Abstract
Introduction
Epilepsy is highly prevalent in tuberous sclerosis complex (TSC), a multi-system genetic disorder. The clinical and economic burden of this condition is expected to be substantial due to treatment challenges, debilitating co-morbidities and the relationship between TSC-related manifestations. This study estimated healthcare resource utilisation (HCRU) and costs for patients with TSC with epilepsy (TSC+E) in the UK.
Methods
Patients with TSC+E in the Clinical Practice Research Datalink (CPRD) linked to Hospital Episodes Statistics were identified from April 1997 to March 2012. Clinical data were extracted over the entire history, and costs were reported over the most recent 3-year period. HCRU was compared with a matched Comparator cohort, and the key cost drivers were identified by regression modelling.
Results
In total, 209 patients with TSC+E were identified, of which 40% recorded ≥2 other primary organ system manifestations and 42% had learning disability. Treatment with ≥2 concomitant antiepileptic drugs (AEDs) was prevalent (60%), potentially suggesting refractory epilepsy. Notwithstanding, many patients with TSC+E (12%) had no record of AED use in their entire history, which may indicate undertreatment for these patients.
Brain surgery was recorded in 12% of patients. Routine electroencephalography and MRI were infrequently performed (30% of patients), yet general practitioner visits, hospitalisations and outpatient visits were more frequent in patients with TSC+E than the Comparator. This translated to threefold higher clinical costs (£14 335 vs £4448), which significantly increased with each additional primary manifestation (p<0.0001).
Conclusions
Patients with TSC+E have increased HCRU compared with the general CPRD population, likely related to manifestations in several organ systems, substantial cognitive impairment and severe epilepsy, which is challenging to treat and may be intractable. Disease surveillance and testing appears to be inadequate with few treatments trialled.
Multidisciplinary care in TSC clinics with specialist neurologist input may alleviate some of the morbidity of patients, but more innovative treatment and management options should be sought.
Keywords: tuberous sclerosis complex, epilepsy, resource use, antiepileptic drugs
Strengths and limitations of this study.
A large cohort of patients was used, which is likely to be representative of UK patients with tuberous sclerosis complex with epilepsy (TSC+E).
Prescription information relates to those issued, but information pertaining to dispensing was not available.
Many patients may seek care outside of primary and specialist care, which was not captured in this study, potentially underestimating healthcare resource utilisation.
A Comparator cohort with epilepsy but without TSC was not used, as the prevalence of epilepsy in the general population was very low and patients with TSC+E are unlikely to only present with epilepsy, evidenced by a large proportion with ≥2 additional manifestation categories.
Introduction
Tuberous sclerosis complex (TSC) is a rare genetic condition estimated to affect nearly 1 million people1 with approximately 8000 patients residing in the UK.2 TSC is diagnosed by the presence of two major clinical features (primary manifestations) or one major and two minor clinical features using the recognised international diagnostic criteria.3 A definitive diagnosis involves the identification of a TSC1 or TSC2 pathogenic mutation4–6 (detected in approximately 85%–90% of cases),7–13 which leads to the activation of the mechanistic target of rapamycin complex 1 (mTORC1) pathway and upregulation of cell growth and protein synthesis.14 TSC commonly affects several organ systems including the brain, skin and kidneys (eg, malignant and non-malignant growths)15 and less frequently affects the heart, eyes and lungs.3 16–18 Manifestations often appear in multiple organ systems and occur at various stages of development.18
In the brain, TSC causes subependymal nodules in the ventricles and the development of tubers in the cortex. The high lifetime prevalence of epilepsy (80%–90% of affected individuals) may be related to the presence of these cerebral cortical tubers, which manifest in over 80% of patients with TSC.12 19 20 The primary aim of epilepsy treatment is to control seizures and to optimise behavioural outcomes, thereby decreasing epilepsy-associated morbidity.21 First-line treatment of epilepsy involves antiepileptic drugs (AEDs), but two-thirds of patients remain refractory22 despite the availability of treatments, which often have short-lived efficacy. In some cases where seizure focus has been shown to coincide with a cortical tuber, epilepsy surgery has been beneficial.12 Vagal nerve stimulation and ketogenic diets are also effective treatment options21 when patients are refractory to AEDs or where surgery is not recommended.
Due to the diverse presentations of TSC and the profound neurodevelopmental and learning disabilities associated with epilepsy, patients are expected to have a substantial requirement for medical attention over their lifetime,23 including provision of adequate transitional healthcare when moving on from paediatric management.24 Continuity of clinical care and on-going monitoring are important due to the age-related changes in manifestations from infancy to adulthood, which require a better planned transition of care from childhood to adult services; this is especially important considering the recommendations for patients with TSC to undergo a variety of expensive investigations including MRI and electroencephalography (EEG).25 Disruption and refocusing of care also often occurs with little emphasis placed on the mental health issues affecting many patients with TSC.26
Presently, little is known about the healthcare resource utilisation (HCRU) of patients with TSC with epilepsy (TSC+E) in comparison with the general population. Previous studies indicate that nervous system manifestations are a significant driver of costs in the general TSC population,27 28 but this burden has not been quantified specifically in patients with TSC+E. This study endeavours to quantify the economic burden of patients with TSC in the UK who have epilepsy in comparison with a general population Comparator.
Methods
Study design and data source
This retrospective cohort study used data from routinely collected electronic medical record datasets in England between January 1987 and June 2013. Primary care data were derived from the Clinical Practice Research Datalink (CPRD), and secondary care was analysed from linked Hospital Episode Statistics (HES) data. Linked data allowed for patients to be tracked through both primary and secondary care. Linkage was available for data collected between April 1997 and March 2012.
CPRD contains approximately 4.4 million active patients from over 670 primary care practices throughout the UK, representing approximately 6.9% national coverage. Previous studies have validated the representativeness of CPRD.29–31
Data in the CPRD include patient demographics, clinical diagnoses, consultations, primary care prescription medications, laboratory tests and specialist referrals. Approximately 50% of CPRD practices are linked to HES data by anonymous patient identifiers. HES includes all inpatient and outpatient visits at National Health Service (NHS) hospitals in England and captures data including patient demographics, clinical diagnoses, procedures, imaging, tests, hospitalisations and discharge details.
Study population
TSC cohort
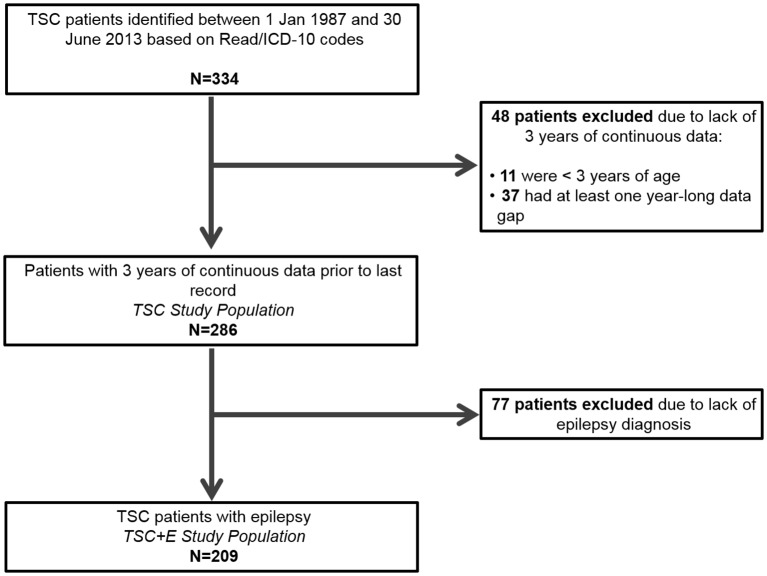
Patients were required to have a recorded diagnosis of TSC (Read codes, PK5.00, PK5.12; ICD-10 code, Q85.1) between 1 January 1987 and 30 June 2013 and at least 3 years of continuous data prior to the last available record (ie, date of transfer out of the CPRD practice, death or other record) during the period of CPRD-HES linkage (1 April 1997 to 31 March 2012).18 Data were collected from the earliest available date in order to capture diagnoses, procedures and tests that may occur infrequently throughout a patient’s lifetime.
