Abstract
Objective
The role of cigarette smoking as an independent risk factor for patients with nasopharyngeal carcinoma (NPC) is controversial. We attempted to provide evidence of a reliable association between cigarette smoking and the risk of NPC.
Design
Meta-analysis.
Data sources
PubMed online and the Cochrane Library of relevant studies published up to February 2016.
Eligibility criteria
All studies had to evaluate the relationship between NPC and cigarette smoking with never smokers as the reference group.
Outcomes
The primary outcome was the adjusted OR, RR or HR of NPC patients comparing smoking with never-smoking; the second was the crude OR, RR or HR.
Results
We identified 17 case–control studies and 4 cohort studies including 5960 NPC cases and 429 464 subjects. Compared with never smokers, current smokers and ever smokers had a 59% and a 56% greater risk of NPC, respectively. A dose–response relationship was identified in that the risk estimate rose by 15% (p<0.001) with every additional 10 pack-years of smoking, and risk increased with intensity of cigarette smoking (>30 cigarettes per day). Significantly increased risk was only found among male smokers (OR, 1.36; 95% CI 1.15 to 1.60), not among female smokers (OR, 1.58; 95% CI 0.99 to 2.53). Significantly increased risk also existed in the differentiated (OR, 2.34; 95% CI 1.77 to 3.09) and the undifferentiated type of NPC (OR, 1.15; 95% CI 0.90 to 1.46). Moreover, people who started smoking at younger age (<18 years) had a greater risk than those starting later for developing NPC (OR, 1.78; 95% CI 1.41 to 2.25).
Conclusions
Cigarette smoking was associated with increased risk of NPC, especially for young smokers. However, we did not find statistical significant risks of NPC in women and in undifferentiated type, which might warrant further researches.
Keywords: cigarette, smoker, nasopharyngeal carcinoma, risk factor, meta-analysis
Strengths and limitations of this study.
Major strengths of our meta-analysis comprise new published studies being included, strict selection criteria, careful literature search, data extraction and analyses by two authors separately.
The main limitations of our meta-analysis are study design, characteristics and size of study population, different outcome and variables used in eligible studies.
Introduction
There were approximately 86 691 incident cases of NPC and 50 831 NPC-related deaths in 2012 worldwide.1 Despite NPC being rare in developed countries, the overall incidence rate in Southeastern Asia is 6.5/100 000 person-years among men and 2.6/100,000 person-years among women.2 Particularly, an age-standardised incidence rate of 20–50 per 100 000 men in south China presented a remarkably high incidence compared with that among white populations.3
Cigarette smoking has been regarded as a risk factor for the occurrence of a wide variety of malignancies, including respiratory tract, gastrointestinal and urogenital systems.4 5 Over the decades, some reports have suggested that cigarette smoking is associated with NPC risk.6 However, the association has not been consistently demonstrated, some studies failed to find such a positive association.7–10 The discrepancies of inconsistent outcome might be owing to variations in study population, methodology, definitions of cigarette smoking and so on. Furthermore, inevitable recall bias and confounding in case–control studies might further complicate the scenario.11 12
One recent meta-analysis of 28 case–control studies and 4 cohort studies reported the adverse effect of cigarette smoking on the incidence of NPC.13 The pooled analysis showed that ever smokers had a 60% greater risk of developing the disease than never smokers. And there was a significant dose-dependent association. However, between-study heterogeneity was strikingly high across the overall analysis and still remained after stratified analyses. Specifically, some included studies might not be appropriate to be combined for synthetic analysis because of their inadequate reports about association between cigarette smoking and NPC risk,14–17 unclear definition of cigarette smoking and health condition of controls,18 19 controls with a history of cancer20 and inappropriate reference group.21 22 These might result in overestimating or underestimating the association of cigarette smoking on NPC risk, and thus the conclusions might be hard to interpret. In addition, new studies have been published recently which warrant an up-to-date analysis.23–26
In this meta-analysis, we sought to provide a summary of available literature to examine the association between cigarette smoking and the risk of NPC, we also assessed the gender and histological type differences in effects of cigarette smoking on the NPC risk.
Methods
Literature search
This meta-analysis was performed on the basis of the Meta-analysis Of Observational Studies in Epidemiology (MOOSE).27 To identify all relevant publications on NPC and cigarette smoking, first, we used the engine ‘Windows Internet Explorer 10.0’ to search the PubMed and Cochrane Library databases with terms ‘(nasopharyngeal carcinoma OR nasopharyngeal cancer OR cancer of nasopharynx) AND (smoking OR cigarette OR tobacco OR nicotine) AND (etiology OR epidemiology OR environment OR risk factor) AND (Humans (Mesh))’, then we scrutinised the references of articles obtained from the database search for additional studies. Only publications in English were included.
Selection criteria
The following criteria were applied for literature selection: (1) the study was case–control or cohort design; (2) controls were cancer-free; (3) cases were patients who were histopathologically confirmed NPC and had no other malignancies; (4) the study evaluated the relationship between NPC and one of various aspects of cigarette smoking, including cigarette smoking status, smoking intensity, cumulative amount of cigarette smoking, age at onset and duration of smoking; (5) studies used never smokers as the reference group; (6) studies provided enough information to estimate the ORs or the relative risk (RR) or HRs with 95% CI for cigarette smoking variable. If multiple articles were on the same study population, the one with adequate information or most related or largest sample size was finally selected; furthermore, when there were separate data for gender or histological type of NPC in one study, they were considered for additional subgroup analysis.
Data extraction
The following data were extracted from eligible studies: first author, publication year, study region, study design, sample size, control source, age of participants (range, mean), gender distribution, categories of smoking (status, intensity, pack-years, age at onset of smoking and so on), method of questionnaire survey, duration of follow-up, endpoint (for cohort study), covariates for adjustment, OR, RR or HR with their 95% CIs for each category of smoking exposure. In case the above effect sizes were not available, crude effect estimates and 95% CIs were calculated by provided number of subjects. All data were independently extracted and analysed by two investigators; any inconsistency was resolved by consensus.
Quality assessment
The qualities of eligible studies were assessed by using the Newcastle-Ottawa Scale (NOS),28 which comprised three parts assigned with a maximum of nine points: selection, comparability, exposures and outcome condition. Two investigators evaluated all eligible publications separately and discrepancies were resolved by discussion.
