Abstract
Objective
We investigated whether serum high-sensitivity C reactive protein (hs-CRP) levels measured in an emergency department (ED) are associated with inhospital mortality in patients with cardiovascular disease (CVD).
Design
A retrospective cohort study.
Setting
ED of a teaching hospital in Japan.
Participants
12 211 patients with CVD aged ≥18 years who presented to the ED by an ambulance between 1 February 2006 and 30 September 2014 were evaluated.
Main outcome measures
Inhospital mortality.
Results
1156 patients had died. The inhospital mortality increased significantly with the hs-CRP levels (<3.0 mg/L: 7.0%, 95% CI 6.4 to 7.6; 3.1–5.4 mg/L: 9.6%, 95% CI 7.9 to 11.3: 5.5–11.5 mg/L: 11.2%, 95% CI 9.4 to 13.0; 11.6–33.2 mg/L: 12.3%, 95% CI 10.5 to 14.1 and ≥33.3 mg/L: 19.9%, 95% CI 17.6 to 22.2). The age-adjusted and sex-adjusted HR for total mortality was increased significantly in the three ≥5.5 mg/L groups compared with the <3.0 mg/L group (5.5–11.5 mg/L: HR=1.32, 95% CI 1.09 to 1.60, p=0.005; 11.6–33.2 mg/L: HR=1.38, 95% CI 1.14 to 1.65, p=0.001 and ≥33.3 mg/L: HR=2.15, 95% CI 1.84 to 2.51, p<0.001). Similar findings were observed for the CVD subtypes of acute myocardial infarction, heart failure, cerebral infarction and intracerebral haemorrhage. This association remained unchanged even after adjustment for age, sex and white cell count and withstood Bonferroni adjustment for multiple testing. When the causes of death were divided into primary CVD and non-CVD deaths, the association between initial hs-CRP levels and mortality remained significant, but the influence of hs-CRP levels was greater in non-CVD deaths than CVD deaths. The percentage of non-CVD deaths increased with hs-CRP levels; among the patients with hs-CRP levels ≥33.3 mg/L, non-CVD deaths accounted for 37.5% of total deaths.
Conclusion
Our findings suggest that increased hs-CRP is a significant risk factor for inhospital mortality among patients with CVD in an ED. Particular attention should be given to our finding that non-CVD death is a major cause of death among patients with CVD with higher hs-CRP levels.
Keywords: heart failure, cerebral infarction, intracerebral haemorrhage, c-reactive protein
Strengths and limitations of this study.
The strengths of our study include its large-scale retrospective cohort design with the examination of 12 211 patients diagnosed with CVD and the measurement of high-sensitivity C reactive protein at baseline.
In addition, this study investigated the causes of deaths of the CVD pataients.
A limitation of our study is the single-centre nature of the study (ie, one teaching hospital).
Another limitaion of our study is the confounders such as haemodynamics, comorbidities and other laboratory data that could not be investigated.
Introduction
Emergency department (ED) patients with cardiovascular disease (CVD) need a timely evaluation for the diagnosis of CVD and the identification of comorbidities. However, the evaluation of patients with CVD transported by an ambulance is often difficult because these patients may have complex medical problems and are sometimes too ill to assist medical staff with important medical information such as time of symptom onset and their medical history. The identification of markers that are associated with inhospital mortality would be useful in the triage of patients with CVD in EDs around the world.
C reactive protein (CRP) is an acute-phase protein produced by the liver, and the serum levels of this protein increase in response to tissue injury, infection, inflammation and neoplastic proliferation. The measurement of serum CRP concentrations is inexpensive and is done routinely to assess patients. In addition, the serum high-sensitivity C reactive protein (hs-CRP) level is known to be a predictive marker of the degree of atherosclerosis and future cardiovascular events.1–4 Several studies have also observed that elevated CRP predicts the prognosis of patients with CVD at the acute stage.5–16
However, there has been controversy over the usefulness of the measurement of CRP as a prognostic marker in ED evaluations in Japan. The objective of the present study was to examine the association between the initial hs-CRP levels and inhospital mortality in patients with CVD and its subtypes, that is, acute myocardial infarction, heart failure, cerebral infarction and intracerebral haemorrhage.
Patients and methods
Study design, setting and population
This was a retrospective cohort study at Iizuka Hospital, a teaching hospital with 1116 beds located at the centre of the Chikuho region of Fukuoka prefecture on Japan’s Kyushu Island. This hospital is the only critical care centre for a population of approximately 430 000 people, and over 8000 cases are transported by emergency vehicle to the hospital each year, accounting for approximately 40% of the emergency-conveyance patients in the Chikuho region. Ethical approval of this study was obtained from the Ethics Committee of Iizuka Hospital (CRM-27015). The requirement of informed consent was waived by the ethics committee because of the retrospective nature of the study.
At our hospital, the cases and records of patients who come to the ED are filed separately and are distinguished from those of the general outpatients. In the present study, the patients were recruited from among the ED files, and we evaluated the cases of 57 443 consecutive patients ≥18 years old who presented to Iizuka Hospital’s ED by an ambulance between 1 February 2006 and 30 September 2014. Among them, 391 patients had repeated ED visits on 2 consecutive days, and only the index case was used, in order to comply with the assumption of independent observations. At the ED, the leading diagnoses were established clinically using the International Classification of Diseases, 10th Revision (ICD-10). We reviewed hospital’s records and cases and excluded 2317 patients who experienced cardiopulmonary arrest and 298 patients who were pregnant.
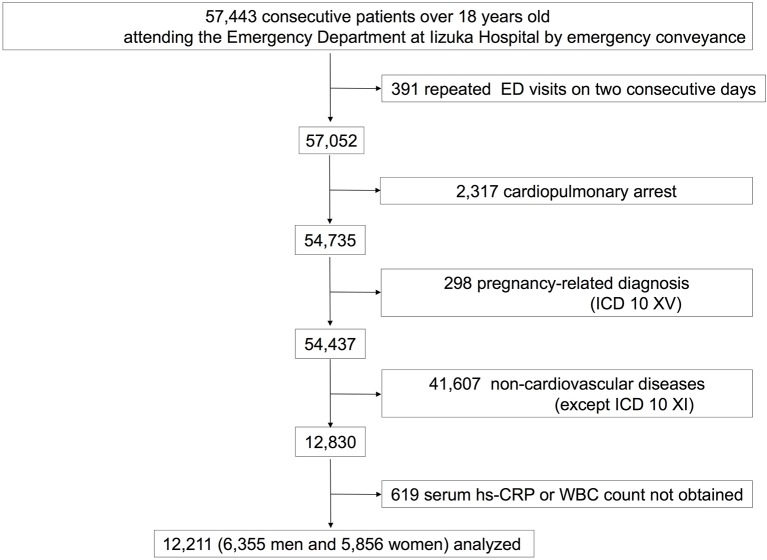
Of the remaining 54 437 records, 12 830 patients were diagnosed with CVD, which was defined as meeting the criteria in diagnosis codes I00–I99 of the ICD-10 at their ED visit. The 619 (4.8%) patients whose hs-CRP level or white cell count (WCC) at baseline was not obtained were excluded. A final total of 12 211 patients with CVD were included in this study (figure 1). We divided the diagnoses with CVD into acute myocardial infarction (I21–I24), heart failure (I50), intracerebral haemorrhage (I61), cerebral infarction (I63) and ‘other’ according to the ICD-10 classification.