Continuous data were required to minimise the effects of suboptimal data recording in the CPRD and HES for the accurate calculation of HCRU and associated costs. Patients with no healthcare records (eg, repeat prescriptions, administrative activities) during the 3-year period were likely to be inactive. Patients with TSC were expected to have healthcare encounters at least yearly (and every 1–3 months for those on continuous medication), and thus, those who did not fulfil this criteria had incomplete records and were excluded. Case report reviews were conducted on the excluded patients.
For the included patients, records were grouped by affected organ system in a TSC clinical code library—the Medical Inventory of TSC Organ System Codes—which was used to systematically assess the population (online supplementary appendix). Seven primary manifestation categories (hereafter referred to as ‘primary manifestations’) affected by TSC were identified: brain (structural), nervous system (including epilepsy), psychiatric, kidney and urinary tract, heart/circulatory system, dermatological system and respiratory system.
bmjopen-2016-015236supp001.pdf (103.3KB, pdf)
Patients with TSC+E cohort
A subpopulation of patients with epilepsy was derived from the TSC cohort (figure 1) according to the presence of selected diagnostic codes. All available history was extracted for each patient with TSC+E, including demographic (gender and age) and clinical data (diagnoses, epilepsy-related co-morbidities, prescription medications, investigations/tests and procedures/surgical interventions).
Figure 1.
Patient exclusion criteria to arrive at the tuberous sclerosis complex (TSC) study population and the TSC with epilepsy (TSC+E) study population.
Prescription information was only available for treatments issued in primary care. Procedures were defined as surgical and palliative interventions (eg, nerve stimulation). General practitioner (GP) visits, outpatient visits and inpatient admissions were also captured by episode.
Epilepsy-specific information including age at first infantile spasm and proportion of patients with a Read code for ‘seizure free’ over a 12-month period was also determined.
Comparator cohort
Comparator patients were randomly matched to each patient with TSC at a 1:5 ratio by age, sex and date of last record (within the same calendar year) to generate 1430 and 1045 Comparator patients for all patients with TSC and TSC+E, respectively. Large Comparator cohorts were extracted to better characterise expenditure and outcomes in a group expected to represent the general UK population and have comparatively fewer healthcare encounters. The Comparator cohort was required to have at least 3 years of data and at least one healthcare record during this period (in addition to the last record) to ensure a more representative and active control Comparator rather than biassing the cohort to include ‘sicker’ patients by requiring an encounter in each of the 3 years. The inclusion of control patients without yearly healthcare encounters is supported by results from a large national GP survey in England that reported that 28% of respondents had not visited their GP during the last 6 months.32
Data analyses
Patient demographics and HCRU
Demographics are reported as summary statistics for the TSC+E and Comparator cohorts. Age was determined at the beginning of the 3-year period of continuous data and was used to stratify patients into adult (≥18 years) and paediatric (<18 years) populations.
Primary care drug prescriptions (by British National Formulary (BNF) chapter), procedures and surgical interventions (eg, specific procedures (nerve stimulation, including vagus nerve stimulation) and operations on brain tissue) and diagnostic tests and investigations are reported throughout the entire patient history and compared between TSC +E and the Comparator cohort. Diagnostics are divided into ‘as required’ and routine according to the TSC consensus recommendations, reflecting tests that should be conducted as deemed necessary and on a regular basis.33
All analyses are reported as summary statistics. Continuous variables are presented as means with SD or 95% CIs or median values with interquartile ranges. Categorical variables are reported as frequencies and percentages, along with 95% CIs, where appropriate.
HCRU was analysed over the 3-year period of continuous data and compared between TSC+E and Comparator cohorts. Drug prescriptions (including repeats), procedures and surgical interventions, diagnostic tests and investigations, GP visits (excluding administration entries), inpatient stays and outpatient visits (by specialty) were determined. The proportion of patients and individual inpatient episodes that were longer than the average indicated in the Healthcare Resource Group (HRG) code used for inpatient admissions were also determined.
Analyses were conducted using SAS V.9.4.
Direct costs analysis
Personal Social Services Research Unit (PSSRU) costs were used to estimate GP consultation costs for an average length of consultation (by consultation type), as derived from the 2006/2007 UK General Practice Workload Survey.34 35 Since PSSRU provided alternative costs for clinical consultations by length of consultation, the lengths recorded in CPRD were used to allocate the correct cost. Secondary care costs and primary care drug therapy costs were estimated using the National Schedule of Reference Costs30 and Health and Social Care Information Centre data,31 respectively. All costs were derived from the most recent available sources and inflated to 2014 costs (using gross domestic product inflators), where required. Healthcare costs outside the NHS (eg, private healthcare) and costs in social care were not included.
Average costs were determined per patient with TSC+E over the 3-year period of continuous data. Costs associated with GP visits, outpatient visits, inpatient stays and drugs are compared with the Comparator cohort.
Costs were also determined for patients with TSC+E without any additional manifestation categories and incrementally assessed when patients presented with 1–4 additional manifestation categories. Specific manifestation combination costs were also calculated, but it should be noted that patients may have presented with other manifestations aside from the specific category (ie, patients with epilepsy and psychiatric manifestations may also have dermatological manifestations).
Key drivers of direct costs in patients with TSC+E
Two regression models were developed to explore the impact of primary manifestations (aside from the nervous system as this includes epilepsy) on healthcare costs in the TSC+E cohort. Gamma distribution models were implemented to account for the non-normal distribution of the dependent variables (ie, total costs over the 3-year period) after comparisons between different distributions were performed to assess which model best fits the data.
The first model assessed the relationship of the primary manifestation categories with total healthcare costs, when presented independently and in combination with other manifestations. Manifestation involvement was determined over the entire history of each TSC +E patient, but cost analyses were conducted over the 3-year period of data only. Pairwise combinations were used to elucidate which organ manifestations jointly impacted costs since patients with TSC have several simultaneous features.
The first model incorporated the following independent variables into the gamma distribution regression: age category (<18 and ≥18 years) for the age at last record, sex and specific primary manifestations involved (brain (structural), circulatory, dermatological, kidney and urinary, and psychiatry). Pairwise interactions were analysed separately for each of the six primary manifestation categories. Respiratory manifestations were excluded as only one patient had a record of a respiratory condition.
The second model was implemented to determine whether increasing the number of primary manifestations (by category) significantly impacted costs. The following independent variables were incorporated into the gamma distribution regression: age category (<18 and ≥18 years) for the age at last record, sex and the number of organ system manifestations per patient (ranging from 1 to ≥4). Subsequently, a comparison was made between the number of primary manifestation categories involved (eg, one primary manifestation category vs two primary manifestation categories).
In each model, p values were derived from Wald tests and values less than 0.05 were considered to be statistically significant.
Ethics
Independent Scientific Advisory Committee for Medicine and Healthcare Products Regulatory Agency Database Research approval was obtained for this study on 20 April 2015 (Protocol 13_146A).
Patient involvement
Patients were not involved in the design of this study, its outcomes or plans for results dissemination.
