Abstract
Objective
To evaluate the health-economic costs and benefits of a guided eHealth intervention (E-health module embedded in Collaborative Occupational healthcare (ECO)) encouraging sick-listed employees to a faster return to work.
Design
A two-armed cluster randomised trial with occupational physicians (OPs) (n=62), clustered and randomised by region into an experimental and a control group, to conduct a health-economic investment appraisal. Online self-reported data were collected from employees at baseline, after 3, 6, 9 and 12 months.
Setting
Occupational health care in the Netherlands.
Participants
Employees from small-sized and medium-sized companies (≥18 years), sick-listed between 4 and 26 weeks with (symptoms of) common mental disorders visiting their OP.
Interventions
In the intervention group, employees (N=131) received an eHealth module aimed at changing cognitions regarding return to work, while OPs were supported by a decision aid for treatment and referral options. Employees in the control condition (N=89) received usual sickness guidance.
Outcomes Measures
Net benefits and return on investment based on absenteeism, presenteeism, health care use and quality-adjusted life years (QALYs) gained.
Results
From the employer’s perspective, the incremental net benefits were €3187 per employee over a single year, representing a return of investment of €11 per invested Euro, with a break-even point at 6 months. The economic case was also favourable from the employee’s perspective, partly because of QALY health gains. The intervention was costing €234 per employee from a health service financier’s perspective. The incremental net benefits from a social perspective were €4210. This amount dropped to €3559 in the sensitivity analysis trimming the 5% highest costs.
Conclusions
The data suggest that the ECO intervention offers good value for money for virtually all stakeholders involved, because initial investments were more than recouped within a single year. The sometimes wide 95% CIs suggest that the costs and benefits are not always very precise estimates and real benefits could vary considerably.
Trial Registration
Keywords: mental disorder, absenteeism, return to work, ehealth, cost-benefit, occupational health
Strengths and limitations of this study.
This study adds to the few available studies that present a trial-based investment appraisal of the economic costs and benefits of a return to work intervention for sick-listed employees.
The trial was only powered to test a difference in sickness absence duration and not for testing economic hypotheses.
The follow-up time is limited to 12 months.
Introduction
Long-term sickness absence has a significant economic impact, largely due to the substantial productivity losses.1 2 Mental disorders are a leading cause of sickness absence,3–6 which is not without economic ramifications.7 Common mental disorders, specifically depression and anxiety, are the most prevalent in the workforce.8
For the treatment of common mental disorders, a range of psychological and pharmaceutical interventions have been shown to be effective and cost-effective.9 10 However, symptomatic recovery does not automatically reduce sickness absence.10–12 To improve occupational outcomes, it is also important to pay attention to return to work during treatment.
In the Netherlands, treatment and sickness certification are separated from each other in social security legislation. Occupational physicians (OPs) play a central role in the sickness guidance of workers by making a problem analysis and giving advice on a return to work plan, whereas treatment is provided by the mental health sector. The legislation was introduced to protect the worker’s privacy and relationship with the curative physician.13 14 A guideline has been developed to suggest directions to OPs to better assist employees with mental health problems in the return to work process. According to this guideline, the OPs need to closely monitor both the mental health problems and the level of functioning. When recovery is slow or hampered, they can consult or refer to a psychiatrist, a psychologist or a social worker.15 A study of Rebergen and colleagues suggested that better adherence to the guideline is associated with earlier return to work.16 However, in practice, adherence appears to be far from optimal,17 18 and there is often a lack of cooperation between the OPs and treatment providers in the mental health sector. Several attempts have been made to bridge this gap. One study about the effect of psychiatric consultation for OPs assisting sick-listed employees did provide results in terms of earlier return to work.19 However, this study was small. Another study evaluating active treatment by an OP within a collaborative care arrangement did improve depressive symptoms, but failed to speed up return to work.20 It appeared that OPs need support in helping sick-listed employees change their attitude towards resuming work and that OPs should monitor symptom improvement and work performance in a more systematic manner.
To overcome these problems and to better manage the return to work of sick-listed employees with (symptoms of) common mental disorders, the ‘E-health module embedded in Collaborative Occupational healthcare’ (ECO) intervention was developed. The ECO intervention was designed to promote return to work by improving work functioning in employees, providing a decision aid for the OP who gives guidance to the employee, and by including the opportunity for psychiatric consultation to the OP.21
The results of a recent trial showed that ECO led to an earlier return to work than usual care (mean duration of 50 days in the ECO group vs 77 days in the care as usual (CAU) group) and higher remission rates of common mental disorder after 9 months in a group of sick-listed employees with (symptoms of) mental disorders.22
Taking the economic perspective, we expect that the ECO intervention is cost-effective as seen from the employer’s viewpoint because ECO is a low-cost self-help intervention with a limited amount of support from the OP and appears to be effective in reducing absenteeism. There is less certainty how cost-effective the intervention would be as seen from the perspective of the sick-listed employees and the healthcare financier (ie, healthcare insurance company in the Dutch context). Therefore, this study conducts a cost–benefit analysis of the ECO intervention from all three stakeholders’ viewpoints, and combines these in an overarching societal perspective. These analyses are important because very few trial-based economic evaluations have been conducted with regard to return-to-work interventions for sick-listed employees with (symptoms of) common mental disorders.12 23
Method
Study design
The ECO study was designed as a two-armed cluster randomised controlled trial, with randomisation at the level of the OP. OPs were either randomised to usual care alone or usual care plus the ECO intervention. The Netherlands Organisation for Health Research and Development funded the study (grant number 171002403 ZonMw Doelmatigheid) together with Achmea, a Dutch insurance company. The Medical Ethics Committee of the University Medical Center Utrecht approved the study protocol in 2011, and the trial was registered at the Netherlands Trial Register under number 2108. The design of the study is described in detail elsewhere.21 22 Here, we provide a brief summary of the main characteristics and focus on the economic aspects.
Randomisation
To prevent contamination, cluster randomisation took place at the area level of the OPs working in the same region across a total of 12 regions. An independent statistician randomised six regions to the ECO condition and the remainder to the control condition using computer-generated randomisation. Since the OPs had to offer the intervention, they could not be blinded for randomisation. The researchers and participants were informed about the allocation after the randomisation procedure.