Figure 1.
Flow diagram of the study. In this study, the cases of 57 443 consecutive patients ≥18 years old who presented to Iizuka Hospital’s emergency department (ED) by an ambulance between 1 February 2006 and 30 September 2014 were evaluated. Excluded from the study were 391 patients who had repeated ED visits on 2 consecutive days, 2317 patients had a history of cardiopulmonary arrest and 298 were pregnant. Of the remaining 54 437 records, 12 830 patients were diagnosed with cardiovascular disease (CVD). Six hundred and nineteen (4.8%) patients whose high-sensitivity C reactive protein (hs-CRP) level or white cell count (WCC) at baseline was not obtained were excluded. A final total of 12 211 patients with CVD were included in this study. ICD 10, International Classification of Diseases, 10th Revision.
Regarding the diagnoses of CVD at the ED, the physicians of the ED performed careful examinations and primary care. The physical examination, electrocardiograph, X-ray, ultrasonography and blood examination were performed immediately. A complete blood cell count and biochemical examination including pH, lactate, creatine kinase-MB, brain natriuretic peptide, D-dimer and troponin assay were available as needed. Brain-imaging data (CT and/or MRI) were also available for patients with neurological abnormalities or a decreased level of consciousness. When a patient was suspected of having CVD, the physicians of the ED were able to contact a cardiologist, neurologist, cardiovascular surgeon or neurosurgeon 24 hours a day, 365 days a year. Based on the examinations by specialists and the test results, the leading diagnosis of CVD complying with ICD-10 was established at the ED. During the study period, no structural changes such as patient uptake, patient flow, the evaluation at the ED and the biomarker analysis occurred.
Data collection and laboratory measurement
The following data were extracted from the medical records for each patient: age, gender, diagnosis in the ED, length of hospital stay (days) and outcome. The cause of death was extracted from the death certificate. Blood samples for initial hs-CRP and WCC were collected soon after the patient arrived at the ED as part of the clinical routine and were analysed immediately for every patient. Serum hs-CRP levels were measured with a latex agglutination turbidimetric immunoassay (CRP-latex X2 Seiken, Denka Seiken, Tokyo). The hs-CRP measurement range was 0.2–320 mg/L, and the normal range is <3 mg/L.
Definition of endpoints
The endpoints were mortality from any cause during hospitalisation. Patients who remained in the hospital on 30 November 2014 were considered alive in this analysis. Patients who did not require hospitalisation and were discharged from the ED were also considered alive, and their stay in the hospital was regarded as 1 day. We divided the causes of death into two main categories using the ICD-10: CVD deaths and non-CVD deaths. CVD death was defined as a death from a cause listed in ICD codes I00–I99, and non-CVD death was defined as a death from a cause other than those listed in codes I00–I99. We classified the non-CVD deaths into the following three subcategories: infection, neoplasm and others. Infection-related deaths included deaths from septicaemia, bacteraemia, endocarditis, pulmonary infections (eg, viral pneumonia, bacterial pneumonia, influenza with respiratory manifestations, abscess of lung or mediastinum), genitourinary infections (eg, urinary tract infection (UTI), pyelonephritis and perinephric abscess), gastrointestinal infections (eg, diverticulitis, C. difficile colitis and perirectal abscess), peritonitis, soft-tissue infections (eg, cellulitis, necrotising fasciitis and gangrene) and joint or bone infections (eg, infective arthritis and osteomyelitis). Neoplasm-related deaths included the deaths from codes C00–D48. Deaths from causes other than those listed above were classified as other deaths.
Statistical analysis
We divided the patients with hs-CRP levels >3.0 mg/L into quartiles. The patients with hs-CRP levels ≤3.0 mg/L were assigned to a separate category which served as the reference group, because the 3.0 mg/L cut-off point corresponds to the ‘high’ CRP level in previous primary prevention studies.17 We calculated the age-adjusted and sex-adjusted or age-adjusted, sex-adjusted and WCC-adjusted HRs for total death from CVD and its subtypes and their 95% CI values using the Cox proportional hazards model. The linear trends of HRs across hs-CRP levels were also tested using the Cox proportional hazards model. The Bonferroni method was used to address issues of multiple subgroup analyses. Bonferroni-corrected statistical significance was defined as p<0.008 = (0.05/6) in the analysis of total death and p<0.004 = (0.05/12) in the analysis of CVD deaths and non-CVD deaths. For the distribution plot of hs-CRP levels in the surviving patients, CVD death and non-CVD death groups, we examined hs-CRP values in each group by performing an analysis of variance. Because the distribution of hs-CRP values was skewed, the hs-CRP levels were natural log transformed for the statistical analyses. All analyses were performed using the SAS software package V.9.4.
Results
The age range of the patients was 18–104 years (median 76 years; IQR 64–84); 52% (6355) were men and 48% (5856) were women. The median baseline serum hs-CRP level was 1.8 mg/L (IQR 0.6–7.5 mg/L). The median number of hospital days including the date of the ED visit was 14 days (IQR 2–32 days). Among these patients, 1156 deaths were recorded, giving an inhospital mortality rate of 9.5% (CI 0.09 to 0.10).
Table 1 shows the inhospital mortality and the age-adjusted and sex-adjusted and age-adjusted, sex-adjusted and WCC-adjusted HRs for the inhospital mortality of CVD according to the patients’ hs-CRP levels. A significant association was observed between hs-CRP levels and the absolute risk of inhospital mortality in the total CVD group (p for trend <0.001). In regard to the subtypes of CVD, the inhospital mortality of the patients with acute myocardial infarction, heart failure, cerebral infarction and intracerebral haemorrhage was also significantly increased as the hs-CRP levels increased (p for trend <0.001).