Data integration
Not all studies included in this meta-analysis provided consistent information about cigarette smoking, so we stipulated smoking status as follows: never smokers (people who did not smoke any tobacco product), ever smokers, current smokers and former smokers. With regard to smoking quantity, we combined data extracted from all eligible publications into new categories: subjects with cigarettes consumption of <30 pack-years were assigned to light smokers, while those who consumed ≥30 pack-years were designated to heavy smokers. Similarly, for age at smoking onset, early group meant that subjects began smoking at <18 years age while later group defined as smoking at ≥18 years age. We also defined that regions with NPC incidence <1 per 100 000 person-years was low incidence rate group, 1–10 per 100 000 person-years was intermediate incidence rate group and >10 per 100 000 was high incidence rate group.
Statistical analysis
Since NPC is considered as a relatively rare outcome, RR and OR were not differentiated, the ORs were used as effect size for all studies. We conducted fixed and random effects meta-analyses and the synthetic estimates did not differ substantially between the two models. Therefore, random-effects (Der Simonian-Laird) model,29 generally regarded as the more conservative method, was applied to calculate point estimates for all analyses. Heterogeneity among articles was estimated by using the I2 statistic and p value associated with Q statistics.30
We conducted dose–response meta-analyses using the generalised least-squares method for trend estimation of summary dose–response data, as described by Greenland and Longnecker.31 For non-linearity relationship, restricted cubic splines with four knots at percentiles 5%, 35%, 65% and 95% of the distribution were created and p value for non-linearity was computed by testing the null hypothesis that the coefficients of the second and the third splines were equal to zero.32
To assess the robustness of our findings and the source of heterogeneity, meta-regression methods and stratified analyses were performed according to study design, incidence rate of regions, adjustment, score of eligible studies, categories of cigarette smoking, gender and NPC histological type (the latter three were only evaluated in stratified analysis). Sensitivity analysis was also conducted by deleting each study in turn to reflect the influence of every single study to the overall estimate. In addition, we evaluated the publication bias in the pooled analysis by Egger’s test and the trim-and-fill method.33 All statistical analyses were performed with Stata SE V.12.0 software, and p value <0.05 (two sides) was considered statistically significant.
Patient involvement
No patients were involved in this study.
Results
Study characteristics
Figure 1 shows the flow chart describing the sequential selection procedures of eligible studies. A total of 342 articles were identified, of which 302 articles were deemed irrelevant after reviewing the titles and abstracts. Subsequently, 40 articles were further scanned by full text. Meanwhile, by searching all references of relevant articles, three additional articles were considered as potentially eligible. Among them, 22 were excluded because of following reasons: 5 studies with inadequate information for data extraction, 4 studies without report of the association between cigarette smoking and NPC risk, 4 studies with overlapped data, 4 studies did not designate never smokers as reference group, 2 studies included improper controls (eg, controls with malignancies or without description of health conditions), 1 without clear definition of cigarette smoking, 1 systematic review and 1 meta-analysis. Finally, 21 articles were eligible for qualitative synthesis, including 17 case–control studies (5673 cases and 8653 controls) and 4 cohort studies (287 cases and 420 811 participants).
Figure 1.
Summary of literature search.
All of the studies in the overall analysis were published between 1985 and 2015. Of these included studies, not all studies reported the estimates for all risk estimates. Nineteen studies reported on ever smoking,7–10 23 25 26 34–45 10 on former smoking,7–9 23 26 38 39 41 46 11 on current smoking,7–10 23 24 26 38 39 41 46 10 on pack-years of smoking7 23 24 26 35 37–39 41 43 and 6 on age at onset of smoking.7 9 10 23 26 46 Additionally, five studies provided separate data of gender35 38 41–43 and five studies reported the risk of NPC histological type associated with cigarette smoking.9 38 39 42 44 As regarding to geographic region, eight studies were conducted in China,7 8 24 26 37 41 43 44 five in the USA,34 35 38 39 46 five in Southeast Asia region,10 23 25 36 40 two in Europe9 45 and one in Africa.42 The summarised characteristics of the 21 studies are presented in tables 1 and 2.
Table 1.
General characteristics of case–control studies used for meta-analysis
| Study | Region | Period | Incidence rate | Cases /Controls |
Man /Woman |
Age range (years old) | Quality score | Source of controls | Matching factors | Adjusting factors |
| Mabuchi et al34 | USA | – | Low | 39/39 | – | – | 7 | Hospital-based | Age, sex, race, Education, occupation, marital status | ─ |
| Yu et al37 | Guangzhou | 1983–1985 | High | 306/306 | 209/97 | Under 45 | 7 | Population-based | Age, sex, residence, education | Birth place, marital status, dietary risk |
| Nam et al35 | USA | 1983–1986 | Low | 204/408 | 141/63 | <65 | 5 | Hospital-based | Age, sex | By multiple logistic regression analysis |
| Sriamporn et al36 | Thailand | 1987–1990 | Moderate | 120/120 | 81/39 | Mean 47.2 | 6 | Hospital-based | Age, sex | Age, sex, education, residence, occupation, consumption of salted fish and alcohol |
| Zhu et al38 | USA | 1984–1988 | Low | 113/1910 | Man | – | 8 | Population-based | ─ | Birth, education, background, medical history, occupation, alcohol intake |
| Vaughan et al39 | USA | 1987–1993 | Low | 231/244 | 154/77 | Mean 55.2 | 9 | Population-based | Age, sex, region | Age, sex, alcohol use, education |
| Cheng et al7 | Taiwan | 1991–1994 | Moderate | 375/327 | 260/115 | Mean 46 (15–74) | 7 | Population-based | Age, sex, residence, education, marital status | Age, sex, race, education, family history of NPC, drinking status |
| Chelleng et al40 | India | 1996–1997 | Moderate | 47/94 | 34/13 | Mean 43.7 | 6 | Population-based | Age, sex, ethnicity | ─ |
| Yuan et al41 | Shanghai | 1987–1991 | Moderate | 935/1032 | 668/267 | Mean 50 | 8 | Population-based | Age, sex, residence | Age, gender, education, intake frequencies of preserved foods, occupational exposure history of chronic ear and nose condition, family history of NPC |
| Zou et al51 | Yangjiang | 1987–1995 | High | 97/192 | 83/14 | Mean 52.6 (30–82) | 7 | Population-based | Age, sex, occupation | ─ |
| Feng et al42 | North Africa | 2002–2005 | Moderate | 440/409 | Man | – | 7 | Hospital-based | Age, sex, ethnicity, centre, childhood household type | Age, socioeconomic status, dietary risk factors |
| Ji et al44 | Wuhan | 1991–2009 | Moderate | 1044/1095 | 755/289 | – | 5 | ─ | Age, sex, ethnicity | Age, gender, cigarette, alcohol intake, family history |
| Polesel et al9 | Italy | 1992–2008 | Low | 150/450 | 119/31 | Median 52 (18–76) | 6 | Hospital-based | Age, sex, residence | Age, sex, place of residence, education, alcohol intake |
| Turkoz et al45 | Turkey | – | Moderate | 183/183 | 122/61 | Mean 44.9 (18–75) | 6 | Hospital-based | Age, sex | Age, sex |
| Fachiroh et al23 | Thailand | 2005–2010 | Moderate | 681/1078 | 504/177 | Mean 49.8 | 6 | Hospital-based | Age, sex, residence | Age group, sex, centre, education, alcohol drinking |
| Lye et al25 | Malaysia | 2007 | Moderate | 356/356 | 276/80 | Mean 53.2 | 6 | Hospital-based | Age, sex, ethnicity | Age, sex, ethnicity, salted fish and alcohol intake |
| Xie et al26 | Hong Kong | 2010–2012 | High | 352/410 | 253/99 | Mean 51.6 | 8 | Population-based | Age, sex, ethnicity, residence district | Age, sex, education, house type, family history of NPC, environmental tobacco smoke exposure, dietary risk, occupational exposure and cooking experience |
NPC, nasopharyngeal carcinoma.