Results
We identified 334 patients with TSC. After applying the inclusion and exclusion criteria, the TSC study cohort included 286 patients and the matched Comparator included 1430 patients. In order to ensure that the included TSC population was not biased, case report reviews were conducted on the 48 excluded patients. Eleven were under the age of 3 and thus had less than 3 years of available data. Of the remaining 37 patients, 50% recorded TSC-related manifestations including epilepsy, renal angiomyolipomas and subependymal giant cell astrocytoma during their entire history (figure 1). The proportion of these patients recording inpatient admissions and outpatient (specialist) visits was comparable to the included patients with TSC (16% excluded vs 16% included; 84% excluded vs 93% included, respectively), but primary care encounters such as prescriptions and GP visits were recorded in much lower proportions (22% excluded vs 73% included).
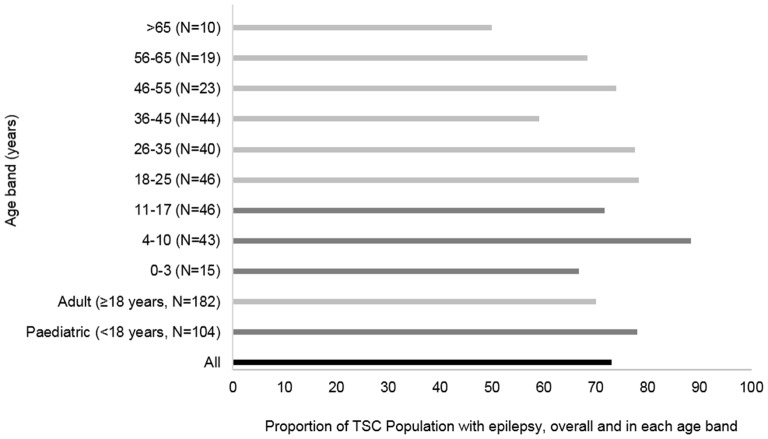
Once patients were identified with codes indicating epilepsy, of the 286 patients with TSC, 209 also had epilepsy (73.1%) (figure 1). When splitting by age groups, epilepsy was recorded in 77.8% of paediatric patients with TSC (n=104) and was most prevalent (88.4%) in paediatric patients with TSC aged 4–10 years (n=43). Active epilepsy was recorded in 50.0% of >65-year-old patients with TSC (n=10) and 59.1% of 36-year-old to 45-year-old patients with TSC (n=44) (figure 2).
Figure 2.
Prevalence of epilepsy in patients with tuberous sclerosis complex (TSC) by age band.
Patient demographics
The mean age at the beginning of the 3-year period was 26.8 years (SD: 17.8), and populations were 48.8% female for both cohorts. A total of 38.8% of patients were under 18, and the median length of history was 18.1 years (IQR: 12.9–23.1) for TSC+E compared with 17.6 years (IQR: 11.7–22.3) in the Comparator cohort.
Clinical characteristics of patients with TSC+E
Of all patients with TSC+E, only 10.1% had recorded an infantile spasm. The mean age at first record of infantile spasm was 0.14 years (95% CI 0.0 to 0.3 years). The vast majority of patients recording infantile spasms also had a record for vigabatrin within 6 months (81.0% (62.6 to 99.3%)). Throughout the entire available history, 57 patients (27.3% (21.2 to 33.4)) had a record of ‘seizure free’ for 12 months at a mean age of 30.5 (25.5 to 35.6) years.
The most prevalent epilepsy-related co-morbidity was learning disability in 42.0% (40.0% to 43.0%) of the paediatric cohort and 60.9% (42.5% to 69.5%) of the adult patients with TSC+E. Psychosis (3.4% (0.9% to 5.8%)) and attention-deficit/hyperactivity disorder (4.3% (1.5% to 7.1%)) were the least frequently recorded co-morbidities (table 1).
Table 1.
Epilepsy-related co-morbidities in patients with tuberous sclerosis complex with epilepsy
| Age (years) | TSC+E (n=209) | |||||||||||||||||||||
| ADHD | Autism | Behavioural disorder | Brain tumour/growth | Dementia | Depression | Intellectual disability | Learning disability | Psychosis | Sleep disorder | Speech and language disorder | ||||||||||||
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) |
n | % (95% CI) |
n | % (95% CI) |
n | % (95% CI) |
n | % (95% CI) |
n | % (95% CI) |
n | % (95% CI) |
n | % (95% CI) |
n | % (95% CI) |
|
| Total | 9 | (4.3 (1.5 to 7.1) | 37 | 17.7 (12.5 to 22.9) | 41 | 19.6 (14.2 to 25.1) | 33 | 15.8 (10.8 to 20.8) | 13 | 6.2 (2.9 to 9.5) | 30 | 14.4 (9.6 to 19.2) | 19 | 9.1 (5.2 to 13.0) | 112 | 53.6 (46.8 to 60.4) | 7 | 3.4 (0.9 to 5.8) | 28 | 13.4 (8.7 to 18.1) | 33 | 15.8 (10.8 to 20.8) |
| <18 | 7 | (8.6 2.4 to 14.9) | 16 | 19.8 (10.9 to 28.6) | 16 | 19.8 (10.9 to 28.6) | 12 | 14.8 (6.9 to 22.7) | 5 | 6.2 (0.8 to 11.5) | 0 | (0) | 3 | 3.7 (0 to 7.9) | 34 | 42.0 (40.0 to 53.0) | 1 | 1.2 (0 to 3.7) | 10 | 12.4 (5.0 to 19.7) | 9 | 11.1 (4.1 to 18.1) |
| ≥18 | 2) | (1.6 0 to 3.7 | 21 | 16.4 (9.9 to 22.9) | 25 | 19.4 (12.6 to 26.5) | 21 | 16.4 (9.9 to 22.9) | 8 | 6.3 (2.0 to 10.5) | 30 | 23.4 (16.0 to 30.9) | 16 | 12.5 (6.7 to 18.3) | 78 | 60.9 (52.4 to 69.5) | 6 | 4.7 (1.0 to 8.4) | 18 | 14.1 (8.0 to 20.2) | 24 | 18.8 (11.9 to 25.6) |
ADHD, attention-deficit/hyperactivity disorder; TSC+E, tuberous sclerosis complex with epilepsy.
The largest proportion of patients with TSC+E (44% (37.2–50.8%)) recorded one additional primary manifestation category aside from their epilepsy diagnosis. Additionally, 40% of patients recorded at least two primary manifestation categories. The majority of patients presented with additional dermatological (n=173, 82.8%) and psychiatric manifestations (n=175, 75.1%).
Drugs prescribed in primary care to TSC+E versus the Comparator cohort
Prescriptions by BNF chapter were examined in the TSC+E and Comparator cohorts over the entire available history and over the most recent 3-year period of data. Over the entire history, AEDs were the most commonly prescribed drug in 88.0% (95% CI 85.3% to 93.7%) of the TSC+E cohort. Hypnotic/antipsychotic prescriptions were recorded over threefold more frequently in paediatric patients with TSC+E than the Comparator paediatric cohort (37.0% (26.3% to 47.8%) vs 12.4% (9.1% to 15.6%) Comparator). The average number of prescriptions throughout the entire history was nearly fivefold greater in patients with TSC+E than the Comparator (351.0 (289.2 to 412.9) vs 64.2 (55.3 to 73.2) average prescriptions per patient). Over the 3-year period of data, the TSC+E cohort recorded over four times as many prescriptions as the Comparator on average per patient (88.2 (68.6 to 107.9) TSC+E vs 17.1 (14.3 to 19.9) Comparator). The adult TSC+E cohort recorded more prescriptions on average per patient (119.1 (89.0 to 149.2)) than the paediatric TSC+E cohort (39.5 (27.1 to 51.8)).
