keloid, which includes 2 special points that are different from other surgical keloid therapy.
Microsurgical Reconstruction
One key element of keloid surgery is to reduce the wound tension, which has been widely considered as a dominant factor for keloid formation.9 A small-sized presternal keloid can be surgically removed and can have a tension reduction suture applied, combined with postoperative radiotherapy.10 A medium-sized or large-sized keloid constitutes a considerable challenge for reconstructive surgeons. Transferring tissues from neighboring or distant areas are the options of choice in such circumstances.
The internal mammary artery perforator (IMAP) flap has been reportedly used for the successful reconstruction of defects in the chest or neck area, mostly for oncoloplastic reasons.11,12 The significant advantages of this regional flap are that it mobilizes a similar tissue and yields aesthetically satisfactory results. It is most suitable for elderly patients with skin laxity.13 The drawbacks are also obvious. It will cause additional scars in the high-tension presternal area. The risk of keloid occurrence will be much higher, especially in young muscular male patients. In female cases, the breast asymmetry after the flap harvest will be apparent; and thus it produces unaesthetic clinical results. We abandoned this flap after we tried a few cases and realized that it was difficult to provide ample tissue for a medium or large area reconstruction in most cases.
Because the presternal area is a high mechanical tension area, reconstruction with additional tissue transplantation instead of a redistribution of tension within the presternal area seems more reasonable. In our early experiences, we adopted a skin graft technique to repair the presternal defect during keloid surgery, because it is the simplest transplantation method in the reconstructive ladder.2 One inherent problem with the skin graft technique is that it excludes the immediate postoperative radiotherapy, which plays a key role in the prevention of keloid recurrence. By introducing the concept of precut and preradiotherapy, we circumvented this issue without any impairment of the therapeutic effect of the radiotherapy. The clinical outcomes seemed satisfactory during the early follow up period. However, severe contractures of the irradiated skin graft took place in the following years, and many patients complained of the uncomfortable feeling of tightness and dryness, in addition to the unsightly patchy appearance (unpublished data). It has been widely accepted that the quality of reconstruction with a skin flap is better than that with a skin graft. So it is reasonable to develop a free flap-based therapeutic protocol for presternal keloid treatment, in place of the previous skin graft-based approach.
The presternal area is actually a perfect place for microsurgical reconstruction. The presternal defects always involve multiple intercostal spaces, from which the internal mammary artery perforators are readily identified. The internal mammary artery perforator, which lies superficially and needs only a minimal dissection maneuver, has been considered a reliable alternative to the internal mammary artery as the recipient vessel in the chest area.14 Some authors have reported successful experiences using the internal mammary artery perforator as the recipient vessel in breast reconstruction.15–17 In this study, we can find at least 2 sets of perforators in most cases. The decision of the optimal recipient vessel will be easily reached when evaluating the vessel diameter and the position of flap inset.
When considering donor sites for keloid reconstruction, we prefer to use the flaps with minimal donor site morbidity. Most workhorse flaps, which result in a conspicuous scar or the permanent impairment of function appear inappropriate for the reconstruction of such benign skin lesions. In this study, we chose the SCIP flap as the reconstructive tool. This flap is credited with a consistent vascular anatomy and has been popularized in recent years. Its donor site morbidity is minimal, and the scar can be concealed very well with underwear.18–21 Although the caliber of the superficial circumflex iliac vessel is not as large as that of conventional workhorse flaps, the success rate of the SCIP flap usually reaches 95% to 100%.4,5 In this study, we transferred 12 flaps and found no major complications perioperatively. We believe the SCIP flap is very suitable for keloid reconstruction.
The Sandwich Therapy
The concept of the sandwich therapy was first reported by Stahl et al22 in 2010. He applied preoperative and postoperative radiotherapy to treat an earlobe keloid, in addition to lesion resection procedures. His observation confirmed that this was an effective keloid treatment. In our study, we further expanded the sandwich therapy by incorporating microsurgical reconstructive procedures. In this treatment approach, the keloid wound received the full dosage of irradiation therapy after the precut, so the proliferation of fibroblasts would be effectively prohibited, and the recurrence rate of keloid would be lowered.23 After microsurgical transplantation, the flap area received only partial dosages of irradiation, which reduces the radiation damage to the flap tissue and preserves a more natural appearance and skin texture than that of the adjoining presternal area.