Table 2.
General characteristics of cohort studies used for meta-analysis
| Study | Region | Period | Incidence rate | Cohort size | No. of cases | Years of follow-up | Endpoint | Quality score | Source of cohort | Adjusting factors |
| Chow et al46 | USA | 1954–1980 | Low | 248 046 | 48 | 26 | Mortality | 6 | Veterans | Age, calendar year |
| Friborg et al10 | Singapore | 1993–2005 | High | 61 320 | 173 | 12 | Morbidity | 9 | Population-based | Age, sex, dialect group, year of interview, education |
| Hsu et al43 | Taiwan | 1984–2006 | Moderate | 9622 | 32 | Mean 18.1 | Incidence | 9 | Population-based | Age, two anti-EBV viral serum-markers |
| Lin et al24 | Guangzhou | 1988–1999 | High | 101 823 | 34 | Mean 7.3 | Incidence | 8 | Factory workers and drivers | Age, sex, education, drinking status, occupation |
EBV, Epstein-Barr virus.
Association between cigarette smoking status and NPC
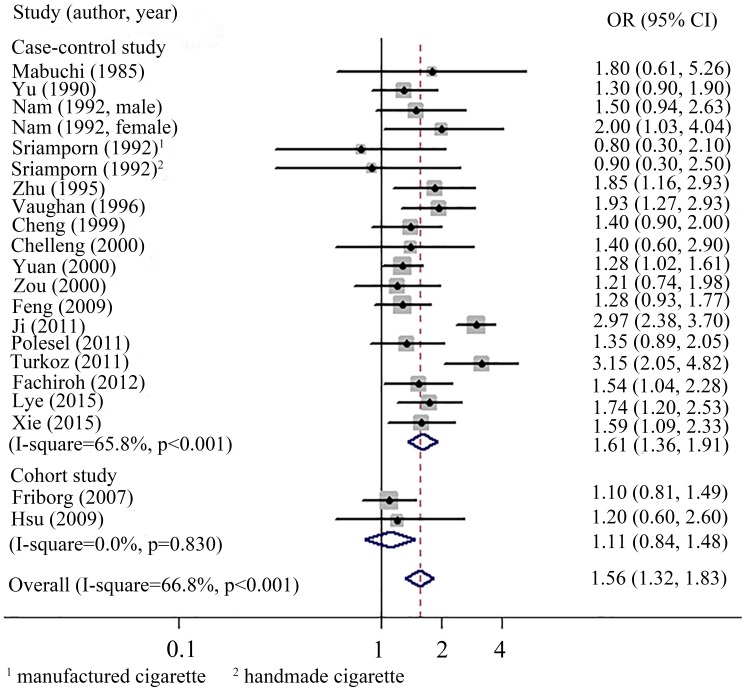
The pooled analysis of nineteen studies revealed a modest but significant increased risk of NPC among ever smokers against never smokers (OR, 1.56; 95% CI 1.32 to 1.83). Heterogeneity was obviously observed across the studies (I2=66.8%, p<0.01). The pooled estimate for case–control studies was 1.61 (95% CI 1.36 to 1.91; heterogeneity: I2=65.8%, p<0.01), whereas cohort studies presented a null association (OR, 1.11; 95% CI 0.84 to 1.48; heterogeneity: I2=0.0%, p=0.83) (figure 2).
Figure 2.
Forest plots for comparing the risk for NPC between ever smokers versus never smokers.
Similarly, 11 studies identified for the comparison of current smokers with NPC risk demonstrated positive result (OR, 1.59; 95% CI 1.35 to 1.89; heterogeneity: I2=32.5%, p=0.14). When analysed by study design, the risk estimates were both statistically significant for case–control and cohort studies. The pooled ORs were 1.67 (95% CI 1.06 to 2.61; heterogeneity: I2=22.6%, p=0.25) and 2.19 (95% CI 1.02 to 4.72; heterogeneity: I2=65.0%, p=0.06), respectively (table 3).
Table 3.