Concomitant AEDs (two drug prescriptions within 2 months of each other in 2 months) prescribed during the entire history were examined for the TSC+E cohort. Overall, the greatest proportion of patients (31.6%) recorded two concomitant AEDs and 12.0% (7.5% to 16.4%) of patients did not record any AEDs ever (table 2). Overall, 59.8% of the patients with TSC+E recorded the use of at least two concomitant AEDs during their history, the reminder (40.2%) receiving none or only one AED; this lack of treatment and trials with AEDs was evident in both the paediatric and adult populations where 12.4% and 11.7%, respectively, had no record of receiving any AEDs (table 2). The most commonly prescribed AEDs were carbamazepine and sodium valproate, both in 48.8% (42.0%–55.6%) of patients. Vigabatrin (commonly prescribed for infantile spasms) was recorded more frequently in paediatric patients with TSC+E than adults (43.2% (32.2% to 54.2%) vs 24.4% (18.5% to 30.3%)).
Table 2.
Numbers of AEDs used by the tuberous sclerosis complex with epilepsy cohort over the entire history
| TSC+E (n=209) | ||||||
| <18 years n=81 | 95% CI | ≥18 years n=128 | 95% CI | Overall n=209 | 95% CI | |
| AEDs used (n) Number, proportion of patients with specified numbers of concomitant AEDs during the entire history | ||||||
| 0 | 10 (12.4) | (5.0 to 19.7) | 15 (11.7) | (6.1 to 17.4) | 25 (12.0) | (7.5 to 16.4) |
| 1 (monotherapy) | 24 (29.6) | (19.5 to 39.8) | 35 (27.3) | (19.5 to 35.2) | 59 (28.2) | (22.1 to 34.4) |
| 2 | 29 (35.8) | (25.1 to 46.5) | 37 (28.9) | (21.0 to 36.9) | 66 (31.6) | (25.2 to 37.9) |
| 3 | 15 (18.5) | (9.9 to 27.2) | 31 (24.2) | (16.7 to 31.7) | 46 (22.0) | (16.4 to 27.7) |
| 4+ | 3 (3.7) | (0 to 7.9) | 10 (7.8) | (3.1 to 12.5) | 13 (6.2) | (2.9 to 9.5) |
AED, antiepileptic drug; TSC+E, tuberous sclerosis complex with epilepsy.
Medical procedures and surgical interventions conducted in TSC+E versus the Comparator cohort
The proportion of patients undergoing selected procedures in TSC+E and Comparator cohorts was determined over the entire available history and the 3-year period. An important proportion of patients with TSC+E had at least one record of undergoing any brain procedures (12.0% (95% CI 7.5% to 16.4%)) over their entire history (online supplementary appendix table 2). Nerve stimulation was performed in more paediatric patients with TSC+E than adults (7.4% (1.6% to 13.2) vs 3.1% (0.1% to 6.2%)). On average, the TSC+E cohort recorded as many procedures as the Comparator cohort (0.1 (0 to 0.2) TSC+E vs 0 (0) Comparator) over the 3-year period (online supplementary appendix table 3).
Diagnostic tests and investigations conducted in patients with TSC+E versus the Comparator cohort
Selected tests and investigations were analysed in the TSC+E and Comparator cohorts over the entire available history and 3-year period. Over one-third (34.0% (27.5% to 40.5%)) of patients with TSC+E recorded no tests ever, and 24.9% (19.0% to 30.8%) of patients recorded only one test. A greater proportion of children recorded MRI than adults (58.0% (95% CI 47.0% to 69.0%) vs 21.1% (95% CI 13.9% to 28.3%)). For ‘as required’ tests, EEG was recorded nearly fivefold more frequently in children than adults with TSC+E (46.9% (35.8% to 58.0%) vs 10.9% (5.5% to 16.4%)).
Over the 3-year period, the TSC+E cohort averaged 1.1 (0.8 to 1.4) tests and investigations. Paediatric patients recorded more than twofold greater MRI investigations on average than adults (0.7 (0.4 to 1.0) children vs 0.3 (0.1 to 0.4) adults). As expected, the Comparator cohort recorded fewer tests than the TSC+E cohort (0.2 (0.2 to 0.3) overall tests per patient, on average).
Healthcare encounters in TSC+E versus the Comparator cohort
Primary and secondary healthcare encounters were analysed for TSC+E and Comparator cohorts over the 3-year period. Overall, patients with TSC+E recorded over threefold more frequent GP visits than the Comparator over the 3-year period (60.8 (95% CI 50.5 to 71.2) vs 25.4 (95% CI 23.3 to 27.5) average visits per patient). Adult patients with TSC+E visited their GP much more frequently than the paediatric TSC+E cohort (71.7 (56.3 to 87.3) vs 34.6 (33.5 to 53.7) average visits per patient) (table 3).
Table 3.
Primary and secondary healthcare encounters in TSC+E and Comparator cohorts during the most recent 3-year period
| TSC+E (n=209) | Comparator (n=1045) | |||||
| <18 years (n=81) |
=18 years (n=128) |
Overall (n=209) |
<18 years (n=405) |
=18 years (n=640) |
Overall (n=1045) |
|
| Mean number of GP visits per patient, by category of consultation (95% CI) | ||||||
| All GP visits* | 43.6 (33.5 to 53.7) | 71.7 (56.3 to 87.2) | 60.8 (50.5 to 71.2) | 18.3 (15.9 to 20.7) | 29.9 (26.8 to 33.0) | 25.4 (23.3 to 27.5) |
| Clinic visits | 24.2 (18.5 to 30.0) | 45.6 (35.7 to 55.4) | 37.3 (30.8 to 43.9) | 11.6 (10.1 to 13.1) | 18.5 (16.6 to 20.5) | 15.9 (14.5 to 17.2) |
| Home visits | 0.1 (0 to 0.2) | 0.3 (0.1 to 0.6) | 0.2 (0.1 to 0.4) | 0 (0) | 0.1 (0 to 0.1) | 0 (0 to 0.1) |
| Telephone consultations | 1.0 (0.3 to 1.8) | 1.5 (0.7 to 2.3) | 1.3 (0.7 to 1.9) | 0.6 (0.5 to 0.8) | 1.0 (0.7 to 1.3) | 0.8 (0.7 to 1.0) |
| Out of hours | 0.3 (0.1 to 0.5) | 0.5 (0.1 to 0.8) | 0.4 (0.2 to 0.6) | 0.3 (0.2 to 0.4) | 0.2 (0.1 to 0.2) | 0.2 (0.2 to 0.3) |
| Other† | 18.0 (13.1 to 22.9) | 23.9 (17.7 to 30.2) | 21.6 (17.4 to 25.9) | 5.7 (4.6 to 6.8) | 10.2 (9.0 to 11.3) | 8.4 (7.6 to 9.3) |