Participants
Participants were recruited from July 2011 to January 2013 from all-cause sick-listed employees working at small-sized and medium-sized companies in the Netherlands who visited an OP. To be eligible for inclusion, the employees had to be at least 18 years of age and on sickness absence between 4 and 26 weeks. This time window was chosen to avoid including employees with spontaneous recovery and to increase the probability of employees ever returning to work.24 In addition, the employees needed to have a score ≥10 on either the depression or the somatisation scale of the Patient Health Questionnaire,25 26 or the Generalised Anxiety Disorder questionnaire.27 Exclusion criteria were (1) poor command of the Dutch language, (2) pregnancy, (3) not having access to the internet and (4) being involved in a legal action against the employer.
Procedure
Initially, an independent statistician randomised 12 regions to either CAU (six regions with 30 OPs) or ECO (six regions with 32 OPs) by using a computer algorithm. Within the cluster of CAU regions, 5875 sick-listed employees were screened for eligibility resulting in 326 screen-positives. In the cluster of ECO regions, 537 screen-positives were obtained from 8740 sick-listed employees. Of these, 89 consenting participants received sickness guidance from OPs who were randomised to CAU and 131 participants from OPs in the ECO cluster. The unequal distribution of participants over the conditions was due to the cluster randomisation of the OPs. Participants received measurements at baseline and at 3, 6, 9 and 12 months post baseline. Dropout occurred in both conditions (see figure 1).
Figure 1.
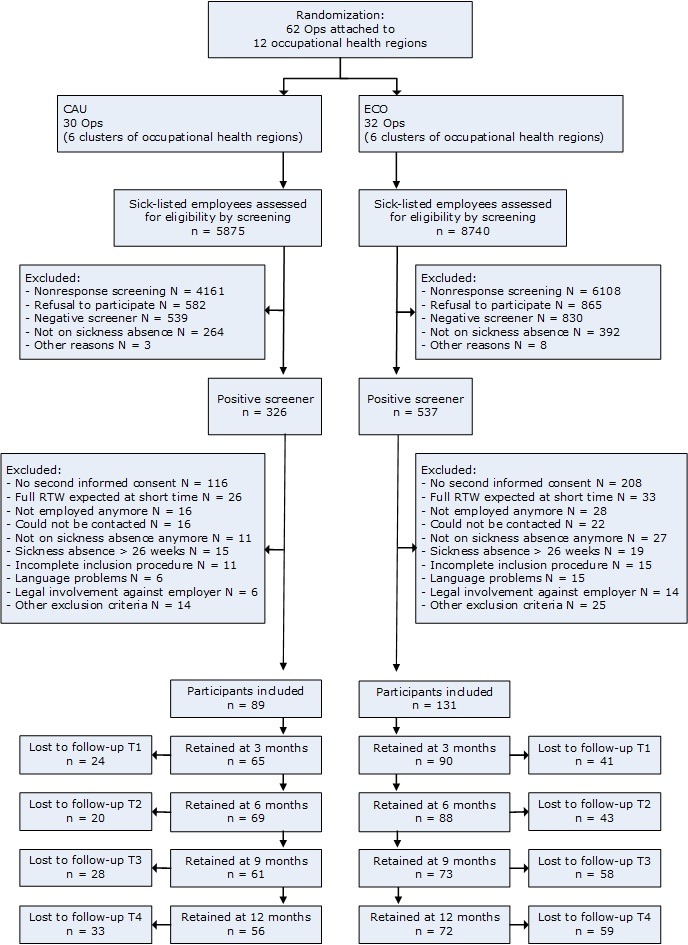
Flowchart of the participants. CAU, care as usual. ECO, E-health module embedded in Collaborative Occupational health care.
Intervention
ECO consists of two components: (1) the eHealth module Return@Work for the employee and (2) an email-based decision aid to support the OP. Return@Work is aimed at improving the self-efficacy of employees and promoting the employee’s intention to return to work. Recent studies have shown that these factors are predictors of actual work resumption.28–30 The decision aid provides the OPs with advice regarding treatment and referral options based on the employee’s outcome monitoring in Return@Work.
The eHealth module starts with an assessment questionnaire. Depending on the results of the questionnaire regarding symptoms and cognitions about return to work of the individual employee, Return@Work presented specific modules and sessions. As a consequence, the amount of modules and sessions offered to the employees differed. In total, Return@Work included five modules composed of 16 sessions, covering: (1) psychoeducation, (2) cognitions regarding return to work while having symptoms (based on principles of cognitive behavioural therapy), (3) problem solving skills, (4) pain and fatigue management and reactivation and (5) relapse prevention. The employees went through the modules independently, but had the possibility to discuss Return@Work modules and assignments with the OP. The OPs were requested to inquire about the employee’s progress in the eHealth module and to provide support if necessary during their regular face-to-face contacts with the employee. Periodic visits between the employee and the OP are part of the guidelines of the Netherlands Society of Occupational Medicine (NVAB),15 to which all OPs were required to adhere.
Besides the modules, Return@Work also contained a monitor of functioning and symptoms on a regular basis. This monitor was used for the second component of ECO, a decision aid to support OPs in the sickness guidance of employees. Based on the outcomes of the monitor in Return@Work, the OPs received automated email messages with advice for next steps in collaborative care. In addition, the decision aid gave OPs the option to consult a psychiatrist in case insufficient progress was made. The OPs in the experimental condition received a 4-hour training about ECO.
In the control condition, the employees received usual sickness guidance. The guidelines of the NVAB were used as a protocol.15 As there is a lack of adherence to the guidelines,17 18 actual care was assessed with a questionnaire by all of the participating employees.