Subgroup analysis on pooled ORs for the association between cigarette smoking and nasopharyngeal carcinoma
| Subgroup | No. of studies | Effect estimate (95% CI) | Heterogeneity I2, p |
Egger’s test p Value | Adjusted for publication bias |
| Smoking status | |||||
| Ever smokers | 19 | 1.56 (1.32 to 1.83) | 66.8%, <0.01 | 0.29 | 1.56 (1.32–1.84) |
| Current smokers | 11 | 1.59 (1.35 to 1.89) | 32.5%, 0.14 | 0.10 | |
| Former smokers | 10 | 1.36 (1.15 to 1.61) | 2.3%, 0.42 | 0.97 | |
| Design | |||||
| Case–control | |||||
| Current smokers | 8 | 1.67 (1.06 to 2.61) | 22.6%, 0.25 | 0.58 | |
| Former smokers | 8 | 1.45 (1.21 to 1.73) | 0.0%, 0.70 | 0.98 | |
| Cohort | |||||
| Current smokers | 3 | 2.19 (1.02 to 4.72) | 65%, 0.06 | 0.16 | |
| Former smokers | 2 | 0.87 (0.54 to 1.41) | 0.0%, 0.37 | ─ | |
| Pack-years | |||||
| <30 | 7 | 1.34 (1.13 to 1.58) | 0.0%, 0.73 | 0.54 | |
| ≥30 | 6 | 2.03 (1.57 to 2.61) | 0.0%, 0.45 | <0.01 | 1.80 (1.37–2.36) |
| Age at onset of smoking (years) | |||||
| <18 | 5 | 1.78 (1.41 to 2.25) | 0.0%, 0.94 | 0.46 | |
| ≥18 | 5 | 1.28 (1.00 to 1.64) | 0.0%, 0.86 | 0.93 | |
| Incidence rate | |||||
| Low | 5 | 1.68 (1.36 to 2.07) | 0.0%, 0.84 | 0.64 | |
| Intermediate | 10 | 1.59 (1.21 to 2.09) | 78.8%, <0.01 | 0.29 | |
| High | 4 | 1.27 (1.05 to 1.53) | 0.0%, 0.52 | 0.63 | |
| Gender | |||||
| Man | 5 | 1.36 (1.15 to 1.60) | 0.0%, 0.68 | 0.48 | |
| Woman | 2 | 1.58 (0.99 to 2.53) | 0.0%, 0.64 | ─ | |
| Histological type | |||||
| Differentiated | 5 | 2.34 (1.77 to 3.09) | 0.0%, 0.72 | 0.64 | |
| Undifferentiated | 4 | 1.15 (0.90 to 1.46) | 0.0%, 0.02 | 0.28 | |
| Adjustment | |||||
| Adjusted | 13 | 1.55 (1.26 to 1.91) | 75.4%, <0.01 | 0.33 | |
| Unadjusted | 6 | 1.57 (1.27 to 1.93) | 0.0%, 0.68 | 0.93 |
When compared with never smokers, former smokers from 10 studies exhibited an increased risk of NPC (OR, 1.36; 95% CI 1.15 to 1.61; heterogeneity: I2=2.3%, p=0.42). However, stratified analysis presented a void association in cohort studies (OR, 0.87; 95% CI 0.54 to 1.41; heterogeneity: I2=0.0%, p=0.37) but a significant association in case–control studies (OR, 1.45; 95% CI 1.21 to 1.73; heterogeneity: I2=0.0%, p=0.70) (table 3).
As for age at cigarette smoking onset, six studies reported the association with NPC risk. The pooled analysis revealed that early group (smoking at <18 years age) had significantly increased risk of NPC (OR, 1.78; 95% CI 1.41 to 2.25; heterogeneity: I2=0.0%, p=0.94), whereas later group (smoking at ≥18 years age) had slightly increased risk of NPC (OR, 1.28; 95% CI 1.00 to 1.64; heterogeneity: I2=0.0%, p=0.86) (table 3).
Dose–response analysis
For the cumulative amount of cigarette smoking, no between-study heterogeneity was found (I2=0.0%, p>0.05) with a pooled OR of 1.34 (95% CI 1.13 to 1.58) for light smokers and 2.03 (95% CI 1.57 to 2.61) for heavy smokers, respectively (table 3). The dose–response analysis showed statistical linear relationship between the number of pack-years and NPC risk (P for linearity=0.83) (figure 3). Smokers had a 15% (OR, 1.15; 95% CI, 1.11 to 1.19, p<0.001) increasing risk of NPC for every additional 10 pack-years smoked in comparison with never smokers (data not shown). When comparing the NPC risk for intensity of cigarettes smoked per day with never smokers, the non-linear dose–response relationship indicated that smokers with high exposure (>30 cigarettes/day) other than with low exposure have higher risk estimate, which presented an upward tendency in steeply rising trend (Pfor non-linearity <0.05) (figure 4).
Figure 3.
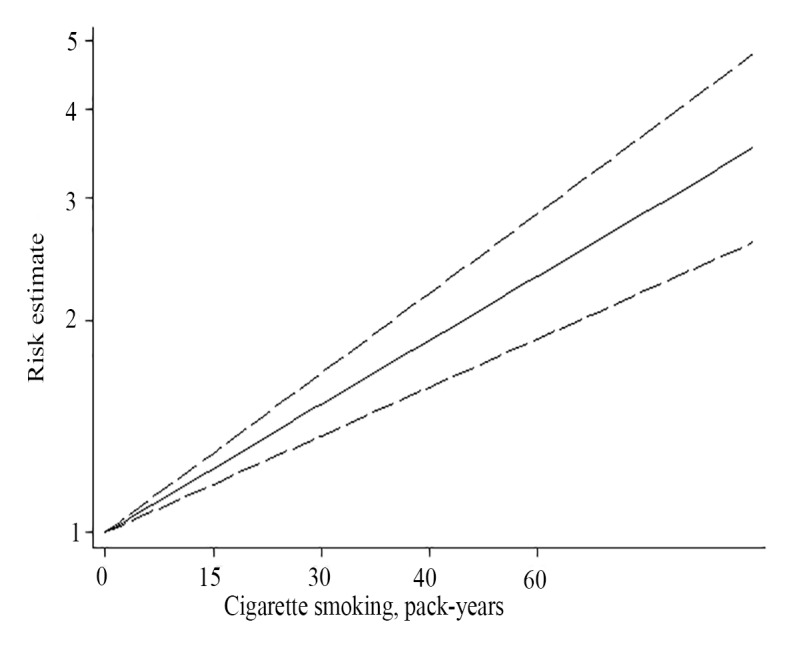
A linear relationship between the cumulative number of pack-years and NPC risk (p for linearity=0.83), with a 15% (95% CI 1.11 to 1.19, p<0.001) increasing risk of NPC for every additional 10 pack-years smoked in comparison with never smokers (the solid line depicts the pooled risk estimate of NPC associated with each 1 pack-year increment of cigarette smoking, the dashed line depicts the upper CI, the dot line depicts the lower CI). NPC, nasopharyngeal carcinoma.
Figure 4.

A non-linear association between intensity of cigarette smoking and NPC risk (p for non-linearity <0.05) (the solid line depicts the pooled risk estimate of NPC associated with each 1 cigarette/day increment, the dashed lines depict the upper and the lower CI, respectively). NPC, nasopharyngeal carcinoma.