Table 1.
Number of patients and deaths, inhospital mortalities and HRs for total death of CVD and its subtypes according to hs-CRP levels
| Diagnosis at the emergency department | Total | hs-CRP levels (mg/L) | p for trend (across categories) | Continuous log scale | p for trend (continous) | ||||
| <3.0 | 3.1–5.4 | 5.5–11.5 | 11.6–33.2 | ≥33.3 | |||||
| CVD | |||||||||
| No of patients | 12 211 | 7375 | 1212 | 1200 | 1216 | 1208 | |||
| No of deaths | 1156 | 517 | 116 | 134 | 149 | 240 | |||
| Inhospital mortality (%) | 9.5 | 7.0 | 9.6 | 11.2 | 12.3 | 19.9 | <0.001 | ||
| Age-adjusted and sex-adjusted HR (95% CI) | 1 (ref.) | 1.18 (0.97 to 1.45) | 1.32*(1.09 to 1.60) | 1.38*(1.14 to 1.65) | 2.15*(1.84 to 2.51) | <0.001 | 1.15*(1.12 to 1.19) | <0.001 | |
| Age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) | 1 (ref.) |
1.15 (0.94 to 1.41) |
1.29 (1.07 to 1.56) |
1.33* (1.11 to 1.60) |
2.00* (1.71 to 2.34) |
<0.001 | 1.14* (1.10 to 1.18) |
<0.001 | |
| Acute myocardial infarction | |||||||||
| No of patients | 1347 | 796 | 145 | 126 | 125 | 155 | |||
| No of deaths | 89 | 28 | 4 | 12 | 9 | 36 | |||
| Inhospital mortality (%) | 6.6 | 3.5 | 2.8 | 9.5 | 7.2 | 23.2 | <0.001 | ||
| Age-adjusted and sex-adjusted HR (95% CI) | 1 (ref.) | 0.65 (0.23 to 1.86) | 1.80 (0.91 to 3.57) | 1.46 (0.68 to 3.13) | 4.33*(2.60 to 7.22) | <0.001 | 1.37*(1.23 to 1.54) | <0.001 | |
| Age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) | 1 (ref.) |
0.62 (0.22 to 1.76) |
1.64 (0.83 to 3.26) |
1.14 (0.51 to 2.54) |
3.44* (2.04 to 5.82) |
<0.001 | 1.31* (1.16 to 1.47) |
<0.001 | |
| Heart failure | |||||||||
| No of patients | 1742 | 620 | 232 | 249 | 310 | 331 | |||
| No of deaths | 148 | 19 | 11 | 28 | 29 | 61 | |||
| Inhospital mortality (%) | 8.5 | 3.1 | 4.7 | 11.2 | 9.4 | 18.4 | <0.001 | ||
| Age-adjusted and sex-adjusted HR (95% CI) | 1 (ref.) | 1.27 (0.60 to 2.66) | 2.29*(1.26 to 4.17) | 1.99*(1.11 to 3.57) | 3.55*(2.11 to 5.97) | <0.001 | 1.35*(1.22 to 1.51) | <0.001 | |
| Age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) | 1 (ref.) |
1.26 (0.60 to 2.64) |
2.34* (1.28 to 4.26) |
2.07* (1.15 to 3.72) |
3.83* (2.26 to 6.49) |
<0.001 | 1.39* (1.24 to 1.55) |
<0.001 | |
| Cerebral infarction | |||||||||
| No of patients | 2879 | 1823 | 277 | 281 | 271 | 227 | |||
| No of deaths | 261 | 109 | 25 | 34 | 43 | 50 | |||
| Inhospital mortality (%) | 9.1 | 6.0 | 9.0 | 12.1 | 15.9 | 22.0 | <0.001 | ||
| Age-adjusted and sex-adjusted HR (95% CI) | 1 (ref.) | 1.15 (0.74to 1.78) | 1.73*(1.18 to 2.55) | 2.04*(1.43 to 2.91) | 3.02*(2.15 to 4.22) | <0.001 | 1.28*(1.19 to 1.37) | <0.001 | |
| Age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) | 1 (ref.) |
1.07 (0.69 to 1.66) |
1.59 (1.08 to 2.34) |
1.63 (1.12 to 2.36) |
2.20* (1.53 to 3.17) |
<0.001 | 1.20* (1.11 to 1.29) |
<0.001 | |
| Intracerebral haemorrhage | |||||||||
| No of patients | 1989 | 1428 | 167 | 153 | 137 | 104 | |||
| No of deaths | 338 | 201 | 39 | 32 | 29 | 37 | |||
| Inhospital mortality (%) | 17.0 | 14.1 | 23.4 | 20.9 | 21.2 | 35.6 | <0.001 | ||
| Age-adjusted and sex-adjusted HR (95% CI) | 1 (ref.) | 1.60 (1.14 to 2.26) | 1.34 (0.92 to 1.95) | 1.33 (0.90 to 1.97) | 2.33*(1.64 to 3.32) | <0.001 | 1.14*(1.07 to 1.21) | <0.001 | |
| Age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) | 1 (ref.) |
1.56 (1.10 to 2.20) |
1.32 (0.91 to 1.92) |
1.29 (0.87 to 1.92) |
2.05* (1.42 to 2.97) |
<0.001 | 1.12* (1.05 to 1.19) |
<0.001 | |
| Others | |||||||||
| No of patients | 4254 | 2708 | 391 | 391 | 373 | 391 | |||
| No of deaths | 320 | 160 | 37 | 28 | 39 | 56 | |||
| Inhospital mortality (%) | 7.5 | 5.9 | 9.5 | 7.2 | 10.5 | 14.3 | 0.002 | ||
| Age-adjusted and sex-adjusted HR (95% CI) | 1 (ref.) | 1.41 (0.98 to 2.01) | 0.95 (0.63 to 1.42) | 1.28 (0.89 to 1.82) | 1.43 (1.05 to 1.95) | 0.032 | 1.08 (1.02 to 1.14) | 0.009 | |
| Age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) | 1 (ref.) |
1.30 (0.91 to 1.86) |
0.74 (0.48 to 1.14) |
1.16 (0.85 to 1.61) |
1.03 (1.02 to 1.04) |
0.381 | 1.04 (0.98 to 1.10) |
0.223 | |
Cardiovascular disease: International Statistical Classification of Diseases and related problems 10th revision (below ICD-10) Diseases of the circulatory system (I00–I99);
acute myocardial infarction: ICD-10 (I21–I24); heart failure: ICD-10 (I50); cerebral infarction: ICD-10 (I63); intracerebral haemorrhage: ICD-10 (I61).