Outcome measures
Participants filled in the Medical Technology Assessment Cost Questionnaire for Psychiatry (TiC-P),31 that among healthcare use also measures absenteeism from work, which is the main outcome variable of this study. The TiC-P is based on self-report, and to cross-check the number of work days lost to absenteeism, we compared the self-reports with administrative data (see Sensitivity analysis section below). Total follow-up time was 12 months with measurements at baseline and after 3, 6, 9 and 12 months. Finally, health gains in terms of quality-adjusted life years (QALYs) were assessed using the 3-level version of the EuroQoL-5D,32 with the Dutch tariff.33
Resource use and costing
Cost data were collected using the TiC-P, including (1) direct medical costs, including the costs of medication, (2) direct non-medical costs (patients’ out-of-pocket costs for trips to health services), (3) costs stemming from productivity losses owing to absenteeism and presenteeism and (4) costs that occurred in the domestic realm (help for housekeeping from family, friends or hired people). Standard costs, expressed in euro (€), were indexed for the reference year 2011 using the consumer price index from Statistics Netherlands. Costs were not discounted because the follow-up period did not exceed 1 year.
Computation of costs
The set costs of the ECO intervention were €300 per user, which is its current (post trial) rate. Direct medical costs were limited to mental health service use. The medical costs were computed by multiplying the number of health service units (sessions, visits, hospital days) with their standard full economic cost price.34 Only medication costs for mental problems were included in the economic analysis. For every type of drug (eg, antidepressants, benzodiazepines, antipsychotics, hypnotics), an average cost price was calculated based on the cost prices per standard daily dose of three drugs most often prescribed to the participants as reported in the Pharmaceutical Compass,35 while taking into account the GP’s prescription costs, the pharmacist’s dispensing costs and the pharmacist’s claw back as per the guideline for cost computations in healthcare.34
The direct non-medical costs consisted of the travel costs that participants had to make to visit OPs and health services. These costs were calculated as the average distance to the specific health service provider multiplied by the costs per km (€0.21) plus parking costs (€3.11) per hour. To the direct non-medical costs, we added the costs of (informal) caregivers (eg, family and friends) due to the employee’s reduced functionality at home, computed by multiplying the number of hours by €12.96.
In the Netherlands, QALY health gains are valued between €20 000 and €80 000 per QALY.36 We used the lower bound of €20 000 to conduct our analysis under conservative assumptions.
Productivity losses comprised the costs of lost work days due to absenteeism and the costs of inefficiency while at work (presenteeism). We used the human capital method to value the productivity costs.37 In the case of absenteeism, this method multiplies the number of days absent by the gender and age-specific average gross wages per employee, as per the Dutch guideline for health economic evaluation.34 To assess the costs of presenteeism, we used the number of days actually worked when ill multiplied by a self-reported inefficiency score. This score ranged from 0 (as effective as in good health) to 1 (totally ineffective). Again, the gender and age-specific average gross wages were used to compute the costs of presenteeism. To illustrate, if an employee reported an inefficiency score of 0.50 for 7 working days, then we assumed that 3.5 working days have been lost due to presenteeism.
Analyses
Following recommendations from the Consolidated Standards of Reporting Trials (CONSORT) and Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statements,38–40 analyses were conducted in agreement with the intention-to-treat principle. Therefore, all participants as randomised were retained in the analysis, and missing observations due to dropout were imputed. For imputation, we used both the estimation-maximisation (EM) algorithm as implemented in SPSS for the main analysis and regression imputation (RI) as implemented in Stata for the sensitivity analysis (see below). In both imputation strategies, we used predictors of outcomes (costs and QALYs) and predictors of dropout (age, gender, partner status, country of birth, number of work loss days). Predictors of the outcomes were included to increase precision in the imputed values, predictors of dropout were incorporated to tackle selection bias, if any, and to meet the missing at random (MAR) assumption underlying most imputation techniques.
The economic evaluation was conducted as an incremental cost–benefit analysis because the primary outcome (duration of sick leave) could directly be expressed in terms of monetary benefits. The costs and benefits were calculated at baseline, 3, 6, 9 and 12 months in the ECO and CAU conditions. The costs in the intermediate months were linearly interpolated. This allowed mapping the monthly cash flows of costs and benefits over the full 12-month period. The cash flows were computed from four perspectives: (1) the employer’s perspective focussing on the net benefits from greater productivity via lesser absenteeism and lesser presenteeism; (2) the healthcare payer’s perspective (in the Netherlands: healthcare insurers) focussing on the direct medical costs due to health service use, including the costs of medication, (3) the employee’s perspective focussing on QALY health gains, fewer out-of-pockets costs and less informal care from family members or friends. Finally, we included the societal perspective (4), including all costs and benefits, regardless of who incurs costs or receives benefits.
The monthly cash flows were used to compute the cumulative costs and cumulative monetary benefits over the full 12 months. Incremental costs, incremental benefits and incremental net benefits were obtained by comparing ECO intervention with CAU. These are the main outcomes of the economic analysis alongside metrics such as the break-even point and the return on investment (ROI).
For assessing the incremental net benefits, we relied on non-parametric bootstrapping (2500 replications) since costs are non-normally distributed. Statistics such as mean costs, 95% CIs, SEs and p values are all based on non-parametric bootstrapping to increase the robustness of our findings. The data were analysed in SPSS (V.22) and Stata (V.13.1).
Sensitivity analysis
The main analysis (using the overarching societal perspective and based on EM imputation) was repeated three times in a series of sensitivity analyses. First, the analysis was conducted again, but now, based on RI to assess the robustness of the findings under a different imputation technique. Second, we crosschecked the self-reported absenteeism against administrative data derived from the registers of the occupational health service or the employer because the main analysis was based on self-reports and some recall bias (under-reporting) could have occurred. Finally, we recalculated the incremental net benefits after trimming the highest 5% of total cumulative costs per employee because the participants with the extremely high costs were only a small minority but may have exercised a disproportional influence on the cost estimates and pushed outcomes to a more favourable outcomes for the ECO intervention. By excluding these participants, primarily from the CAU condition, the net benefits were re-estimated but now under conservative assumptions.