Stratified analysis
When conducted stratified analysis by regions with different incidence rate, there were 19 studies compared NPC risk for ever smokers with that for never smokers. Among them, five studies carried out in regions with low NPC incidence rate yielded the highest risk (OR, 1.68; 95% CI 1.36 to 2.07; heterogeneity: I2=0.0%, p=0.84). The pooled estimates were 1.59 (95% CI 1.21 to 2.09; heterogeneity: I2=78.8%, p<0.01) for regions (10 studies) with intermediate NPC incidence rate and 1.27 (95% CI 1.05 to 1.53; heterogeneity: I2=0.0%, p=0.52) for regions (4 studies) with high incidence rate, respectively (table 3).
We also performed stratified analysis by status of adjustment for confounding variables. Thirteen studies provided adjusted ORs for pooled analysis. But six studies either reported unadjusted ORs or reported the number of cases and controls which could be used to calculate the ORs. The estimates for the association of cigarette smoking and NPC risk in adjusted group (OR, 1.55; 95% CI 1.26 to 1.91; heterogeneity: I2=75.3%, p<0.01) and in unadjusted group (OR, 1.57, 95% CI 1.27 to 1.93; heterogeneity: I2=0.0%, p=0.68) were similar (table 3).
When the meta-regression analyses were applied to assess the sources of heterogeneity and their impacts on the NPC risk, we found that the publication year, study design, regions of different incidence rate and quality of studies were not significant sources of heterogeneity (p=0.55, data not shown).
Association between cigarette smoking and histological type of NPC
Specifically, the effects of cigarette smoking on NPC histological types were different. We found that significant association was only noted for differentiated squamous-cell NPC (OR, 2.34; 95% CI 1.77 to 3.09; heterogeneity: I2=0.0%, p=0.72). Contrarily, the risk estimate for undifferentiated carcinoma of NPC in smokers was statistically insignificant though the OR was 1.15 (95% CI 0.90 to 1.46; heterogeneity: I2=0.0%, p=0.02) (table 3).
Association between cigarette smoking of gender and NPC
Seven studies addressed the association between cigarette smoking and NPC risk by gender, including five in males and two in women. Compared with never smokers, increased risk for male smokers was noted (OR, 1.36; 95% CI, 1.15 to 1.60). However, an insignificant association (OR, 1.58; 95% CI, 0.99 to 2.53) was observed for female smokers (table 3).
Sensitivity analysis and publication bias
Sensitivity analysis revealed that44 study42 was the source of statistical heterogeneity in the pooled analysis for ever smokers. When this outlier study was removed, between-study heterogeneity dropped strikingly to 27.3% in the remaining studies, whereas the ORs (OR, 1.47; 95% CI 1.31 to 1.66) changed moderately but remained significant. As for case–control studies, the OR changed from 1.61 (95% CI 1.36 to 1.91) to 1.52 (95% CI 1.35 to 1.72) with heterogeneity fallen from 65.8% to 23.5% (figure 5). The findings were further verified in the intermediated incidence rate group (OR, 1.49, 95% CI 1.21 to 1.82; heterogeneity: I2=49.6%, p=0.04) and in the adjusted group (OR, 1.45; 95% CI 1.25 to 1.69; heterogeneity: I2=41.8%, p=0.05) (data not shown). However, the heterogeneity reduced partly when the study of Turkoz et al was removed (OR, 1.50; 95% CI 1.28 to 1.76; heterogeneity: I2=62.4%, p<0.01) (data not shown).
Figure 5.
Forest plots for comparing the risk for NPC between ever smokers versus never smokers after deleting the Ji et al study.
Publication bias was evaluated by Egger test and Trim-and-Fill method. Except for subgroup analyses with ever smokers and heavy smokers, no prominently significant publication bias (with p>0.05 in Egger test) was observed in our meta-analysis. After adjusted for publication bias, the risk of NPC remained stable with an OR of 1.56 (95% CI 1.32 to 1.84) for ever smokers, but changed slightly (OR, 1.80, 95% CI 1.37 to 2.36) for heavy smokers (table 3).
Discussion
The results from this meta-analysis, based on 17 case–control studies and 4 cohort studies, supported that there was moderate association between cigarette smoking and nasopharyngeal carcinoma risk, which was consistent with the result of previous meta-analysis.13
Interpretation
The pooled risk estimate for cohort studies comparing ever smokers to never smokers was not statistically significant. When conducted similar stratified analyses for current smokers and former smokers, we found that current smoking was significantly related to the risk of NPC while former smoking had an insignificant association with NPC risk. Considering the findings of stratified analyses, it might be the result from former smoking that contributed to the discrepancy between pooled analysis for cohort studies and overall analysis. In addition, this meta-analysis demonstrated relatively high heterogeneity both for the overall analysis and subgroup analyses. When the Ji et al study44 was removed from the synthetic analysis, heterogeneity was strikingly reduced in stratified analysis by study design and regions with different NPC incidence rate. Furthermore, the meta-regression analyses indicated that heterogeneity did not prominently result from publication year, study design, regions of different incident rate and quality of studies. To our knowledge, multiple lines of epidemiological studies had found that the development of NPC could be influenced by varieties of aetiologies including Epstein-Barr virus (EBV), genetic components and other environmental factors, like preserved food, socioeconomic status, occupation, so on and so forth.6 47–50 Therefore, it might be its inappropriate subjects that contributed to selection bias which resulted in the high heterogeneity in the44 Ji et al’s study, though it had a large sample size with risk estimates adjusted by age, gender, alcohol intake and family history.
One large cohort study,10 conducted in high-incidence region and comprised the majority of undifferentiated NPC (nearly 90% cases), did not reported statistically increased risk of NPC among current smokers compared with never smokers. The difference in the effect of current smoking on NPC risk may be due to its histological type of NPC because undifferentiated carcinoma in high-risk areas seemed more strongly related to EBV infection other than cigarette smoking.48 Meanwhile, some case–control studies with small sample size of current smokers also had null results,7–9 38 39 of which two studies pointed out that significantly higher risk only existed for smokers with considerable levels of cigarette smoking (>20 cigarettes/day or >30 pack-years).38 39 Nonetheless, the result of our integrated analysis for current smokers versus never smokers was generally consistent with that of the previous meta-analyses.13
For former smokers, the less consistent risk estimates might result from small number of studies with adequate sample size. The estimates for former smokers in eight studies7–10 26 39 41 46 presented null association on NPC risk which was parallel to the results of stratified analysis by study design, and only two studies23 38 demonstrated statistically positive results. The discrepancies in the effects of former cigarette smoking on NPC risk might arise from the following aspects: the group of former smokers may have included people who had quit for a long time, and thus their risk might diminish or even reach the level of never smokers; the minimum period of time since quitting smoking in former smokers varied by study, which could result in judgement bias on the interviewed subjects in some studies.