*Statistically significant after applying a Bonferroni correction.
CVD, cardiovascular disease; hs-CRP, high-sensitivity C reactive protein; WCC, white cell count.
The age-adjusted and sex-adjusted HRs for total inhospital mortality in the patients with hs-CRP levels ≥5.5 mg/L were significantly higher compared with those in the patients with levels <3.0 mg/L (5.5–11.5 mg/L: HR=1.32, 95% CI 1.09 to 1.60, p=0.005; 11.6–33.2 mg/L: HR=1.38, 95% CI 1.14 to 1.65, p=0.001; and ≥33.3 mg/L: HR=2.15, 95% CI 1.84 to 2.51, p<0.001). In the same way, the age-adjusted and sex-adjusted HRs for the inhospital mortality of the patients with acute myocardial infarction, heart failure, cerebral infarction and intracerebral haemorrhage also increased with increasing hs-CRP levels, and were significantly higher in the patients with hs-CRP levels ≥33.3 mg/L compared with those with levels <3.0 mg/L.
In addition, when we determined the age-adjusted and sex-adjusted HRs for one increment in log-transformed hs-CRP concentrations, we observed significant upward trends for inhospital mortality for both total CVD and its subtypes. This association remained unchanged even after adjustment for age, sex and WCC and withstood Bonferroni adjustment for multiple testing.
As shown in table 2, when divided the cases separately into CVD deaths and non-CVD deaths, the age-adjusted and sex-adjusted HRs of the cause-specific inhospital deaths increased with increasing hs-CRP levels in both the subgroup of CVD deaths and that of non-CVD deaths (p for trend <0.001). In the patients with hs-CRP levels ≥33.3 mg/L, the age-adjusted and sex-adjusted HR for CVD death was 1.44 (95% CI 1.20 to 1.73, p<0.001) compared with that in the patients with hs-CRP <3.0 mg/L. The HR for non-CVD death, on the other hand, was 12.05 (95% CI 8.06 to 18.04, p<0.001) in this group. The relationship between the hs-CRP levels and non-CVD deaths was thus much stronger than that between the hs-CRP levels and CVD deaths. Regarding the subtypes of CVD, similar relationships were observed.
Table 2.
Number of cause-specific deaths, HRs for cause-specific death of CVD and its subtypes according to hs-CRP levels
| Diagnosis at the emergency department | Total | hs-CRP levels (mg/L) | p for trend (across categories) | Continuous log scale | p for trend (continuous) |
||||
| <3.0 | 3.1–5.4 | 5.5–11.5 | 11.6–33.2 | ≥33.3 | |||||
| CVD | |||||||||
| No of patients | 12 211 | 7375 | 1212 | 1200 | 1216 | 1208 | |||
| No of CVD deaths | 976 | 484 | 106 | 116 | 120 | 150 | |||
| CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
1.16 (0.94 to 1.43) |
1.23 (1.00 to 1.51) |
1.20 (0.98 to 1.46) |
1.44* (1.20 to 1.73) |
<0.001 | 1.08* (1.03 to 1.11) |
<0.001 | |
| CVD-related, Age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
1.13 (0.92 to 1.40) |
1.20 (1.00 to 1.48) |
1.16 (0.95 to 1.42) |
1.33* (1.10 to 1.61) |
0.002 | 1.05* (1.01 to 1.09) |
0.004 | |
| No of non-CVD deaths | 180 | 33 | 10 | 18 | 29 | 90 | |||
| Non-CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
1.55 (0.76 to 3.15) |
2.67* (1.50 to 4.75) |
3.84* (2.32 to 6.35) |
12.05* (8.05 to 18.04) |
<0.001 | 1.77* (1.63 to 1.93) |
<0.001 | |
| Non-CVD-related, age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
1.52 (0.75 to 3.09) |
2.61* (1.47 to 4.65) |
3.72* (2.25 to 6.16) |
11.49* (7.66 to 17.23) |
<0.001 | 1.75* (1.61 to 1.91) |
<0.001 | |
| Acute myocardial infarction | |||||||||
| No of patients | 1347 | 796 | 145 | 126 | 125 | 155 | |||
| No of CVD deaths | 70 | 25 | 2 | 11 | 7 | 25 | |||
| CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
0.37 (0.09 to 1.56) |
1.93 (0.94 to 3.95) |
1.31 (0.56 to 3.05) |
3.51* (1.98 to 6.23) |
<0.001 | 1.30* (1.14 to 1.48) |
<0.001 | |
| CVD-related, age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
0.35 (0.08 to 1.47) |
1.74 (0.85 to 3.58) |
1.01 (0.41 to 2.47) |
2.71* (1.50 to 4.90) |
0.001 | 1.23* (1.08 to 1.40) |
0.002 | |
| No of non-CVD deaths | 19 | 3 | 2 | 1 | 2 | 11 | |||
| Non-CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
2.82 (0.47 to 17.04) |
1.21 (0.12 to 11.75) |
2.59 (0.42 to 15.84) |
10.14* (2.75 to 37.33) |
<0.001 | 1.73* (1.32 to 2.28) |
<0.001 | |
| Non-CVD-related, age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
2.71 (0.45 to 16.45) |
1.11 (0.11 to 10.81) |
2.07 (0.31 to 13.72) |
8.64* (2.32 to 32.27) |
0.001 | 1.67* (1.27 to 2.21) |
<0.001 | |
| Heart failure | |||||||||