| Mean number of inpatient admissions per patient, by category of admission (95% CI) | ||||||
| All admissions | 4.3 (1.0 to 7.5) | 2.9 (2.2 to 3.5) | 3.4 (2.1 to 4.7) | 0.8 (0.5 to 1.1) | 1.5 (1.2 to 1.7) | 1.2 (1.0 to 1.4) |
| Elective admission | 3.3 (0.1 to 6.4) | 1.1 (0.8 to 1.5) | 2.0 (0.7 to 3.2) | 0.3 (0.2 to 0.4) | 0.7 (0.6 to 0.9) | 0.6 (0.5 to 0.7) |
| A&E admission | 1.0 (0.4 to 1.7) | 1.6 (1.1 to 2.1) | 1.4 (1.0 to 1.8) | 0.3 (0.2 to 0.4) | 0.5 (0.4 to 0.6) | 0.4 (0.3 to 0.5) |
| Other admission | 0 (0) | 0.2 (0 to 0.4) | 0.1 (0 to 0.2) | 0.2 (0 to 0.4) | 0.3 (0.2 to 0.3) | 0.2 (0.1 to 0.3) |
| Mean length of stay in hospital (days) per patient (95% CI) | ||||||
| All admissions | 2.7 (2.3 to 3.0) | 7.2 (3.1 to 11.4) | 5.0 (2.9 to 7.2) | 2.2 (1.9 to 2.6) | 4.8 (3.7 to 6.0) | 4.2 (3.3 to 5.1) |
| Elective admission | 2.1 (1.9 to 2.2) | 8.4 (0 to 18.8) | 4.3 (0.7 to 7.9) | 2.0 (1.5 to 2.5) | 4 (2.2 to 5.8) | 3.6 (2.1 to 5.0) |
| A&E admission | 4.5 (3.1 to 6.0) | 6.8 (5.0 to 8.7) | 6.1 (4.8 to 7.5) | 2.8 (2.0 to 3.5) | 7.4 (5.3 to 9.5) | 6.1 (4.6 to 7.6) |
| Other admission | 0 (0) | 2.9 (1.8 to 4.0) | 2.9 (1.8 to 4.0) | 1.8 (1.4 to 2.1) | 2.3 (2.1 to 2.6) | 2.1 (1.9 to 2.4) |
| Top five most frequently visited outpatient specialties in TSC (mean visits, 95% CI) | ||||||
| All outpatient visits | 16.8 (13.9 to 19.7) | 14.4 (11.1 to 17.7) | 15.3 (13.0 to 17.6) | 3.3 (2.7 to 3.9) | 5.3 (4.7 to 6.0) | 4.5 (4.1 to 5.0) |
| Neurology/neurosurgery | 2.9 (2.1 to 3.7) | 2.6 (1.8 to 3.4) | 2.7 (2.2 to 3.3) | 0.1 (0 to 0.1) | 0.1 (0.1 to 0.2) | 0.1 (0.1 to 0.2) |
| Paediatrics | 5.6 (4.5 to 6.6) | 0.5 (0.2 to 0.7) | 2.4 (1.9 to 3.0) | 0.6 (0.4 to 0.7) | 0 (0) | 0.2 (0.2 to 0.3) |
| Psychiatry/mental disorders | 1.0 (0.1 to 2.0) | 2.9 (1.0 to 4.8) | 2.2 (0.9 to 3.4) | 0.1 (0 to 0.3) | 0.3 (0.1 to 0.5) | 0.2 (0.1 to 0.4) |
| Allied health professional | 1.4 (0.4 to 2.5) | 0.7 (0.4 to 1.1) | 1.0 (0.6 to 1.5) | 0.2 (0.1 to 0.4) | 0.4 (0.3 to 0.6) | 0.4 (0.3 to 0.5) |
| Ophthalmology | 1.7 (1.1 to 2.3) | 0.5 (0.2 to 0.8) | 1.0 (0.7 to 1.3) | 0.2 (0.1 to 0.4) | 0.3 (0.2 to 0.5) | 0.3 (0.2 to 0.4) |
A&E, accident and emergency; GP, general practitioner; TSC, tuberous sclerosis complex; TSC+E, TSC with epilepsy.
*Analyses exclude administration encounters. With administration, GP visits are: 53.9 (41.9–66.0) for paediatric TSC+E, 89.6 (70.9–108.4) for adult TSC+E, 75.8 (63.2–88.3) for overall TSC+E populations. For Comparator: 22.4 (19.5–25.3) paediatric, 37.5 (33.7–41.4) adult, 31.7 (29.0–34.3) overall.
†Other includes: non-consultation data, non-consultation medication data, radiology result, hospital inpatient report, third-party consultation, mail to patient, results recording, letter from outpatients, discharge details, mail from patient.
Secondary care was also used more frequently by patients with TSC+E, especially in the paediatric population. The TSC+E cohort averaged 3.4 (2.1–4.7) inpatient admissions over the 3-year period in comparison to the 1.2 (1.0–1.4) admissions recorded by the Comparator. Paediatric patients with TSC+E recorded 3.3 (0.1–6.4) elective admissions and 1.0 (0.4–1.7) accident and emergency (A&E) admissions on average, while the Comparator paediatric cohort only recorded 0.3 (0.2–0.4) admissions for both elective and A&E hospitalisations (table 3).
Mean lengths of stay in hospital were similar between TSC+E and Comparator cohorts (table 3). Of all hospital admissions, 30.3% (27.0%–33.6%) were longer than the average for patients with TSC+E in comparison to 21.0% (18.8%–23.2%) in the Comparator.
The TSC+E cohort consulted with outpatient specialists over threefold more frequently than the Comparator cohort overall (15.3 (13.0–17.6) vs 4.5 (4.1–5.0) average visits per patient). Neurology/neurosurgery, paediatrics and psychiatry/mental disorder specialists consulted most often with patients with TSC+E and substantially more frequently than the Comparator (table 3). However, patients recorded fewer than three visits over the 3-year period on average, aside from paediatrics (5.6 (4.5–6.6) average visits per patient); when this was examined further, one-quarter of the paediatric TSC+E cohort did not record any visits with a paediatrician. With regards to neurology consultations, 88.5% of the overall TSC+E population did not see a specialist during the 3-year period.
Directs costs of TSC+E versus the Comparator cohort
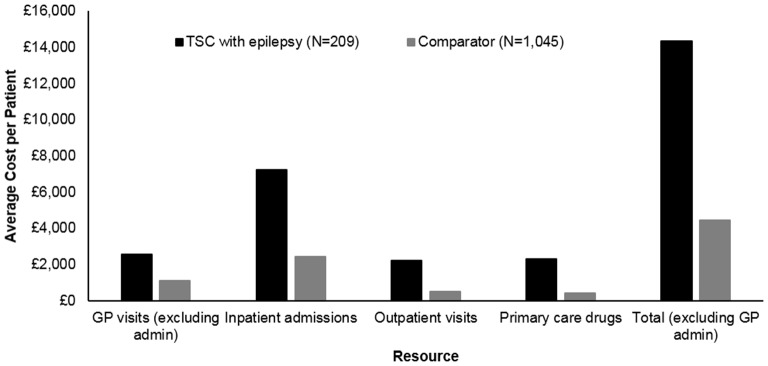
Costs associated with primary and secondary care HCRU were calculated for the TSC+E and Comparator cohorts over the 3-year period. Overall, patients with TSC+E incurred costs of £14 335 on average during the 3-year period, a figure more than threefold greater than costs for the Comparator (£4448) (figure 3). The most substantial proportion of costs was attributed to inpatient admissions for both the TSC+E (50.5%) and Comparator (54.6%) cohorts.
Figure 3.
Mean costs per patient with tuberous sclerosis complex (TSC) with epilepsy over the 3-year period by type of healthcare resource used. Only includes the cost of general practitioner (GP) visits (costs of tests conducted at the GP practice are not included). GP costs including administration encounters: £2970 TSC vs £1263 Comparator. Total including GP administration encounters: £14 753 TSC vs £4623 Comparator.