There is an apparent disadvantage to sandwich therapy. The precut procedure brought an additional surgical intervention to the patients, which was not only inflicted on the patients but also presented as time-consuming and medical resource–consuming. We even tried to make some modifications to simplify the sandwich therapy in another group. In limited cases, we initiated irradiation within 24 hours postmicrosurgical transplantation. However, we soon encountered 1 case with an acute irradiation injury to the flap, which unfortunately prolonged the healing process. We believed that, because no optimal radiation modality has been proposed with high-quality evidence yet, we should practice with more prudence and caution for safety concerns.
Overall, we proposed a new type of surgical modality for keloid treatment. We incorporated 2 new elements into the traditional keloid resection method: reconstruction with a free SCIP flap and preradiation therapy. The advantages for the microsurgical intervention in the presternal area are straightforward. First, to introduce a distant tissue for wound coverage could provide minimal wound tension after keloid resection, which is a key point for keloid prevention. Theoretically, this is a superior method over local perforator flaps, regarding the high predisposition to keloid formation in the presternal area. Second, the microsurgical reconstruction process is not complicated, because the vascular anatomy in both the recipient and the donor sites are consistent. The dissections in both sites could be performed superficially, directly and less time consuming. These procedures leave the patients with minimal trauma, finally, and are particularly suitable for those with benign lesions.
Another new element, preradiation therapy, may be more controversial. Some may consider that it complicated this whole treatment modality. However, at present, because no consensus has been reached on how to combine the radiation therapy with free flap transplantation, the preradiation therapy remains a reasonable tool to curb the recurrence of keloids effectively and safely.
CONCLUSIONS
The sandwich therapy includes 2 new elements. The microsurgical transfer of the SCIP flap can reduce the incisional tension, whereas the perioperative radiation therapy could lower the risk of keloid recurrence during wound healing. The sandwich therapy constitutes a new treatment modality for presternal keloid.
Footnotes
Conflicts of interest and sources of funding: none declared.
A.Z. and K.S. are first authors.
REFERENCES
- 1.Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010;125:557–68. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Wang Y, Wang X, et al. A keloid edge precut, preradiotherapy method in large keloid skin graft treatment. Dermatol Surg. 2014;40:52–7. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SH, Oni G, Guevara R, et al. Latissimus dorsi donor-site morbidity: the combination of quilting and fibrin sealant reduce length of drain placement and seroma rate. Ann Plast Surg. 2012;68:555–8. [DOI] [PubMed] [Google Scholar]
- 4.Goh TL, Park SW, Cho JY, et al. The search for the ideal thin skin flap: superficial circumflex iliac artery perforator flap—a review of 210 cases. Plast Reconstr Surg. 2015;135:592–601. [DOI] [PubMed] [Google Scholar]
- 5.Iida T, Mihara M, Yoshimatsu H, et al. Versatility of the superficial circumflex iliac artery perforator flap in head and neck reconstruction. Ann Plast Surg. 2014;72:332–6. [DOI] [PubMed] [Google Scholar]
- 6.Koshima I, Nanba Y, Tsutsui T, et al. Superficial circumflex iliac artery perforator flap for reconstruction of limb defects. Plast Reconstr Surg. 2004;113:233–40. [DOI] [PubMed] [Google Scholar]