| No of patients | 1742 | 620 | 232 | 249 | 310 | 331 | |||
| No of CVD deaths | 98 | 15 | 10 | 21 | 19 | 33 | |||
| CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
1.49 (0.67 to 3.33) |
2.27 (1.14 to 4.51) |
1.76 (0.88 to 3.48) |
2.58* (1.39 to 4.79) |
0.004 | 1.23* (1.09 to 1.39) |
0.001 | |
| CVD-related age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
1.49 (0.67 to 3.32) |
2.29 (1.15 to 4.56) |
1.78 (0.90 to 3.54) |
2.66* (1.42 to 4.97) |
0.003 | 1.24* (1.09 to 1.41) |
0.001 | |
| No of non-CVD deaths | 50 | 4 | 1 | 7 | 10 | 28 | |||
| Age-adjusted and sex-adjusted HR (95% CI) | 1 (ref.) |
0.52 (0.06 to 4.68) |
2.50 (0.72 to 8.74) |
2.92 (0.91 to 9.42) |
6.80* (2.36 to 19.57) |
<0.001 | 1.69* (1.38 to 2.08) |
<0.001 | |
| Non-CVD-related, age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
0.51 (0.06 to 4.57) |
2.52 (0.72 to 8.80) |
3.21 (0.99 to 10.33) |
8.19* (2.82 to 23.80) |
<0.001 | 1.84* (1.47 to 2.29) |
<0.001 | |
| Cerebral infarction | |||||||||
| No of patients | 2879 | 1823 | 277 | 281 | 271 | 227 | |||
| No of CVD deaths | 230 | 103 | 22 | 33 | 38 | 34 | |||
| CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
1.08 (0.68 to 1.72) |
1.76 (1.19 to 2.61) |
1.95* (1.34 to 2.84) |
2.13* (1.44 to 3.14) |
<0.001 | 1.21* (1.12 to 1.30) |
<0.001 | |
| CVD-related age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
0.99 (0.62 to 1.57) |
1.59 (1.07 to 2.35) |
1.48 (1.00 to 2.19) |
1.44 (0.95 to 2.20) |
0.012 | 1.11 (1.03 to 1.21) |
0.008 | |
| No of non-CVD deaths | 31 | 6 | 3 | 1 | 5 | 16 | |||
| Non-CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
2.07 (0.51 to 8.41) |
0.92 (0.11 to 7.63) |
3.08 (0.91 to 10.49) |
18.78* (7.27 to 48.51) |
<0.001 | 1.95* (1.57 to 2.41) |
<0.001 | |
| Non-CVD-related age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
2.20 (0.54 to 8.99) |
0.99 (0.12 to 8.29) |
3.87 (1.10 to 13.62) |
24.08* (8.62 to 67.25) |
<0.001 | 2.08* (1.65 to 2.64) |
<0.001 | |
| Intracerebral haemorrhage | |||||||||
| No of patients | 1989 | 1428 | 167 | 153 | 137 | 104 | |||
| No of CVD deaths | 313 | 196 | 37 | 29 | 28 | 23 | |||
| CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
1.56 (1.10 to 2.22) |
1.25 (0.84 to 1.84) |
1.32 (0.88 to 1.97) |
1.49 (0.96 to 2.30) |
0.019 | 1.07 (1.00 to 1.14) |
0.061 | |
| CVD-related, age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
1.52 (1.07 to 2.17) |
1.23 (0.83 to 1.82) |
1.29 (0.86 to 1.92) |
1.30 (0.82 to 2.04) |
0.020 | 1.05 (0.98 to 1.13) |
0.166 | |
| No of non-CVD deaths | 25 | 5 | 2 | 3 | 1 | 14 | |||
| Non-CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
3.17 (0.62 to 16.37) |
5.13 (1.22 to 21.58) |
1.85 (0.21 to 16.09) |
32.57* (11.41 to 92.97) |
<0.001 | 2.26* (1.78 to 2.86) |
<0.001 | |
| Non-CVD-related age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
3.05 (0.59 to 15.77) |
4.97 (1.18 to 20.89) |
1.77 (0.20 to 15.38) |
28.22* (9.76 to 81.58) |
<0.001 | 2.21* (1.73 to 2.81) |
<0.001 | |
| Others | |||||||||
| No of patients | 4254 | 2708 | 391 | 391 | 373 | 391 | |||
| No of CVD deaths | 265 | 145 | 35 | 22 | 28 | 35 | |||
| CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
1.45 (1.00 to 2.11) |
0.83 (0.53 to 1.30) |
1.02 (0.67 to 1.54) |
1.01 (0.67 to 1.54) |
0.894 | 1.01 (0.95 to 1.08) |
0.750 | |
| CVD-related age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
1.32 (0.91 to 1.91) |
0.57 (0.35 to 0.93) |
0.77 (0.53 to 1.14) |
1.03 (1.02 to 1.04) |
0.119 | 0.96 (0.90 to 1.03) |
0.212 | |
| No of non-CVD deaths | 55 | 15 | 2 | 6 | 11 | 21 | |||
| Non-CVD-related age-adjusted and sex-adjusted HR (95% CI) |
1 (ref.) |
0.86 (0.20 to 3.76) |
2.08 (0.80 to 5.40) |
3.62* (1.63 to 8.05) |
4.87* (2.47 to 9.61) |
<0.001 | 1.44* (1.25 to 1.65) |
<0.001 | |
| Non-CVD-related age-adjusted, sex-adjusted and WCC-adjusted HR (95% CI) |
1 (ref.) |
0.90 (0.21 to 3.93) |
2.24 (0.86 to 5.85) |
3.87* (1.73 to 8.64) |
5.30* (2.66 to 10.54) |
<0.001 | 1.47* (1.28 to 1.69) |
<0.001 | |
*Statistically significant after applying a Bonferroni correction.
Acute myocardial infarction: ICD-10 (I21–I24); cerebral infarction: ICD-10 (I63); CVD: International Statistical Classification of Diseases and related problems 10th revision (below ICD-10) Diseases of the circulatory system (I00-I99); heart failure: ICD-10 (I50); intracerebral haemorrhage: ICD-10 (I61).
CVD, cardiovascular disease; hs-CRP, high-sensitivity C reactive protein; WCC, white cell count.