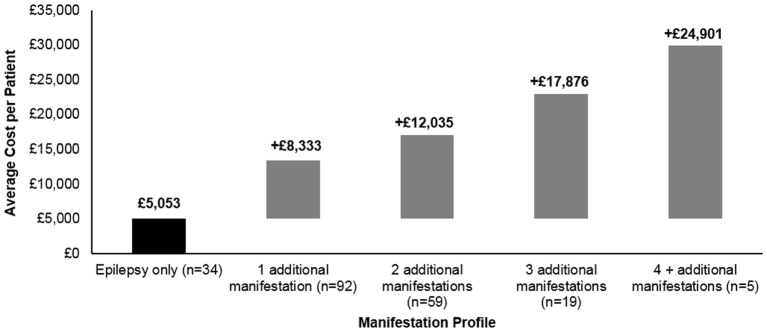
Incremental costs were evaluated for the TSC+E population overall during the 3-year period. Patients with epilepsy and no additional manifestations incurred mean costs totalling £5053, and the addition of one additional primary organ system manifestation added £8333 to baseline epilepsy costs. Costs increased incrementally as patients presented with more manifestations, with four additional manifestations incurring an excess of £24 901 to baseline TSC+E costs (figure 4).
Figure 4.
Mean cost of additional manifestation categories to patients with tuberous sclerosis complex with epilepsy over the 3-year period.
Patients with specific primary organ system manifestations were also costed in order to determine cost effects of adding at least the specific manifestation category to patients only recording epilepsy. Dermatological manifestations increased costs by £8,878, while the circulatory system (£13,319), brain (+£19,082) and respiratory (+£34,278) manifestations increased costs substantially. However, it should be noted that only one patient had a respiratory manifestation and costs for each category can include patients who recorded other additional manifestations.
Key drivers of direct costs in patients with TSC+E
Two regression models were developed in order to estimate which manifestations were likely to drive cost in patients with TSC+E. No individual primary manifestation categories were found to have a significant impact on costs in the TSC+E cohort (table 4), but the combination of kidney and urinary/dermatological manifestations significantly contributed to costs (p=0.0012). The number of manifestations significantly affected costs (p<0.0001) and had a consistently significant relationship beyond the comparison of 2 manifestations vs 1 (table 5).
Table 4.
Model 1: key drivers of direct costs in patients with tuberous sclerosis complex with epilepsy during the most recent 3-year period
| Variable | p Value |
| Single manifestation categories | |
| Brain | 0.9533 |
| Circulatory | 0.5882 |
| Dermatological | 0.2704 |
| Kidney and urinary | 0.1282 |
| Psychiatric | 0.7628 |
| Respiratory | 0.5999 |
| Combinations of manifestation categories | |
| Brain/circulatory | 0.1846 |
| Brain/dermatological | 0.5965 |
| Brain/kidney and urinary | 0.5621 |
| Brain/psychiatric | 0.2749 |
| Dermatological/circulatory | 0.5965 |
| Dermatological/kidney and urinary | 0.0012 |
| Dermatology/psychiatric | 0.1023 |
| Kidney and urinary/circulatory | 0.1341 |
| Kidney and urinary/psychiatric | 0.6255 |
p Values are generated from a gamma distribution regression assessing the significance of manifestations in driving tuberous sclerosis complex-associated costs.
Table 5.
Model 2: key drivers of direct costs in patients with tuberous sclerosis complex with epilepsy during the most recent 3-year period
| Parameter* | p Value |
| Age (<18 and ≥18) | 0.6089 |
| Sex | 0.2285 |
| Manifestation categories (n) | <0.0001 |
| 1 manifestation* vs 2 manifestations† | 0.3098 |
| 1 manifestation vs 3 manifestations‡ | 0.0495 |
| 1 manifestation vs ≥4 manifestations§ | 0.0059 |
| 2 manifestations vs 3 manifestations | 0.0111 |
| 2 manifestations vs ≥4 manifestations | <0.0001 |
| 3 manifestations vs ≥4 manifestations | 0.0101 |
| 1 manifestation* vs 2 manifestations† | 0.3098 |
*n=34 for one manifestation.
†n=92 for two manifestations.
‡n=19 for three manifestations.
§n=5 for ≥4 manifestations.
Discussion
The study undertaken is the first to examine HCRU of patients with TSC+E in the UK. The results suggest that the TSC+E population is presented with challenges related to disease management, as there is still evidence of a lack of monitoring and appropriate management of these patients. Patients with TSC+E often develop numerous other manifestations that collectively contribute to cost and HCRU.27
Comparison with other studies
The prevalence of epilepsy in the TSC population was slightly lower than the 80%–90% lifetime prevalence quoted by many papers.12 19 However, our study is limited by its estimation of period prevalence and the recording of active epilepsy in the cohort. As the population included adults, this prevalence may also reflect the resolution of epilepsy in adulthood or previous mild epilepsy during childhood, which may not have been well recorded prior to the release of the newer TSC guidelines.3 25
Infantile spasms were also recorded in a lower proportion of patients with TSC+E than is generally reported,21 22 but the high frequency of vigabatrin prescriptions in children may reflect poor recording of infantile spasms. Additionally, most patients with infantile spasms recorded a prescription for vigabatrin, which agrees with clinical recommendations.36 Over one-quarter of all patients with TSC+E recorded seizure freedom, which is comparable to a chart review study,22 but with the limitation that the duration of epilepsy remission beyond 12 months is unknown. However, a small percentage of patients recorded no AEDs ever, which could reflect control of epileptic seizures but also of missing data.
The TSC +E cohort also had a high prevalence of common TSC co-morbidities including learning disabilities, behavioural disorders and autism, which agrees with previous studies that have found high proportions of epileptic patients with cognitive impairment.22 The presence of these co-morbidities leads to significant disability.37
In addition to co-morbidities, the majority of patients with TSC+E also recorded at least one other primary manifestation category, which agrees with previous studies indicating that TSC most often affects a broad spectrum of organs.17 18 27 Most patients recorded psychiatric and dermatological manifestations, which were found to substantially increase costs of the average patient with TSC who only records epilepsy.
Due to the complexity of epilepsy treatment in TSC, patients had a substantial requirement for AEDs and hypnotics/antipsychotics. However, it is surprising that 12% of active patients with TSC+E did not record any AED use throughout their history and a further 28% were prescribed only one AED at a time. This could potentially reflect undertreatment for these patients, although treatment gap or patients receiving medication outside of primary care (which would not be captured) cannot be ruled out. Active epilepsy is still reported in 50% of elderly patients (>65 years), and refractory epilepsy must have been present in a greater proportion in younger patients, especially considering that seizure freedom may occur less frequently in patients with cognitive impairment.22 Similar proportions of children also recorded several concomitant AEDs, suggesting lack of specialist neurology referrals for controlling their disease.
Brain procedures were infrequently recorded but were more commonly conducted in the paediatric TSC+E cohort. Current guidelines recommend surgical procedures and interventions (including nerve stimulation) in patients who are inadequately controlled after two trials of AEDs.36 38 Similar proportions of paediatric and adult patients with TSC+E recorded concomitant AED use, which could be suggestive of refractory epilepsy, but surgical interventions were more common in the paediatric cohort. This may reflect early consideration of surgery in drug-refractory epilepsy as recommended in treatment guidelines.36
Similarly, low proportions of patients with TSC+E recorded routine tests and investigations suggested between 1–3 years in the TSC consensus recommendations.25 MRI was only recorded in approximately one-third of patients ever but is recommended to be performed in all patients with suspected epilepsy in order to assess the presence of cortical tubers and other brain abnormalities.25 This may be indicative of undermonitoring in these patients. However, MRI was performed in twofold more children than adults, potentially reflecting a change in clinical practice following the release of the international consensus recommendations. The general lack of MRI in the TSC+E cohort may also reflect the high burden of intellectual co-morbidities, which could influence the decision to undertake imaging. Paediatric patients with seizures are also recommended to undergo baseline EEG,25 but this recorded in less than half of paediatric patients ever, possibly explained by the high proportion of patients with learning disabilities. Surveillance and monitoring appear to be lacking in the TSC+E cohort; this may have an impact on the increased HCRU and costs, especially inpatient admissions.