- 7.van de Kar AL, Kreulen M, van Zuijlen PP, et al. The results of surgical excision and adjuvant irradiation for therapy-resistant keloids: a prospective clinical outcome study. Plast Reconstr Surg. 2007;119:2248–54. [DOI] [PubMed] [Google Scholar]
- 8.Emad M, Omidvari S, Dastgheib L, et al. Surgical excision and immediate postoperative radiotherapy versus cryotherapy and intralesional steroids in the management of keloids: a prospective clinical trial. Med Princ Pract. 2010;19:402–5. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa R, Akaishi S, Huang C, et al. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch. 2011;78:68–76. [DOI] [PubMed] [Google Scholar]
- 10.Wang LZ, Ding JP, Yang MY, et al. Forty-five cases of chest keloids treated with subcutaneous super-tension-reduction suture combined with postoperative electron-beam irradiation. Dermatol Surg. 2014;40:1378–84. [DOI] [PubMed] [Google Scholar]
- 11.Schellekens PP, Hage JJ, Paes EC, et al. The internal mammary artery perforator pedicled island flap for reconstruction of the lower head and neck and supraclavicular region: how we do it. Clin Otolaryngol. 2010;35:332–6. [DOI] [PubMed] [Google Scholar]
- 12.Yu BT, Hsieh CH, Feng GM, et al. Clinical application of the internal mammary artery perforator flap in head and neck reconstruction. Plast Reconstr Surg. 2013;131:520e–6e. [DOI] [PubMed] [Google Scholar]
- 13.Koulaxouzidis G, Orhun A, Stavrakis T, et al. Second intercostal internal mammary artery perforator (IMAP) fasciocutaneous flap as an alternative choice for the treatment of deep sternal wound infections (DSWI). J Plast Reconstr Aesthet Surg. 2015;68:1262–7. [DOI] [PubMed] [Google Scholar]
- 14.Fansa H, Schirmer S, Cervelli A, et al. Computed tomographic angiography imaging and clinical implications of internal mammary artery perforator vessels as recipient vessels in autologous breast reconstruction. Ann Plast Surg. 2013;71:533–7. [DOI] [PubMed] [Google Scholar]
- 15.Halim AS, Alwi AA. Internal mammary perforators as recipient vessels for deep inferior epigastric perforator and muscle-sparing free transverse rectus abdominis musculocutaneous flap breast reconstruction in an Asian population. Ann Plast Surg. 2014;73:170–3. [DOI] [PubMed] [Google Scholar]
- 16.Munhoz AM. Internal mammary perforator recipient vessels for breast reconstruction using free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg. 2008;122:315–6. [DOI] [PubMed] [Google Scholar]
- 17.Saint-Cyr M, Chang DW, Robb GL, et al. Internal mammary perforator recipient vessels for breast reconstruction using free TRAM, DIEP, and SIEA flaps. Plast Reconstr Surg. 2007;120:1769–73. [DOI] [PubMed] [Google Scholar]
- 18.He Y, Jin S, Tian Z, et al. Superficial circumflex iliac artery perforator flap's imaging, anatomy and clinical applications in oral maxillofacial reconstruction. J Craniomaxillofac Surg. 2016;44:242–8. [DOI] [PubMed] [Google Scholar]
- 19.Hong JP, Sun SH, Ben-Nakhi M. Modified superficial circumflex iliac artery perforator flap and supermicrosurgery technique for lower extremity reconstruction: a new approach for moderate-sized defects. Ann Plast Surg. 2013;71:380–3. [DOI] [PubMed] [Google Scholar]
- 20.Hsu WM, Chao WN, Yang C, et al. Evolution of the free groin flap: the superficial circumflex iliac artery perforator flap. Plast Reconstr Surg. 2007;119:1491–8. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Kim KN, Yoon CS. Reconstruction of moderate-sized distal limb defects using a superthin superficial circumflex iliac artery perforator flap. J Reconstr Microsurg. 2015;31:631–5. [DOI] [PubMed] [Google Scholar]
- 22.Stahl S, Barnea Y, Weiss J, et al. Treatment of earlobe keloids by extralesional excision combined with preoperative and postoperative “sandwich” radiotherapy. Plast Reconstr Surg. 2010;125:135–41. [DOI] [PubMed] [Google Scholar]
- 23.Gauglitz GG, Korting HC, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]