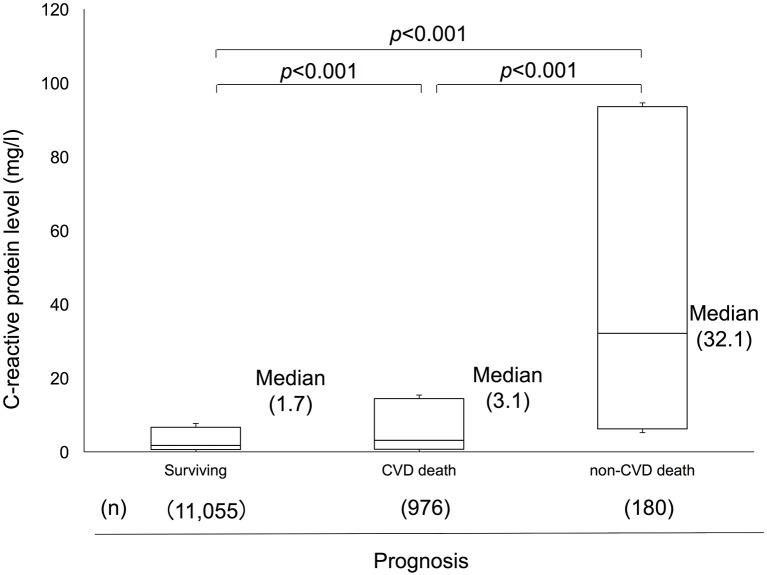
Figure 2 shows a box plot of the distribution of hs-CRP levels in the surviving patients and the CVD death and non-CVD death groups. There were significant differences in the hs-CRP levels among the three groups (p<0.001, respectively). The association was unchanged even after Bonferroni adjustment. The median hs-CRP value was 1.7 mg/L in the surviving group, 3.1 mg/L in the CVD death group, and 32.1 mg/L in the non-CVD death group. Table 3 shows number of total death, CVD death, non-CVD death and its subtypes and according to hs-CRP levels. The proportions of non-CVD death increased with hs-CRP levels: 6.4%, 8.6%, 13.5%, 19.3% and 37.5% for the above-described hs-CRP groups. Among the deaths of patients with hs-CRP levels ≥33.3 mg/L, 17.1% deaths were caused by infection, 12.1% deaths were caused by neoplasm, and 8.3% deaths were caused by other causes. The number of infection deaths showed a significant positive linear trend with the hs-CRP levels (χ2=101.7, p<0.001). Similar associations were observed for neoplasms (χ2 = 67.4, p<0.001) and the other causes group (χ2=15.7, p=0.003).
Figure 2.
A box plot of the distribution of high-sensitivity C reactive protein (hs-CRP) levels in the surviving patients and the cardiovascular disease (CVD) death and non-CVD death groups. There were significant differences in the hs-CRP levels among the three groups (p<0.001, respectively). The association was unchanged even after Bonferroni adjustment. The median hs-CRP value was 1.7 mg/L in the surviving group, 3.1 mg/L in the CVD death group and 32.1 mg/L in the non-CVD death group.
Table 3.
No of total death, CVD death, non-CVD death and its subtypes according to hs-CRP levels
| Cause of death | Total | hs-CRP levels (mg/L) | χ2test | p value | ||||
| <3.0 | 3.1–5.4 | 5.5–11.5 | 11.6–33.2 | ≥33.3 | ||||
| No of total deaths | 1156 | 517 | 116 | 134 | 149 | 240 | ||
| No of CVD deaths, n (%) | 976 (84.4) | 484 (93.6) | 106 (91.4) | 116 (86.6) | 120 (80.5) | 150 (62.5) | ||
| No of non-CVD deaths, n (%) | 180 (15.6) | 33 (6.8) | 10 (8.6) | 18 (13.4) | 29 (19.5) | 90 (37.5) | 127.9 | <0.001 |
| No of infection deaths, n (%) | 64 (5.5) | 6 (1.2) | 3 (2.6) | 5 (3.7) | 9 (6.0) | 41 (17.1) | 101.7 | <0.001 |
| No of neoplasm deaths, n (%) | 49 (4.2) | 6 (1.2) | 2 (1.7) | 5 (3.7) | 7 (4.7) | 29 (12.1) | 67.4 | <0.001 |
| No of other deaths, n (%) | 67 (5.8) | 21 (4.1) | 5 (4.3) | 8 (5.9) | 13 (8.7) | 20 (8.3) | 15.7 | 0.003 |
Infection deaths: deaths from septicaemia, bacteraemia, endocarditis, pulmonary infections (eg, viral pneumonia, bacterial pneumonia, influenza with respiratory manifestations and abscess of lung or mediastinum), genitourinary infections (eg, urinary tract infection, pyelonephritis and perinephric abscess), gastrointestinal infections (eg, diverticulitis, C. difficile colitis and perirectal abscess), peritonitis, soft-tissue infections (eg, cellulitis, necrotising fasciitis and gangrene) and joint or bone infections (eg, infective arthritis and osteomyelitis). Neoplasm deaths: deaths from International Statistical Classification of Diseases and related problems 10th revision C00–D48. Other deaths: deaths from other than the causes listed above.
CVD, cardiovascular disease; hs-CRP, high-sensitivity C reactive protein.
Discussion
The results of this large retrospective cohort study at a local Japanese teaching hospital clearly demonstrated that the risk among patients with CVD for inhospital mortality increased significantly with increasing initial hs-CRP levels taken in the ED. As with total deaths, the risks for cause-specific inhospital mortality from CVD death and non-CVD death also increased significantly as the hs-CRP levels increased. The influence of hs-CRP levels on mortality was greater in the non-CVD deaths than in the CVD deaths.
These findings provide important information regarding critical care for patients with CVD. Prompt risk stratification is important in the management of patients with CVD in an ED. Hs-CRP is a sensitive and non-specific marker of systemic inflammation. A patient’s initial hs-CRP level may prove to be a simple and readily available adjunct that could help the emergency care staff to identify patients with CVD who may be at a high risk of death. Several studies have shown that elevated CRP levels at admission in patients with CVD, including those with acute coronary syndrome, ischaemic stroke and acute heart failure, are associated with their mortality.6 7 11 13 14 In addition, several studies have examined the utility of hs-CRP for predicting all-cause mortality in different settings.18–20 These results, together with ours, imply that CRP is a valuable biomarker for identifying patients with CVD at high risk of total inhospital death. Although hs-CRP levels are much lower in Japanese populations compared with Western populations,21 our present findings confirmed the utility of measuring the initial hs-CRP in ED settings in Japan. In the present study, initial hs-CRP levels ≥5.5 mg/L were associated with greater mortality in patients with CVD. In addition, the addition of the WCC in the adjustment did not substantially change the HRs in this study. This might be caused by the presence of leucopenia, that is, a low WCC. Although both the WCC and CRP are used as inflammatory biomarkers, patients with leucopenia as the result of a severe inflammatory response or immune suppression might have poorer prognoses.