Results
Sample characteristics and baseline costs
Baseline characteristics of the sample (including baseline costs) are presented in table 1. The mean age of the 220 participants was 44 years and 59% was women. No important differences were observed at baseline in demographic characteristics and quality of life, but baseline costs were somewhat higher in the ECO condition, suggesting that the ECO group had a slightly disadvantageous start. We will return to this issue in the Discussion. As described by Volker and colleagues,22 job characteristics and sickness absence duration at baseline were also comparable between the intervention condition and control condition, indicating that the randomisation was generally well balanced.
Table 1.
Baseline characteristics in the care as usual (CAU) and the ECO intervention group
| CAU (n=89) | ECO (n=131) | |
| Age, mean (SD) | 45.5 (10.7) | 43.3 (9.5) |
| Female, N (%) | 53 (59.6) | 77 (58.8) |
| Married/living together, N (%) | 62 (69.7) | 91 (69.5) |
| Educational level, N (%) | ||
| Low | 32 (36.0) | 48 (36.6) |
| Average | 31 (34.8) | 47 (35.9) |
| High | 26 (29.2) | 36 (27.5) |
| Country of birth: The Netherlands, N (%) | 83 (93.3) | 123 (93.9) |
| Direct medical costs, mean (SD) | 645 (58) | 602 (49) |
| Direct non-medical costs, mean (SD) | 35 (2) | 33 (2) |
| Absenteeism, mean (SD) | 2850 (146) | 3078 (125) |
| Presenteeism, mean (SD) | 34 (16) | 20 (14) |
| Costs in the domestic realm, mean (SD) | 143 (26) | 133 (20) |
| Medication, mean (SD) | 8 (2) | 12 (3) |
| Total costs, mean (SD) | 3716 (154) | 3879 (141) |
| Quality of life, mean (SD) | 0.57 (0.027) | 0.54 (0.024) |
ECO, E-health module embedded in Collaborative Occupational healthcare.
Loss to follow-up
The measurements at 3, 6, 9 and 12 months were completed by 155 (70.5%), 157 (71.4%), 134 (60.9%) and 128 (58.2%) of the participants. The dropout rate over the 12-month trial period was higher in the ECO condition (45.0%) than the control condition (37.1%), but this difference was not statistically significant (χ2=1.38; df=1; p=0.240). As indicated, we looked for variables that predict dropout and included these as predictors in the EM and RI imputations. This was done to counter selection bias (if any) and to better meet the MAR assumption underpinning the imputation strategies.
On the topic of treatment adherence, 90 of the 131 participants in the ECO condition (69%) finished the introduction and started with the intervention. These participants had a mean number of total log-ins of 7.8. Forty per cent (36/90) completed at least half of the modules and 23% (21/90) finished at least 70% of the prescribed number of sessions.22
Costs and QALYs at 3, 6, 9 and 12 months
The next step of the cost–benefit analyses was to ascertain costs and quality of life at the follow-up measurements (table 2). Cost differences were highest for absenteeism. At 12 months, all the cost differences were statistically significant and in favour of the ECO condition. The total costs difference at the 12-month follow-up amounted to €919 (SE=205; z=4.48; p<0.001), mainly due to reduced absenteeism.
Table 2.
Average monthly costs in the care as usual (CAU) and the ECO intervention group at 3, 6, and 9 months (in 2011 Euro)*†
| 3 months | 6 months | 9 months | 12 months | |
| Direct medical costs | ||||
| CAU | 474 | 321 | 383 | 296 |
| ECO | 463 | 476 | 333 | 148 |
| Cost difference | 11 | −155 | 50 | 148 |
| Direct non-medical costs | ||||
| CAU | 135 | 74 | 102 | 98 |
| ECO | 104 | 89 | 67 | 45 |
| Cost difference | 31 | −15 | 35 | 53 |
| Productivity losses | ||||
| Absenteeism | ||||
| CAU | 2120 | 1699 | 1276 | 1118 |
| ECO | 1887 | 1264 | 725 | 572 |
| Cost difference | 233 | 435 | 551 | 546 |
| Presenteeism | ||||
| CAU | 166 | 233 | 269 | 493 |
| ECO | 357 | 408 | 322 | 325 |
| Cost difference | −191 | −175 | −53 | 168 |
| Total costs | ||||
| CAU | 2895 | 2328 | 2029 | 2005 |
| ECO | 2811 | 2238 | 1446 | 1090 |
| Cost difference | 84 | 90 | 583 | 915 |
| Quality of life (utility) | ||||
| CAU | 0.65 | 0.68 | 0.68 | 0.73 |
| ECO | 0.65 | 0.72 | 0.76 | 0.77 |
| Difference in utilities | 0 | 0.04 | 0.08 | 0.04 |
*Between-group differences in italics are statistically significant at p<0.05.
†Numbers may not add due to rounding.
ECO, E-health module embedded in Collaborative Occupational healthcare.
Cost–benefit analysis: employer’s perspective
For the employer’s perspective, only the intervention costs and costs stemming from absenteeism and presenteeism were included, thus assuming that the employer would be interested to know the pay out of this investment when paying for the intervention. Cumulated over the 12-month period, the incremental benefits were €3487 in favour of the ECO condition (Bootstrapped 95% CI −418 to 7390; SE=1992; z=1.75; p=0.080), which was mainly due to a larger reduction in absenteeism over 12 months compared with CAU (bootstrapped M=4291; 95% CI 2908 to 8292; SE=2041; z=2.10; p=0.036). Next, we calculated incremental net benefits by subtracting the intervention costs (€300) from the incremental benefits. As shown in table 3, the incremental net benefits over 12 months were €3187 per employee in favour of the ECO condition, but there is significant uncertainty in the estimate (bootstrapped 95% CI −656 to 7029; SE=1961; z=1.63; p=0.104). We return to this issue in the Discussion. The break-even point for the employer, the moment in time where the investment of €300 is recouped, is around 6 months. The ROI is 3187/300=10.62, indicating that for every euro invested, the pay-out is €10.6.
Table 3.