Patients with TSC+E consulted with primary and secondary care much more frequently than the Comparator, which translated into higher costs. The notably higher frequency of GP visits in adult patients with TSC+E versus paediatrics suggests that this transfer of care is suboptimal. It is also notable that one-quarter of paediatric patients had not visited a paediatrician in the 3-year period and the vast majority of all patients had never visited a neurologist. The increased burden on GP visits may reflect a lack of appropriate management in specialist care and consequently fewer tests and procedures, which are more likely to be performed in an outpatient setting. High inpatient costs and longer-than-average (according to HRG code) inpatient admissions are suggestive of invasive procedures and the need for specialised care often required in TSC,39 which were not present in the Comparator cohort.
The high costs incurred by the TSC+E cohort are likely to reflect the diverse and severe manifestation profile of patients, further showcased by the increased incremental costs resulting from the addition of specific primary organ system manifestation categories. Additionally, the presentation of more than two manifestations was found to significantly drive costs according to the regression model. High costs may reflect a small number of patients with substantial HCRU requirements, but the clinical profiles of these patients suggest that the population is likely to have considerable resource use due to the presence of several manifestations.
The results of this study indicate that the TSC+E population in CPRD has a clinical profile agreeing with many contemporary studies with the added knowledge indicating a substantial requirement for healthcare resources reflective of TSC-related epilepsy, their diverse manifestation profile and debilitating co-morbidities. It is likely that many of these patients experienced refractory epilepsy as the majority of patients recorded several concomitant AEDs, which was unlikely to resolve due to the high prevalence of learning disabilities and a deficit of suitable options. The lack of management in specialist care and inadequate testing may contribute to an increased burden on GPs, secondary care and the requirement for longer-than-average stays in hospital. As epilepsy presents in childhood, it is especially important for guidelines to be followed in order to ensure a smooth transition into adult care, emphasising the need for specialised centres such as TSC clinics and innovative treatments.
Strengths and limitations
The strength of this study is its sourcing of information from actual clinical practice in CPRD and HES. Data are captured in both primary and secondary care to provide the most complete picture of patient care and associated costs. CPRD has also been shown to be representative of patients in the UK29 30; thus, the findings in this study are considered generalisable to the UK TSC population. Large Comparator cohorts matched at a ratio of 5:1 were also used to bolster statistical analyses.
However, the requirement for continuous data may have biased results towards a comparison of sicker patients with TSC with healthier patients with CPRD. To account for this, excluded patients with TSC were examined on a case-by-case basis, which indicated that patients had incomplete primary care data due to age under 3 years, repeated transfers in and out of the CPRD and poor recording of data, rather than having mild cases of TSC. When comparing secondary care HES data, these patients were comparable to the included patients with TSC, and were thus assumed to have transferred out of their primary care contributing practice, leading to gaps in data. Costs and HCRU would have been underestimated if these patients were included due to their missing data. It is not possible to track patients outside of CPRD-contributing practices, and as such, this approach was considered most amenable to an accurate estimation of healthcare resource use. Multiple imputation was not performed due to potentially unclear patterns of missingness. As such, missing-at-random assumptions were not fulfilled, which would implicate the reliability of imputing information.
Limitations in this study are typical of those using real-world data since the information recorded is dependent on the accuracy of the information input by the healthcare professional. Only primary care drugs and associated costs were captured in CPRD, while those prescribed in secondary and tertiary care settings are not; as such, drug costs and utilisation (such as the relatively low percentage of AED prescriptions) are likely to be underestimated. It should, however, be noted that drugs commonly used in secondary care are captured within inpatient and outpatient codes, and so only expensive or unusual therapies would be completely missed.
Patients seeking care outside of the GP and hospitalisations (eg, mental health clinics, social care for behavioural and educational needs) will also not be captured in the data. A&E admissions that do not result in inpatient admission will not be included in the data; this could lead to underestimation of hospital admissions for patient with epilepsy who may visit A&E when experiencing seizures but who are discharged prior to inpatient admission.
It was not possible to assess completeness or representativeness of TSC-related data recording as the study is limited to only those patients with a recorded diagnosis of TSC and epilepsy. Additionally, primary care HCRU would not be captured for patients who transferred out of CPRD during the study period.
A comparison was not performed between a general epilepsy cohort without TSC as only 34 patients in the TSC cohort had epilepsy without any other diagnosed manifestations and the prevalence of epilepsy in the Comparator cohort was very low. Additionally, the purpose of this study was to characterise the disease burden of patients with TSC with epilepsy who are very likely to present with multiple primary organ system manifestation categories.
Conclusion
Patients with TSC+E have a substantial requirement for primary and secondary HCRU and drugs. However, patients are still recording fewer important diagnostic tests (such as MRIs and EEGs) and specialist consultations than recommended in the current national guidelines for TSC management. In addition, transition of care from paediatric to adult services seems to be suboptimal, evidenced by fewer tests, brain procedures and specialist visits in adults compared with paediatric patients.
Most patients are treated with multiple, concurrent AEDs, which may be reflective of severe epilepsy unresponsive to treatment. Nonetheless, there are also many patients who appear to be undertreated or not treated at all.
Undermonitoring and inappropriate management—along with a diverse profile of manifestations, intellectual co-morbidities and epilepsy severity—may be contributing to healthcare expenditure.
Future adequately powered studies could endeavour to study patients with TSC and non-TSC disorder with epilepsy and also patients with TSC with and without epilepsy in order to characterise the additional cost burden of epilepsy. Additionally, real-world studies should examine seizure frequency in relation to refractory epilepsy and treatments in order to better understand the clinical profile of these patients.
Supplementary Material
Footnotes
Contributors: All authors designed the study. KP and MM conducted the analyses. MM drafted the manuscript with review and input from all authors. All authors interpreted the data.
Funding: This research was funded by Novartis Pharmaceuticals.
Competing interests: The authors declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article. CS and MK have received honoraria and study participation fees from Novartis. MM, KP and CM are employees of IMS Health, a consulting company that received funding from Novartis to conduct the study. EG, VS and MN are employees of Novartis Pharmaceuticals. All authors have completed the International Committee of Medical Journal Editors uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from Novartis Pharmaceuticals Corporation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work. The lead author affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Study data are unavailable for sharing due to patient confidentiality restrictions.
References
- 1. TSA Tuberous Sclerosis Alliance. What is TSC? http://www.tsalliance.org/pages.aspx?content=2 (accessed June 01 2015).
- 2. TSA Tuberous Sclerosis Alliance. Consultation on the United Kingdom plan for rare diseases: submission from the tuberous sclerosis association May 2012. URL:http://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=1&ved=0CCIQFjAA&url=http://www.tuberous-sclerosis.org/_literature_124492/TSA_Publications_-_Consultation_on_the_UK_Plan_for_Rare_Diseases&ei=9Y5cVf7-B8jwUqjAgegB&usg=AFQjCN Published 2012. (accessed June 01 2015).