We also observed an association between hs-CRP levels and cause-specific inhospital mortality from CVD death in this study. When the CVD cases were divided into subtypes of CVD, similar relationships were observed. The mechanisms underlying the association between hs-CRP levels and the risk of atherosclerotic CVD death are still unknown. However, there is a possibility that elevated levels of CRP reflect the extent of infarction and inflammation related to the pathobiology of ischaemic tissue damage.8 10 22 In terms of heart failure, it is known that inflammatory markers such as tumour necrosis factor, interleukin (IL)−6 and CRP are elevated in patients with congestive heart failure and correlate with the degree of heart failure.23–25 These findings and our present results raise the possibility that CRP levels are associated with the severity of CVDs that are related to broad vascular damage. An evaluation of inflammatory risk in patients with CVD should thus be routinely performed to identify high-risk patients in need of additional close monitoring. Although the results of the present study suggested that hs-CRP was a strong predictor of cardiovascular mortality, and several studies have indicated the value of determining the CRP level in patients with CVD, CRP itself is be unlikely to provide an effective target for intervention and is known to be a downstream surrogate inflammatory marker. Moving upstream in the inflammatory cascade from CRP to IL-6 and IL-1 might provide novel therapeutic opportunities to reduce the cardiovascular event rate.26 The results of ongoing clinical trials of inflammation inhibition (such as those of the phase II trial data on canakinumab, a human monoclonal antibody that targets IL-1β27) are worthy of attention.
Our present analyses also showed an association between hs-CRP levels and non-CVD deaths, and the influence of hs-CRP levels was much stronger in the non-CVD deaths than in the CVD deaths. In addition, the proportion of non-CVD deaths increased with the increase in the hs-CRP level. Although the actual number of non-CVD deaths was not very large, non-CVD deaths accounted for 37.5% of the total deaths among the patients with hs-CRP levels ≥33.3 mg/L. In addition, the median hs-CRP value was the highest in the non-CVD death group, 32.1 mg/L. hs-CRP can be elevated by underlying conditions other than CVD, such as infection, neoplasm and other diseases. In the present patient series, infection and neoplasm were major causes of non-CVD death. However, a non-CVD death cause might be regarded as a misclassification or a complication of a well-classified CVD. Generally, CVD is a common cause of death globally and has shown a relationship with CRP levels, but CRP is an extremely sensitive marker for many diagnoses—not just CVD. Therefore, the patients with CVD and elevated hs-CRP levels in the present study might have had comorbidities at the ED.
Concerning neoplasms, several studies have reported that CRP levels have prognostic value in a wide variety of operable and inoperable cancers.28–30 In the present study, some patients with CVD with elevated hs-CRP levels on admission might have had a poorer prognosis for cancer and an increased risk of death. Infections, pneumonia and urinary tract infections (UTIs) are the most common infectious complications of ischaemic stroke, and they are independently associated with stroke outcome.31 Current guidelines for the early management of patients with acute ischaemic stroke recommend that patients with suspected pneumonia or UTIs should be treated promptly with appropriate antibiotics.32 However, until, there has been no specific recommendation for the treatment of infectious complications in other subtypes of CVD, such as acute myocardial infarction, heart failure and intracerebral haemorrhage.
In the present study, in addition to cerebral infarction, similar associations were observed between hs-CRP levels and non-CVD death in other subtypes of CVD. Similarly, a prospective cohort study demonstrated that, among patients with ischaemic stroke, elevated CRP levels on admission is a predictor of pneumonia and UTI within 5 days.33 These findings imply that a search for infections and tailored treatment without delay may be indicated for all types of patients with CVD with elevated hs-CRP levels.
There are several limitations of our study that must be acknowledged. The first limitation is that this was a retrospective cohort study at a single hospital. It is possible that our medical care may be different from that at other hospitals throughout the world. However, to maintain its standard of medical care, Iizuka Hospital has affiliations with overseas medical institutions: the University of Pittsburgh Medical Center, El Camino Hospital and Virginia Mason Institute. In addition, our hospital has been designated a residency training hospital since 1989, and it is renowned in Japan as an educational hospital. We thus believe that standard medical care is provided at our hospital.
Second, in this study, the evaluation of hs-CRP values was based on a single measurement in the ED. Since the time to reach the peak hs-CRP level may differ according to the underlying diseases in individual patients, it is possible that the initial hs-CRP levels do not precisely reflect the pathological condition in each disease. This could have weakened the association found in this study, biasing the results towards the null hypothesis. Therefore, the true association may be stronger than that shown in our study.
Third, confounders and covariates other than age, sex and WCC could not be adjusted in this study. It is not our intention to suggest that CRP can replace the clinical evaluation of individual patients with CVD. Rather, we simply report that, in a large cohort, the hs-CRP level was associated with inhospital mortality. Ideally, our analysis would have assessed whether the hs-CRP measurement added prognostic information beyond commonly used risk assessment scoring systems such as the Acute Physiology and Chronic Health Evaluation. Unfortunately, data such as haemodynamics and comorbidities could not be analysed in this study, so we elected instead to focus our analysis only on laboratory data that are routinely available. Further clinical and laboratory investigations are required to explain the association between mortality and the initial ED-measured hs-CRP level in patients with CVD.
Conclusion
The results of the present study clearly demonstrated the potential utility of hs-CRP measurement in the ED triage for patients with CVD as well as its subtypes, that is, acute myocardial infarction, heart failure, cerebral infarction and intracerebral haemorrhage. The assessment of hs-CRP at baseline even in patients with CVD may improve the ability to identify patients at high risk of death from not only the primary CVD but also other systemic complications.
Supplementary Material
Footnotes
Contributors: Conceptualisation, methodology, validation, writing and visualisation: RY, YD, KA and SI. Formal analysis: RY, YD and SI. Supervision: YD, KA and SI.
Competing interests: None declared.
Patient consent: The requirement of informed consent was waived by the ethic committee because of retrospective nature of the study.