This meta-analysis revealed that there was a clear dose–response relationship between cigarette smoking and the risk of NPC. That is, the more cigarette smoking (intensity of cigarettes smoked per day and the amount of pack-years), the higher risk for the development of NPC. Note that similar results have been widely observed for pancreatic cancer, liver cancer, renal carcinoma and gall bladder disease.51–54 The exact explanation of this dose-dependent effect remains vague, it could be hypothesised that the more cigarette smoking, the greater impact on the epithelial cells of nasopharynx. Therefore, the risk of NPC would be higher in those who smoked more cigarettes. The actual mechanism about the relationship of the amount of smoking and NPC risk had been searched by molecular studies,55 56 which pointed out that smoking is a factor for tumour growth and acts as a mutagen and DNA damaging agent that drives tumour initiation in normal epithelial cells of nasopharynx.
In this analysis, a statistically significant effect of smoking on NPC risk was observed in males but not in women. The gender difference in response to smoking might be related to interaction between protective endogenous or exogenous oestrogens among women compared with men,57 and could also be explained by maturity of smoking trends among men and but not among women. Men might have exposure to smoking for a longer duration as compared with women (34% of the men vs 11% of the women had started smoking before the age of 15 years).58 However, the result of women being ever smokers might not be adequately stable because only two studies reported the association between cigarette smoking and the risk of NPC for women.35 41
Additionally, we found that the younger age people began to smoke, the higher risk they developed NPC. Our results showed that the pooled ORs were 1.78 (95% CI 1.41 to 2.25) for smokers in early group and 1.28 (95% CI 1.00 to 1.64) in later group, respectively. Interestingly, the findings of previous meta-analysis appeared totally opposite with ORs of 1.17 (95% CI 0.78 to 1.75) for early group and 1.58 (95% CI 1.10 to 2.26) for later group.13 Like many other cancers, NPC may take decades to develop from premalignant cells to detectable solid tumour. Thus, the exposure to carcinogenic agents early in life could have substantial impacts on the development of NPC.6 59 Moreover, the incidence of NPC peaks at age of 50–59 years in high-risk regions, whereas in western countries, the incidence of NPC peaks somewhat later (≥65 years old).59 As a result, the number of NPC patients in terms of age distribution could considerably vary in our eligible studies that were conducted in different countries.
When stratified by histological type of NPC, the pooled analysis presented a higher risk of differentiated NPC than that of undifferentiated NPC, and the later had an insignificant risk estimate. This difference might be owing to fewer studies included in the pooled analysis for undifferentiated NPC because we excluded those ineligible studies either for no report of the association between cigarette smoking and NPC risk16 or for overlapped data.60 It might avoid incorrect estimation of smoking effects on NPC risk. Moreover, we found that the risk estimates adversely associated with the NPC incidence rate. For example, the pooled OR for high incidence rate areas to low incidence rate areas ranged from 1.27 to 1.68. This might suggest there are substantial heterogeneity between NPC risk and smoking by histological types and geographical variations. Undifferentiated carcinoma of the nasopharynx is the predominant type in high-risk areas, and it is consistently associated with EBV infection, which may increase the carcinogenic effect of cigarette smoking.48
Generalisability
The magnitude of association between cigarette smoking and the NPC risk was not as big as those for other smoking-related cancers like lung cancer and gastrointestinal malignancies.4 However, NPC was quite epidemic in Southeastern Asia especially in cities in southern China, and China was one of the largest tobacco producing and consuming countries in the world.61 Besides, we found current smokers are more related to the development of NPC with a higher risk estimate as compared with former smokers. These emphasised the importance and urgency of efforts to initiate the control of cigarette smoking to improve public health. Any efficient tobacco control programme would be helpful to reduce morbidity and mortality of smoking-related cancers worldwide.
Limitations
The results of this meta-analysis should be explicated in the context of several limitations. For example, the design of included studies varied in source of subjects recruited, standardisation for categories of cigarette smoking, ambiguous definition of tobacco products and adjusted factors. Additionally, our meta-analysis was a mix of retrospective studies and prospective studies, and was lack of individual participant data for adjustment of potential confounders. Generally, EBV infection was thought to be highly related to NPC risk.62 However, a 22-year follow-up study carried out by Hsu et al revealed that EBV was less likely to modify the estimate for smoking associated with NPC risk.43 And the links of other risk factors like dietary and social practices were often inconsistent between studies.62 Moreover, the risk estimates of NPC resembled both in the group with adjusted OR and in the group with unadjusted OR in our meta-analysis.
Conclusions
This meta-analysis demonstrated that cigarette smoking is associated with a modest, but statistically significant increased risk of NPC. Yet, further prospective studies are needed to elucidate the NPC risk in terms of gender, histological type and for former smokers and smoking onset age.
Supplementary Material
Footnotes
Contributors: LONG and LI: did the literate research and selected the eligible articles separately; LI and NIE: extracted the whole data and assessed the quality of our selected articles; LONG: integrated and analyzed data, and wrote the manuscript. FU: designed and revised the manuscript. All authors: read and approved the final manuscript.