- 3. Northrup H, Krueger DA. International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational tuberous sclerosis complex consensus conference. Pediatr Neurol 2013;49:243–54. 10.1016/j.pediatrneurol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Slegtenhorst M, de Hoogt R, Hermans C, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 1997;277:805–8. 10.1126/science.277.5327.805 [DOI] [PubMed] [Google Scholar]
- 5. Huang J, Manning BD. The TSC1–TSC2 complex: a molecular switchboard controlling cell growth. Biochem J 2008;412:179–90. 10.1042/BJ20080281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993;75:1305–15. [DOI] [PubMed] [Google Scholar]
- 7. Sancak O, Nellist M, Goedbloed M, et al. Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype—phenotype correlations and comparison of diagnostic DNA techniques in Tuberous Sclerosis Complex. Eur J Hum Genet 2005;13:731–41. 10.1038/sj.ejhg.5201402 [DOI] [PubMed] [Google Scholar]
- 8. Niida Y, Lawrence-Smith N, Banwell A, et al. Analysis of both TSC1 and TSC2 for germline mutations in 126 unrelated patients with tuberous sclerosis. Hum Mutat 1999;14:412–22. [DOI] [PubMed] [Google Scholar]
- 9. Kozlowski P, Roberts P, Dabora S, et al. Identification of 54 large deletions/duplications in TSC1 and TSC2 using MLPA, and genotype–phenotype correlations. Hum Genet 2007;121:389–400. 10.1007/s00439-006-0308-9 [DOI] [PubMed] [Google Scholar]
- 10. Jones AC, Shyamsundar MM, Thomas MW, et al. Comprehensive mutation analysis of TSC1 and TSC2—and phenotypic correlations in 150 families with tuberous sclerosis. Am J Hum Genet 1999;64:1305–15. 10.1086/302381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dabora SL, Jozwiak S, Franz DN, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 2001;68:64–80. 10.1086/316951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345–56. 10.1056/NEJMra055323 [DOI] [PubMed] [Google Scholar]
- 13. Au KS, Williams AT, Roach ES, et al. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med 2007;9:88–100. doi:10.1097GIM.0b013e31803068c7 [DOI] [PubMed] [Google Scholar]
- 14. Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013;381:125–32. 10.1016/S0140-6736(12)61134-9 [DOI] [PubMed] [Google Scholar]
- 15. Henske EP, Ewalt DH, Bissler JJ. Renal manifestations in tuberous sclerosis complex. http://www.tsalliance.org/documents/Renal Manifestations in TSC.pdf.
- 16. Rosser T, Panigrahy A, McClintock W. The diverse clinical manifestations of tuberous sclerosis complex: a review. Semin Pediatr Neurol 2006;13:27–36. 10.1016/j.spen.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 17. Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet 2008;372:657–68. 10.1016/S0140-6736(08)61279-9 [DOI] [PubMed] [Google Scholar]
- 18. Kingswood C, Bolton P, Crawford P, et al. The clinical profile of tuberous sclerosis complex (TSC) in the United Kingdom: a retrospective cohort study in the Clinical Practice Research Datalink (CPRD). Eur J Paediatr Neurol 2016;20 10.1016/j.ejpn.2015.11.011 [DOI] [PubMed] [Google Scholar]
- 19. Shepherd CW, Houser OW, Gomez MR. MR findings in tuberous sclerosis complex and correlation with seizure development and mental impairment. AJNR Am J Neuroradiol 1995;16:149-55 http://www.ajnr.org/content/16/1/149.full.pdf. Published 1995. [PMC free article] [PubMed] [Google Scholar]
- 20. Ridler K, Suckling J, Higgins N, et al. Standardized whole brain mapping of tubers and subependymal nodules in tuberous sclerosis complex. J Child Neurol 2004;19:658–65. 10.1177/08830738040190090501 [DOI] [PubMed] [Google Scholar]
- 21. Thiele EA. Managing epilepsy in tuberous sclerosis complex. J Child Neurol 2004;19:680–6 http://www.ncbi.nlm.nih.gov/pubmed/15563014 10.1177/08830738040190090801 [DOI] [PubMed] [Google Scholar]
- 22. Chu-Shore CJ, Major P, Camposano S, et al. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia 2010;51:1236–41. 10.1111/j.1528-1167.2009.02474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rentz AM, Skalicky AM, Liu Z, et al. Tuberous sclerosis complex: a survey of health care resource use and health burden. Pediatr Neurol 2015;52:435–41. 10.1016/j.pediatrneurol.2014.11.013 [DOI] [PubMed] [Google Scholar]
- 24. Park MJ, Adams SH, Irwin CE. Health care services and the transition to young adulthood: challenges and opportunities. Acad Pediatr 2011;11:115–22. 10.1016/j.acap.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 25. Krueger DA, Northrup H; International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013;49:255–65. 10.1016/j.pediatrneurol.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thiele EA, Granata T, Matricardi S, et al. Transition into adulthood: tuberous sclerosis complex, Sturge-Weber syndrome, and Rasmussen encephalitis. Epilepsia 2014;55 Suppl 3:29–33. 10.1111/epi.12722 [DOI] [PubMed] [Google Scholar]
- 27. Kingswood JC, Crawford P, Johnson SR, et al. The economic burden of tuberous sclerosis complex in the UK: a retrospective cohort study in the Clinical Practice Research Datalink. J Med Econ 2016. 12. [DOI] [PubMed] [Google Scholar]
- 28. Kingswood JC, Nasuti P, Patel K, et al. The economic burden of tuberous sclerosis complex in UK patients with renal manifestations: a retrospective cohort study in the clinical practice research datalink (CPRD). J Med Econ 2016. 11. [DOI] [PubMed] [Google Scholar]
- 29. García Rodríguez LA, Pérez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol 1998;45:419–25. 10.1046/j.1365-2125.1998.00701.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams T, van Staa T, Puri S, et al. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012;3:89–99. 10.1177/2042098611435911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–36. 10.1093/ije/dyv098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mori I. GP patient survey—national summary report. http://gp-survey-production.s3.amazonaws.com/archive/2014/July/1301375001_Y8W2 National Summary Report_FINAL v1.pdf Published 2014. (accessed Aug 06 2015).
- 33. Northrup H, Koenig MK, Pearson DA, et al. Tuberous sclerosis complex. 2015. (accessed Nov 13 2015).
- 34. NHS Business Services Authority NHS Drug Tariff. http://www.nhsbsa.nhs.uk/PrescriptionServices/4940.aspx Published 2012 (accessed Aug 01 2015)
- 35. Department of Health. NHS Reference Costs. https://www.gov.uk/government/collections/nhs-reference-costs (accessed Aug 1 2015).
- 36. Curatolo P, Jóźwiak S, Nabbout R. TSC Consensus Meeting for SEGA and Epilepsy Management. Management of epilepsy associated with tuberous sclerosis complex (TSC): clinical recommendations. Eur J Paediatr Neurol 2012;16:582–6. 10.1016/j.ejpn.2012.05.004 [DOI] [PubMed] [Google Scholar]
- 37. Prather P, de Vries PJ. Behavioral and cognitive aspects of tuberous sclerosis complex. J Child Neurol 2004;19:666–74. 10.1177/08830738040190090601 [DOI] [PubMed] [Google Scholar]
- 38. Moavero R, Cerminara C, Curatolo P. Epilepsy secondary to tuberous sclerosis: lessons learned and current challenges. Childs Nerv Syst 2010;26:1495–504. 10.1007/s00381-010-1128-8 [DOI] [PubMed] [Google Scholar]
- 39. Wilson TA, Rodgers S, Tanweer O, et al. Tuberous sclerosis healthcare utilization based on the national inpatient sample database: a review of 5,655 hospitalizations. World Neurosurg 2016;91:97–105. 10.1016/j.wneu.2016.03.043 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-015236supp001.pdf (103.3KB, pdf)