Ethics approval: Ethics Committee of Iizuka Hospital.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Ridker PM, Cushman M, Stampfer MJ, et al. . Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 1997;336:973–9. 10.1056/NEJM199704033361401 [DOI] [PubMed] [Google Scholar]
- 2. Lindahl B, Toss H, Siegbahn A, et al. . Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC Study Group. Fragmin during Instability in Coronary Artery Disease. N Engl J Med 2000;343:1139–47. 10.1056/NEJM200010193431602 [DOI] [PubMed] [Google Scholar]
- 3. Boekholdt SM, Hack CE, Sandhu MS, et al. . C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women: the EPIC-Norfolk prospective population study 1993-2003. Atherosclerosis 2006;187:415–22. 10.1016/j.atherosclerosis.2005.09.023 [DOI] [PubMed] [Google Scholar]
- 4. Sabatine MS, Morrow DA, Jablonski KA, et al. . Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation 2007;115:1528–36. 10.1161/CIRCULATIONAHA.106.649939 [DOI] [PubMed] [Google Scholar]
- 5. Liuzzo G, Biasucci LM, Gallimore JR, et al. . The prognostic value of C-reactive protein and serum amyloid a protein in severe unstable angina. N Engl J Med 1994;331:417–24. 10.1056/NEJM199408183310701 [DOI] [PubMed] [Google Scholar]
- 6. Nikfardjam M, Müllner M, Schreiber W, et al. . The association between C-reactive protein on admission and mortality in patients with acute myocardial infarction. J Intern Med 2000;247:341–5. 10.1046/j.1365-2796.2000.00670.x [DOI] [PubMed] [Google Scholar]
- 7. Chew DP, Bhatt DL, Robbins MA, et al. . Incremental prognostic value of elevated baseline C-reactive protein among established markers of risk in percutaneous coronary intervention. Circulation 2001;104:992–7. 10.1161/hc3401.095074 [DOI] [PubMed] [Google Scholar]
- 8. Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke 2001;32:917–24. 10.1161/01.STR.32.4.917 [DOI] [PubMed] [Google Scholar]
- 9. Audebert HJ, Rott MM, Eck T, et al. . Systemic inflammatory response depends on initial stroke severity but is attenuated by successful thrombolysis. Stroke 2004;35:2128–33. 10.1161/01.STR.0000137607.61697.77 [DOI] [PubMed] [Google Scholar]
- 10. Keskin O, Ulusoy RE, Kalemoglu M, et al. . White blood cell count and C-reactive protein predict short-term prognosis in acute myocardial infarction. J Int Med Res 2004;32:646–54. 10.1177/147323000403200610 [DOI] [PubMed] [Google Scholar]
- 11. Masotti L, Ceccarelli E, Forconi S, et al. . Prognostic role of C-reactive protein in very old patients with acute ischaemic stroke. J Intern Med 2005;258:145–52. 10.1111/j.1365-2796.2005.01514.x [DOI] [PubMed] [Google Scholar]
- 12. Canale ML, Stroppa S, Caravelli P, et al. . Admission C-reactive protein serum levels and survival in patients with acute myocardial infarction with persistent ST elevation. Coron Artery Dis 2006;17:693–8. 10.1097/01.mca.0000236286.48812.8c [DOI] [PubMed] [Google Scholar]
- 13. Elkind MS, Tai W, Coates K, et al. . High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med 2006;166:2073–80. 10.1001/archinte.166.19.2073 [DOI] [PubMed] [Google Scholar]
- 14. Idicula TT, Brogger J, Naess H, et al. . Admission C-reactive protein after acute ischemic stroke is associated with stroke severity and mortality: the ’Bergen stroke study'. BMC Neurol 2009;9:18 10.1186/1471-2377-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sheikh AS, Yahya S, Sheikh NS, et al. . C-reactive Protein as a Predictor of Adverse outcome in Patients with Acute Coronary Syndrome. Heart Views 2012;13:7–12. 10.4103/1995-705X.96660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park JJ, Choi DJ, Yoon CH, et al. . Prognostic value of C-reactive protein as an inflammatory and N-terminal probrain natriuretic peptide as a neurohumoral marker in acute heart failure (from the Korean Heart Failure registry). Am J Cardiol 2014;113:511–7. 10.1016/j.amjcard.2013.10.022 [DOI] [PubMed] [Google Scholar]
- 17. Buckley DI, Fu R, Freeman M, et al. . C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med 2009;151:483–95. 10.7326/0003-4819-151-7-200910060-00009 [DOI] [PubMed] [Google Scholar]
- 18. Koenig W, Khuseyinova N, Baumert J, et al. . Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984-1998. Clin Chem 2008;54:335–42. 10.1373/clinchem.2007.100271 [DOI] [PubMed] [Google Scholar]
- 19. Marsik C, Kazemi-Shirazi L, Schickbauer T, et al. . C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem 2008;54:343–9. 10.1373/clinchem.2007.091959 [DOI] [PubMed] [Google Scholar]
- 20. Ridker PM. High-sensitivity C-reactive protein as a predictor of all-cause mortality: implications for research and patient care. Clin Chem 2008;54:234–7. 10.1373/clinchem.2007.099465 [DOI] [PubMed] [Google Scholar]
- 21. Kaptoge S, Di Angelantonio E, Lowe G, et al. . C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 2010;375:132–40. 10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 1999;22:391–7. 10.1016/S0166-2236(99)01401-0 [DOI] [PubMed] [Google Scholar]
- 23. Tsutamoto T, Hisanaga T, Wada A, et al. . Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol 1998;31:391–8. 10.1016/S0735-1097(97)00494-4 [DOI] [PubMed] [Google Scholar]
- 24. Vasan RS, Sullivan LM, Roubenoff R, et al. . Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation 2003;107:1486–91. 10.1161/01.CIR.0000057810.48709.F6 [DOI] [PubMed] [Google Scholar]
- 25. Yin WH, Chen JW, Jen HL, et al. . Independent prognostic value of elevated high-sensitivity C-reactive protein in chronic heart failure. Am Heart J 2004;147:931–8. 10.1016/j.ahj.2003.11.021 [DOI] [PubMed] [Google Scholar]
- 26. Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res 2016;118:145–56. 10.1161/CIRCRESAHA.115.306656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ridker PM, Howard CP, Walter V, et al. . Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation 2012;126:2739–48. 10.1161/CIRCULATIONAHA.112.122556 [DOI] [PubMed] [Google Scholar]
- 28. Heikkilä K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health 2007;61:824–33. 10.1136/jech.2006.051292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149–63. 10.2217/fon.09.136 [DOI] [PubMed] [Google Scholar]
- 30. Clarke SJ, Chua W, Moore M, et al. . Use of inflammatory markers to guide cancer treatment. Clin Pharmacol Ther 2011;90:475–8. 10.1038/clpt.2011.122 [DOI] [PubMed] [Google Scholar]
- 31. Aslanyan S, Weir CJ, Diener HC, et al. . Pneumonia and urinary tract infection after acute ischaemic stroke: a tertiary analysis of the GAIN International trial. Eur J Neurol 2004;11:49–53. 10.1046/j.1468-1331.2003.00749.x [DOI] [PubMed] [Google Scholar]
- 32. Jauch EC, Saver JL, Adams HP, et al. . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 33. Fluri F, Morgenthaler NG, Mueller B, et al. . Copeptin, procalcitonin and routine inflammatory markers-predictors of infection after stroke. PLoS One 2012;7:e48309 10.1371/journal.pone.0048309 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.