Monthly per patient costs in the care as usual (CAU) and the ECO intervention group from an employer’s perspective (in 2011 Euro)
| Month | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Cumulative |
| CAU | |||||||||||||
| Absenteeism | 2850 | 2485 | 2120 | 1910 | 1910 | 1699 | 1487 | 1487 | 1276 | 1197 | 1197 | 1118 | 20 736 |
| Presenteeism | 34 | 100 | 166 | 199 | 199 | 233 | 251 | 251 | 269 | 380 | 380 | 493 | 2955 |
| Total costs | 2884 | 2585 | 2286 | 2109 | 2109 | 1932 | 1738 | 1738 | 1545 | 1577 | 1577 | 1611 | 23 691 |
| ECO | |||||||||||||
| Absenteeism | 3078 | 2483 | 1887 | 1576 | 1576 | 1264 | 994 | 994 | 725 | 648 | 648 | 572 | 16 445 |
| Presenteeism | 20 | 188 | 357 | 382 | 382 | 408 | 365 | 365 | 322 | 323 | 323 | 325 | 3760 |
| Total costs | 3098 | 2671 | 2244 | 1958 | 1958 | 1672 | 1359 | 1359 | 1047 | 971 | 971 | 897 | 20 205 |
| Incremental benefits | −214 | −86 | 42 | 151 | 151 | 260 | 379 | 379 | 498 | 606 | 606 | 714 | 3486 |
| Intervention costs | 300 | ||||||||||||
| Incremental net benefits | −514 | −600 | −558 | −407 | −256 | 4 | 383 | 762 | 1260 | 1866 | 2472 | 3186 | |
| Return on investment | 10.6 | ||||||||||||
ECO, E-health module embedded in Collaborative Occupational health carehealthcare.
Cost–benefit analysis: healthcare payer’s perspective
For the perspective of the healthcare financier, we looked at the direct medical costs including the costs for medication. We computed the monthly cash flows and compared these between the ECO and CAU conditions as before. The cumulative costs over 12 months were more or less the same for each condition with a small difference of €66 in favour of the ECO condition. Assuming that the health insurer would pay for the intervention, the intervention costs of €300 have to be subtracted from these benefits in order to obtain the net benefits. This generated a negative value of €234, implying that the ECO intervention is not cost saving from a healthcare insurer’s perspective (bootstrapped 95% CI −1379 to 911; SE=584; z=−0.40; p=0.689).
Cost–benefit analysis: employee’s perspective
Employee’s costs and benefits included direct non-medical costs (ie, the patient’s out-of-pocket costs and costs in the domestic realm) and QALY health gains. Cumulated over 12 months, the incremental benefits for the ECO group were €262 regarding non-medical costs and €696 due to QALY gains (0.035*€20 000). The incremental net benefits were €958–€300=658 (bootstrapped 95% CI 2901 to 025; SE=187; z=3.51; p=0.000). The break-even point occurred at 8 months and the ROI was 658/300=2.2.
Cost–benefit analysis: societal perspective
For the societal perspective, we included the costs and benefits of all stakeholders. The difference between conditions of the cumulative benefits was €29 893–€25 383=€4510 in favour of the intervention condition (bootstrapped 95% CI 103 to 8918; SE=2249; z=2.01 p=0.045). Subtraction of the intervention costs of €300 yielded incremental net benefits from a social perspective of €4210 (bootstrapped 95% CI −259 to 8674; SE=22 77; z=1.85; p=0.064). Break-even was achieved at 7 months and the ROI was 4210/300=14.0.
Sensitivity analyses
For the main analysis, we used EM imputation; now, we recomputed the estimates under RI. Taking the societal perspective, the incremental net benefits became €4093 (bootstrapped 95% CI −2798 to 465; SE=2231; z=1.83; p=0.067) and the ROI was 4093/300=13.6, which is close to the EM-based analysis (see table 4).
Table 4.
Incremental net benefit and return on investment from societal perspective for base case and sensitivity analyses (in 2011 Euro)
| Incremental net benefit | Return on investment | |
| Base case analysis | 4210 (−259 to 8674) | 14.0 |
| Sensitivity analysis regression imputation | 4093 (−279 to 8465) | 13.6 |
| Sensitivity analysis administrative data | 5316 (−2590 to 13,222) | 17.7 |
| Sensitivity analysis trimming highest 5% | 3559 (−611 to 7729) | 11.9 |
The incremental net benefits in the main analyses were dominated by the costs offsets due to reduced absenteeism, but these were based on self-reported data. Crosschecking the self-reported data against administrative data derived from the registers of the occupational health service or employer showed that the estimates for days absent were lower in the analysis based on self-report data than on administrative data (72 work days absent based on self-reported data vs an average of 102 work days absent based on administrative data). When basing the analysis on administrative data, the total cumulative incremental net benefits became €5316 (bootstrapped 95% CI −2590 to 13 222; SE=4034; z=1.32; p=0.188), which is higher by a factor 1.3 than the corresponding estimate presented in the main analysis. The main analysis thus represents a safer (lower) estimate.
Finally, we repeated the main analysis by replacing the total costs of the respondents with the top 5% highest total costs due to absenteeism by the highest amount witnessed in the other 95% respondents. The top 5% outliers were mainly situated in the CAU condition, raising the average costs for this group. The incremental net-benefits based on the trimmed costs dropped from €4210 to €3559 (SE 95% CI= −6117,729; SE=2128; z=1.67; p=0.094), which can be regarded as a more conservative lower bound.
Discussion
Principal findings
This study was set out to evaluate the cost-effectiveness of an intervention that encourages sick-listed employees with (symptoms of) common mental disorders to make an early return to their work. The economic evaluation was conducted as an incremental cost–benefit analysis and reports on the incremental cost to benefit ratio, the return on investment, the break-even point and the incremental monetary net benefits, as customarily seen in business cases and investment appraisals. These metrics were computed from various perspectives: the perspective of the employer, the employee, the healthcare financier and society. The main findings can now be summarised as follows:
Taking the employer’s perspective, the focus of the economic evaluation was placed on the intervention costs and changes in productivity owing to changes in absenteeism and presenteeism. Assuming that the employer would make the investment in the ECO intervention of €300 per employee, the incremental net benefits were €3187 per employee over a year. This was equivalent to a ROI of €11 per invested Euro. Benefits largely stemmed from reduced absenteeism and exceeded the investment costs after 6 months.