Funding: This study was partially supported by grants 81472971 from the National Natural Science Foundation of China.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. . Cancer incidence and mortality worldwide: IARC cancer base. v1.0: GLOBOCAN, 2012. [DOI] [PubMed] [Google Scholar]
- 2. Curado MP, Edwards MP, Shin MP, et al. . Cancer incidence in five continents. Geneva: WHO Press, 2007. [Google Scholar]
- 3. Jemal A, Bray F, Center MM, et al. . Global cancer statistics. CA Cancer J Clin 2011;61:69–90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 4. Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer 2004;45:S3–S9. 10.1016/j.lungcan.2004.07.998 [DOI] [PubMed] [Google Scholar]
- 5. Sandler RS, Sandler DP, Comstock GW, et al. . Cigarette smoking and the risk of colorectal cancer in women. J Natl Cancer Inst 1988;80:1329–33. 10.1093/jnci/80.16.1329 [DOI] [PubMed] [Google Scholar]
- 6. Jia WH, Qin HD. Non-viral environmental risk factors for nasopharyngeal carcinoma: a systematic review. Semin Cancer Biol 2012;22:117–26. 10.1016/j.semcancer.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 7. Cheng YJ, Hildesheim A, Hsu MM, et al. . Cigarette smoking, alcohol consumption and risk of nasopharyngeal carcinoma in Taiwan. Cancer Causes Control 1999;10:201–7. 10.1023/A:1008893109257 [DOI] [PubMed] [Google Scholar]
- 8. Zou J, Sun Q, Akiba S, et al. . A case-control study of nasopharyngeal carcinoma in the high background radiation areas of Yangjiang, China. J Radiat Res 2000;41:53–62. 10.1269/jrr.41.S53 [DOI] [PubMed] [Google Scholar]
- 9. Polesel J, Franceschi S, Talamini R, et al. . Tobacco smoking, alcohol drinking, and the risk of different histological types of nasopharyngeal cancer in a low-risk population. Oral Oncol 2011;47:541–5. 10.1016/j.oraloncology.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 10. Friborg JT, Yuan JM, Wang R, et al. . A prospective study of tobacco and alcohol use as risk factors for pharyngeal carcinomas in Singapore Chinese. Cancer 2007;109:1183–91. 10.1002/cncr.22501 [DOI] [PubMed] [Google Scholar]
- 11. Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet 2002;359:248–52. 10.1016/S0140-6736(02)07451-2 [DOI] [PubMed] [Google Scholar]
- 12. Weinberg CR. Toward a clearer definition of confounding. Am J Epidemiol 1993;137:1–8. 10.1093/oxfordjournals.aje.a116591 [DOI] [PubMed] [Google Scholar]
- 13. Xue WQ, Qin HD, Ruan HL, et al. . Quantitative association of tobacco smoking with the risk of nasopharyngeal carcinoma: a comprehensive meta-analysis of studies conducted between 1979 and 2011. Am J Epidemiol 2013;178:325–38. 10.1093/aje/kws479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin TM, Yang CS, Tu SM, et al. . Interaction of factors associated with cancer of the nasopharynx. Cancer 1979;44:1419–23. [DOI] [PubMed] [Google Scholar]
- 15. Chen CJ, Liang KY, Chang YS, et al. . Multiple risk factors of nasopharyngeal carcinoma: Epstein-Barr virus, malarial infection, cigarette smoking and familial tendency. Anticancer Res 1990;10:547–53. [PubMed] [Google Scholar]
- 16. Nesić V, Sipetić S, Vlajinac H, et al. . Risk factors for the occurrence of undifferentiated carcinoma of nasopharyngeal type: a case-control study. Srp Arh Celok Lek 2010;138:6–10. 10.2298/SARH1002006N [DOI] [PubMed] [Google Scholar]
- 17. Ren ZF, Liu WS, Qin HD, et al. . Effect of family history of cancers and environmental factors on risk of nasopharyngeal carcinoma in Guangdong, China. Cancer Epidemiol 2010;34:419–24. 10.1016/j.canep.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 18. Armstrong RW, Armstrong MJ, Yu MC, et al. . Salted fish and inhalants as risk factors for nasopharyngeal carcinoma in Malaysian Chinese. Cancer Res 1983;43:2967–70. [PubMed] [Google Scholar]
- 19. West S, Hildesheim A, Dosemeci M. Non-viral risk factors for nasopharyngeal carcinoma in the Philippines: results from a case-control study. Int J Cancer 1993;55:722–7. 10.1002/ijc.2910550504 [DOI] [PubMed] [Google Scholar]
- 20. Ng TP, Tp N. A case-referent study of cancer of the nasal cavity and sinuses in Hong Kong. Int J Epidemiol 1986;15:171–5. [DOI] [PubMed] [Google Scholar]
- 21. Guo X, Johnson RC, Deng H, et al. . Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int J Cancer 2009;124:2942–7. 10.1002/ijc.24293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma F, Zhang H, Zhai Y, et al. . Functional polymorphism -31C/G in the promoter of BIRC5 gene and risk of nasopharyngeal carcinoma among chinese. PLoS One 2011;6:e16748 10.1371/journal.pone.0016748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fachiroh J, Sangrajrang S, Johansson M, et al. . Tobacco consumption and genetic susceptibility to nasopharyngeal carcinoma (NPC) in Thailand. Cancer Causes Control 2012;23:1995–2002. 10.1007/s10552-012-0077-9 [DOI] [PubMed] [Google Scholar]
- 24. Lin JH, Jiang CQ, Ho SY, et al. . Smoking and nasopharyngeal carcinoma mortality: a cohort study of 101,823 adults in Guangzhou, China. BMC Cancer 2015;15:906 10.1186/s12885-015-1902-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lye MS, Visuvanathan S, Chong PP, et al. . Homozygous wildtype of XPD K751Q polymorphism Is associated with increased risk of nasopharyngeal carcinoma in Malaysian population. PLoS One 2015;10:e0130530 10.1371/journal.pone.0130530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xie SH, Yu IT, Tse LA, et al. . Tobacco smoking, family history, and the risk of nasopharyngeal carcinoma: a case-referent study in Hong Kong Chinese. Cancer Causes Control 2015;26:913–21. 10.1007/s10552-015-0572-x [DOI] [PubMed] [Google Scholar]
- 27. Stroup DF, Berlin JA, Morton SC, et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- 28. Sbea WG. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa, Ontario, Canada: Ottawa Health Research Institute, 2000. [Google Scholar]
- 29. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 31. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992;135:1301–9. 10.1093/oxfordjournals.aje.a116237 [DOI] [PubMed] [Google Scholar]
- 32. Orsini NGS, et al. . A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J 2011;11:29. [Google Scholar]