From the perspective of the healthcare payer, the incremental net benefits were negative, amounting to additional costs of €234 per employee on average.
As seen from the employee, the net benefits, including the value of the employee’s QALY health gains, exceeded the costs by €658.
From the societal perspective, the initial investment was also more than recouped. Considering all costs and benefits, the incremental net benefits were €4210, with a break-even point at 7 months. Every euro invested yielded €14. Trimming the 5% highest costs, mostly from the care as usual condition, reduced the incremental net benefits to €3559.
Limitations
This study has several limitations, which are reported and discussed here.
First, cost data are often non-normally distributed with some people generating very high costs. This results in large SD in the cost estimates and less precise estimates of average costs. In such a context, it would require a very large sample size to power the trial for testing economic hypotheses. However, our study was only powered to test a difference in sickness absence duration. As a consequence, the wide 95% CIs indicate that the cost estimates are subject to much uncertainty. More specifically, trimming the highest 5% of the costs in one of our sensitivity analysis showed that the incremental net benefits became €3559, which is 85% of the original estimate of €4210. This suggests that our study needs replication, preferably in a larger study.
Second, loss-to follow-up was substantial. To handle dropout, missing data were imputed using EM. To ascertain the robustness of our findings, we also used RI. With RI, we arrived at similar conclusions: €4093 (vs €4210 under EM), attesting to the robustness in our findings. Nonetheless, selection bias introduced by (selective) dropout cannot be ruled out completely and could have influenced the outcomes that we obtained.
Third, costs at baseline were higher in the ECO condition. We could have adjusted for the baseline differences, but this would most likely have led to even better outcomes in favour of the ECO condition. Ignoring the baseline differences has therefore put our main analyses on a more conservative footing.
Fourth, the main driver of costs and benefits was absenteeism and, in the main analysis, these were based on self-report. This may have introduced some recall bias, but self-reports of absenteeism usually involve under-reporting, thus leading to conservative outcomes. Still, we crosschecked the self-reports against administrative data from the registers of the occupational health service and the employer. As expected, the benefits were lower when based on self-reports than on administrative data.
Fifth, it should be noted that the cost–benefit analysis did not include the future costs of implementing the ECO intervention on a wider scale. As the main component is a low cost self-help intervention (Return@Work) and the training of OPs only lasts a few hours, the implementation costs are expected to be low, but should be considered when the intervention is disseminated on a wider scale.
Finally, the follow-up time is limited to 12 months. We do not know what the net benefits would be over a longer time span. However, costs differences were highest in the last months. This may imply that a longer follow-up period would have seen more profitable outcomes.
Results in context
Reviews about the effectiveness of psychological return to work interventions for employees with mental health problems show mixed outcomes in reducing sickness absence and promoting an earlier return to work.12 23 Moreover, only a few of the reviewed studies that appeared to be effective report a full economic evaluation. Of these, none evaluated a guided eHealth intervention for return to work. One study that is somewhat comparable with our study is from Schene and colleagues. Schene et al describe the economic evaluation of an intervention for employees with major depression, who were sick-listed between 10 weeks and 2 years.41 The experimental condition received occupational therapy in addition to usual outpatient treatment for depression. Their intervention increased the number of hours worked accumulating in a median economic gain of US$4000–5000 per patient per year, which is in line with our findings regarding the reduction in absenteeism. The study of Schene et al was smaller (n=62), was directed at a more severely depressed population and the intervention was not delivered online but as an intensive face-to-face therapy consisting of 24 group sessions and 15 individual sessions.
Lerner and colleagues evaluated a brief telephonic programme to improve work functioning for employees with major depressive disorder or dysthymia with an at-work productivity loss of at least 5% in the past 2 weeks.42 Compared with usual care, annualised cost savings averaged at $6042 per participant, but these savings were extrapolated from a shorter (4 months) follow-up. These cost savings are higher than the cost-savings observed in our study. Nonetheless, Lerner’s et al extrapolation from 4 to 12 months might have overstated the savings if the treatment effect was not sustained.
Arends and colleagues evaluated the costs and benefits of a problem-solving intervention provided by OPs to prevent recurrent sickness absence in workers with common mental disorders.43Compared with care as usual, the intervention was more effective but also more expensive. From an employer’s perspective, the intervention showed no economic benefits, which is in contrast to our study.
Noben and colleagues conducted a cost–benefit analysis from the employer’s perspective of a preventive intervention in the work setting among nurses with an elevated risk of mental complaints.44 The authors concluded that the intervention was a good investment as the net benefits (stemming from reduced absenteeism and presenteeism) were positive (€651) and the ROI was €11 per Euro spent. This return on investment is comparable with ours.
In contrast to Noben and colleagues and several other studies,45 we found negative results for presenteeism in the short run (first 9 months), but these were alleviated in the longer run (at the end of the year). An explanation for the initially negative results on presenteeism might be that employees who returned to work early were not completely fit and as productive as normally. In other words, there was an initial trade-off between reduced absenteeism and increased presenteeism. However, after the first 9 months, the additional costs caused by presenteeism ceased to exist and were reversed into benefits. This change is possibly driven by an improvement in quality of life when people work.
The literature suggests that in terms of economic costs, presenteeism often is a larger problem than absenteeism. Our results are not in line with these findings. This could be due to the Dutch system in which employees receive a substantial percentage of their wage during the first 2 years of their illness. In many other countries, the fall in income is more acute when employees stay absent from their work, increasing the incentive to keep on working—even when work is then associated with greater levels of presenteeism.
The results of our study can only be generalised to employees who have been sick-listed for 4–26 weeks, working in small- to medium-sized companies.
Conclusions and implications
In the Netherlands, employers have an incentive to invest in sickness management as they have the responsibility to pay 70%–100% of the salary of sick-listed employees for up to 2 years. Employees who are on sickness absence have to visit an occupational physician, paid by the employer within the first 6 weeks. Both the employee and employer have to agree on an action plan. In this plan, the responsibilities of both parties are defined to ensure a quick return to work of the employee. In this context, the ECO intervention can be seen as an effective intervention that, in addition, has a high probability of offering good value for money because the initial investment (of €300) is more than recouped within a single year as seen from the employer’s perspective, while the employee derives benefits in the form of increased quality of life when returning to work sooner rather than later. As noted, some 95% CIs of our estimates are wide. By implication, one should not rely too much on the point estimates of net benefits, ROI ratios, break-even points, because they lack precision. In other words, our estimates have some degree of uncertainty but suggest that the ECO intervention has a high likelihood to be an appealing business case as seen from most stakeholder perspectives.
Supplementary Material
Acknowledgments
We acknowledge with many thanks H Anema (VU University) for advice on the design of the study, G van Lomwel (ACHMEA/UWV) for advice on design of the study and providing access to data and M Blankers for help with statistical analyses.
Footnotes
Contributors: CFC initiated the collaborative clinical trial project. MZV, AB and CFC contributed to the design of the study and obtained the funding. DV, MZV and CFC were responsible for the acquisition of the data. SL and FS conducted the statistical analysis and drafted the first manuscript. DV, MZV, EB, BB, AB and CFC critically revised the mansucript. All authors read and approved the final manuscript. SL, FS and CFC are guarantors.
Funding: This study was financially supported by The Netherlands Organization for Health Research and Development (ZonMw) (grant number 171002403) and Achmea, a Dutch health insurance company. The funding sources had no role in the data analysis and interpretation and in the writing of this paper.
Competing interests: SL, DV, MZV, BB, FS report personal fees from employment at the Trimbos Institute, the Netherlands Institute of Mental Health and Addiction, a not-for-profit organisation. CFC has received research grants from Eli Lilly outside the submitted work.
Ethics approval: The study protocol was approved by the Medical Ethics Committee of the University Medical Center Utrecht, The Netherlands, in February 2011. All participants provided written informed consent before taking part.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Lerner D, Amick BC, Lee JC, et al. . Relationship of employee-reported work limitations to work productivity. Med Care 2003;41:649–59. 10.1097/01.MLR.0000062551.76504.A9 [DOI] [PubMed] [Google Scholar]
- 2. Adler DA, McLaughlin TJ, Rogers WH, et al. . Job performance deficits due to depression. Am J Psychiatry 2006;163:1569–76. 10.1176/ajp.2006.163.9.1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moncrieff J, Pomerleau J. Trends in sickness benefits in Great Britain and the contribution of mental disorders. J Public Health Med 2000;22:59–67. 10.1093/pubmed/22.1.59 [DOI] [PubMed] [Google Scholar]
- 4. Shiels C, Gabbay MB, Ford FM. Patient factors associated with duration of certified sickness absence and transition to long-term incapacity. Br J Gen Pract 2004;54:86–91. [PMC free article] [PubMed] [Google Scholar]
- 5. Cattrell A, Harris EC, Palmer KT, et al. . Regional trends in awards of incapacity benefit by cause. Occup Med 2011;61:148–51. 10.1093/occmed/kqr008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murray CJ, Vos T, Lozano R, et al. . Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. 10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 7. de Graaf R, Tuithof M, van Dorsselaer S, et al. . Sick leave due to psychological and physical illnesses among employees: results of the ‘Netherlands Mental Health Survey and Incidence Study-2’ (NEMESIS-2) [in Dutch: Verzuim door psychische en somatisch aandoeningen bij werkenden: resultaten van de ‘Netherlands Mental Health Survey and Incidence Study-2’ (NEMESIS-2)]. Utrecht: Trimbos-instituut 2011. [Google Scholar]
- 8. Lelliott P, Tulloch S, Boardman J, et al. . Mental Health and Work. London: Cross Government Health Work and Well-being Programme, 2008. [Google Scholar]
- 9. Chisholm D, Sanderson K, Ayuso-Mateos JL, et al. . Reducing the global burden of depression. Br J Psychiatry 2004;184:393–403. 10.1192/bjp.184.5.393 [DOI] [PubMed] [Google Scholar]
- 10. Harvey SB, Joyce S, Modini M, et al. . Work and depression/anxiety disorders: a systematic review of reviews. Melbourne, Australia: Beyondblue, 2013. [Google Scholar]
- 11. Ejeby K, Savitskij R, Ost LG, et al. . Symptom reduction due to psychosocial interventions is not accompanied by a reduction in sick leave: results from a randomized controlled trial in primary care. Scand J Prim Health Care 2014;32:67–72. 10.3109/02813432.2014.909163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nieuwenhuijsen K, Faber B, Verbeek JH, et al. . Interventions to improve return to work in depressed people. Cochrane Database Syst Rev 2014;12:CD006237 10.1002/14651858.CD006237.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. OECD. Mental Health and Work: Netherlands, Mental Health and Work. Paris: OECD Publishing, 2014. [Google Scholar]
- 14. Willems JHBM, Doppegieter RMS. De scheiding van ‘behandeling en controle’: aan actualisering toe? Tijdschrift voor Bedrijfs- en Verzekeringsgeneeskunde 2007;15:184–7. 10.1007/BF03074555 [DOI] [Google Scholar]
- 15. Nederlandse Vereniging voor Arbeids- en Bedrijfsgeneeskunde (NVAB). Richtlijn: Handelen van de bedrijfsarts bij werkenden met psychische problemen. (Guideline: The management of mental health problems of workers by occupational physicians). Eindhoven: NVAB (Netherlands Society of Occupational Medicine), 2007. [Google Scholar]