- 33. Egger M, Davey Smith G, Schneider M, et al. . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mabuchi K, Bross DS, Kessler II. Cigarette smoking and nasopharyngeal carcinoma. Cancer 1985;55:2874–6. [DOI] [PubMed] [Google Scholar]
- 35. Nam JM, McLaughlin JK, Blot WJ. Cigarette smoking, alcohol, and nasopharyngeal carcinoma: a case-control study among U.S. whites. J Natl Cancer Inst 1992;84:619–22. 10.1093/jnci/84.8.619 [DOI] [PubMed] [Google Scholar]
- 36. Sriamporn S, Vatanasapt V, Pisani P, et al. . Environmental risk factors for nasopharyngeal carcinoma: a case-control study in northeastern Thailand. Cancer Epidemiol Biomarkers Prev 1992;1:345–8. [PubMed] [Google Scholar]
- 37. Yu MC, Garabrant DH, Huang TB, et al. . Occupational and other non-dietary risk factors for nasopharyngeal carcinoma in Guangzhou, China. Int J Cancer 1990;45:1033–9. [DOI] [PubMed] [Google Scholar]
- 38. Zhu K, Levine RS, Brann EA, et al. . A population-based case-control study of the relationship between cigarette smoking and nasopharyngeal cancer (United States). Cancer Causes Control 1995;6:507–12. 10.1007/BF00054158 [DOI] [PubMed] [Google Scholar]
- 39. Vaughan TL, Shapiro JA, Burt RD, et al. . Nasopharyngeal cancer in a low-risk population: defining risk factors by histological type. Cancer Epidemiol Biomarkers Prev 1996;5:587–93. [PubMed] [Google Scholar]
- 40. Chelleng PK, Narain K, Das HK, et al. . Risk factors for cancer nasopharynx: a case-control study from Nagaland, India. Natl Med J India 2000;13:6–8. [PubMed] [Google Scholar]
- 41. Yuan JM, Wang XL, Xiang YB, et al. . Non-dietary risk factors for nasopharyngeal carcinoma in Shanghai, China. Int J Cancer 2000;85:364–9. [DOI] [PubMed] [Google Scholar]
- 42. Feng BJ, Khyatti M, Ben-Ayoub W, et al. . Cannabis, tobacco and domestic fumes intake are associated with nasopharyngeal carcinoma in North Africa. Br J Cancer 2009;101:1207–12. 10.1038/sj.bjc.6605281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hsu WL, Chen JY, Chien YC, et al. . Independent effect of EBV and cigarette smoking on nasopharyngeal carcinoma: a 20-year follow-up study on 9,622 males without family history in Taiwan. Cancer Epidemiol Biomarkers Prev 2009;18:1218–26. 10.1158/1055-9965.EPI-08-1175 [DOI] [PubMed] [Google Scholar]
- 44. Ji X, Zhang W, Xie C, et al. . Nasopharyngeal carcinoma risk by histologic type in central China: impact of smoking, alcohol and family history. Int J Cancer 2011;129:724–32. 10.1002/ijc.25696 [DOI] [PubMed] [Google Scholar]
- 45. Turkoz FP, Celenkoglu G, Dogu GG, et al. . Risk factors of nasopharyngeal carcinoma in Turkey-an epidemiological survey of the Anatolian Society of Medical Oncology. Asian Pac J Cancer Prev 2011;12:3017–21. [PubMed] [Google Scholar]
- 46. Chow WH, McLaughlin JK, Hrubec Z, et al. . Tobacco use and nasopharyngeal carcinoma in a cohort of US veterans. Int J Cancer 1993;55:538–40. 10.1002/ijc.2910550403 [DOI] [PubMed] [Google Scholar]
- 47. Hildesheim A, West S, DeVeyra E, et al. . Herbal medicine use, Epstein-Barr virus, and risk of nasopharyngeal carcinoma. Cancer Res 1992;52:3048–51. [PubMed] [Google Scholar]
- 48. Tsao SW, Tsang CM, Pang PS, et al. . The biology of EBV infection in human epithelial cells. Semin Cancer Biol 2012;22:137–43. 10.1016/j.semcancer.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 49. Polesel J, Serraino D, Negri E, et al. . Consumption of fruit, vegetables, and other food groups and the risk of nasopharyngeal carcinoma. Cancer Causes Control 2013;24:1157–65. 10.1007/s10552-013-0195-z [DOI] [PubMed] [Google Scholar]
- 50. He YQ, Xue WQ, Shen GP, et al. . Household inhalants exposure and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC Cancer 2015;15:1022 10.1186/s12885-015-2035-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zou L, Zhong R, Shen N, et al. . Non-linear dose-response relationship between cigarette smoking and pancreatic cancer risk: evidence from a meta-analysis of 42 observational studies. Eur J Cancer 2014;50:193–203. 10.1016/j.ejca.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 52. Lee YC, Cohet C, Yang YC, et al. . Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol 2009;38:1497–511. 10.1093/ije/dyp280 [DOI] [PubMed] [Google Scholar]
- 53. Hunt JD, van der Hel OL, McMillan GP, et al. . Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer 2005;114:101–8. 10.1002/ijc.20618 [DOI] [PubMed] [Google Scholar]
- 54. Aune D, Vatten LJ, Boffetta P. Tobacco smoking and the risk of gallbladder disease. Eur J Epidemiol 2016;31:643–53. 10.1007/s10654-016-0124-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Furmanski P. Revealing the mechanism of tissue damage due to tobacco use: finally, a smoking gun? Am J Pathol 2013;182:1489–93. 10.1016/j.ajpath.2013.02.004 [DOI] [PubMed] [Google Scholar]
- 56. Salem AF, Al-Zoubi MS, Whitaker-Menezes D, et al. . Cigarette smoke metabolically promotes cancer, via autophagy and premature aging in the host stromal microenvironment. Cell Cycle 2013;12:818–25. 10.4161/cc.23722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Corrao G, Zambon A, Conti V, et al. . Menopause hormone replacement therapy and cancer risk: an Italian record linkage investigation. Ann Oncol 2008;19:150–5. 10.1093/annonc/mdm404 [DOI] [PubMed] [Google Scholar]
- 58. Peto R, Darby S, Deo H, et al. . Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000;321:323–9. 10.1136/bmj.321.7257.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 2006;15:1765–77. 10.1158/1055-9965.EPI-06-0353 [DOI] [PubMed] [Google Scholar]
- 60. Nazar-Stewart V, Vaughan TL, Burt RD, et al. . Glutathione S-transferase M1 and susceptibility to nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 1999;8:547–51. [PubMed] [Google Scholar]
- 61. Mackay J, Ritthiphakdee B. Reddy KS: Tobacco control in Asia. Lancet 2013;381:1581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chua MLK, Wee JTS, Hui EP, et al. . Nasopharyngeal carcinoma. Lancet 2016;387:1012–24. 10.1016/S0140-6736(15)00055-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.