- 16. Rebergen DS, Bruinvels DJ, Bos CM, et al. . Return to work and occupational physicians' management of common mental health problems–process evaluation of a randomized controlled trial. Scand J Work Environ Health 2010;36:488–98. 10.5271/sjweh.3084 [DOI] [PubMed] [Google Scholar]
- 17. Rebergen D, Hoenen J, Heinemans A, et al. . Adherence to mental health guidelines by Dutch occupational physicians. Occup Med 2006;56:461–8. 10.1093/occmed/kql042 [DOI] [PubMed] [Google Scholar]
- 18. Rebergen DS, Bruinvels DJ, Bezemer PD, et al. . Guideline-based care of common mental disorders by occupational physicians (CO-OP study): a randomized controlled trial. J Occup Environ Med 2009;51:305–12. 10.1097/JOM.0b013e3181990d32 [DOI] [PubMed] [Google Scholar]
- 19. van der Feltz-Cornelis CM, Hoedeman R, de Jong FJ, et al. . Faster return to work after psychiatric consultation for sicklisted employees with common mental disorders compared to care as usual. A randomized clinical trial. Neuropsychiatr Dis Treat 2010;6:375–85. 10.2147/NDT.S11832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vlasveld MC, van der Feltz-Cornelis CM, Adèr HJ, et al. . Collaborative care for sick-listed workers with major depressive disorder: a randomised controlled trial from the Netherlands Depression Initiative aimed at return to work and depressive symptoms. Occup Environ Med 2013;70:223–30. 10.1136/oemed-2012-100793 [DOI] [PubMed] [Google Scholar]
- 21. Volker D, Vlasveld MC, Anema JR, et al. . Blended E-health module on return to work embedded in collaborative occupational health care for common mental disorders: design of a cluster randomized controlled trial. Neuropsychiatr Dis Treat 2013;9:529–37. 10.2147/NDT.S43969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Volker D, Zijlstra-Vlasveld MC, Anema JR, et al. . Effectiveness of a blended web-based intervention on return to work for sick-listed employees with common mental disorders: results of a cluster randomized controlled trial. J Med Internet Res 2015;17:e116 10.2196/jmir.4097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arends I, Bruinvels DJ, Rebergen DS, et al. . Interventions to facilitate return to work in adults with adjustment disorders. Cochrane Database Syst Rev 2012;12:CD006389 10.1002/14651858.CD006389.pub2 [DOI] [PubMed] [Google Scholar]
- 24. Henderson M, Glozier N, Holland Elliott K. Long term sickness absence. BMJ 2005;330:802–3. 10.1136/bmj.330.7495.802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258–66. 10.1097/00006842-200203000-00008 [DOI] [PubMed] [Google Scholar]
- 27. Spitzer RL, Kroenke K, Williams JB, et al. . A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 28. Nieuwenhuijsen K, Noordik E, van Dijk FJ, et al. . Return to work perceptions and actual return to work in workers with common mental disorders. J Occup Rehabil 2013;23:290–9. 10.1007/s10926-012-9389-6 [DOI] [PubMed] [Google Scholar]
- 29. van Oostrom SH, van Mechelen W, Terluin B, et al. . A workplace intervention for sick-listed employees with distress: results of a randomised controlled trial. Occup Environ Med 2010;67:596–602. 10.1136/oem.2009.050849 [DOI] [PubMed] [Google Scholar]
- 30. Volker D, Zijlstra-Vlasveld MC, Brouwers EP, et al. . Return-to-work self-efficacy and actual return to work among long-term sick-listed employees. J Occup Rehabil 2015;25:423–31. 10.1007/s10926-014-9552-3 [DOI] [PubMed] [Google Scholar]
- 31. Hakkaart-van Roijen L. Manual Trimbos/iMTA questionnaire for costs associated with psychiatric illness (in Dutch). Rotterdam: Institute for Medical Technology Assessment, 2002. [Google Scholar]
- 32. Euroqol Group. Eq-5D User Guide. Rotterdam, The Netherlands: Sanders Instituut, EUR, 1995. [Google Scholar]
- 33. Lamers LM, McDonnell J, Stalmeier PF, et al. . The Dutch tariff: results and arguments for an effective design for national EQ-5D valuation studies. Health Econ 2006;15:1121–32. 10.1002/hec.1124 [DOI] [PubMed] [Google Scholar]
- 34. Hakkaart L, Tan S, Bouwmans C. Manual for cost research. Methods and standard costs for economic evaluations in healthcare (in Dutch). Rotterdam: Institute for Medical Technology Assessment, Erasmus University Rotterdam, 2010. [Google Scholar]
- 35. Zorginstituut Nederland. Dutch Health Care Insurance Board (in Dutch). 2014. http://www.medicijnkosten.nl/ (accessed 7 May 2014).
- 36. Zwaap J, Knies S, van der Meijden C, et al. . Kosteneffectiviteit in de praktijk. Diemen: Zorginstituut Nederland, 2015. [Google Scholar]
- 37. Rice DP, Cooper BS. The economic value of human life. Am J Public Health Nations Health 1967;57:1954–66. 10.2105/AJPH.57.11.1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schulz KF, Altman DG, Moher D. Consort 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;2010:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campbell MK, Piaggio G, Elbourne DR, et al. . Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 40. Husereau D, Drummond M, Petrou S, et al. . Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BJOG 2013;120:765–70. 10.1111/1471-0528.12241 [DOI] [PubMed] [Google Scholar]
- 41. Schene AH, Koeter MW, Kikkert MJ, et al. . Adjuvant occupational therapy for work-related major depression works: randomized trial including economic evaluation. Psychol Med 2007;37:351–62. 10.1017/S0033291706009366 [DOI] [PubMed] [Google Scholar]
- 42. Lerner D, Adler D, Hermann RC, et al. . Impact of a work-focused intervention on the productivity and symptoms of employees with depression. J Occup Environ Med 2012;54:128–35. 10.1097/JOM.0b013e31824409d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arends I, Bültmann U, van Rhenen W, et al. . Economic evaluation of a problem solving intervention to prevent recurrent sickness absence in workers with common mental disorders. PLoS One 2013;8:e71937 10.1371/journal.pone.0071937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Noben C, Evers S, Nieuwenhuijsen K, et al. . Protecting and promoting mental health of nurses in the hospital setting: Is it cost-effective from an employer’s perspective? Int J Occup Med Environ Health 2015;28:891–900. 10.13075/ijomeh.1896.00465 [DOI] [PubMed] [Google Scholar]
- 45. Salomon JA, Vos T, Hogan DR, et al. . Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet 2012;380:2129–43. 10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


