Abstract
Introduction
The growing number of smartphone health applications available in the app stores makes these apps a promising tool to help reduce the global problem of non-adherence to long-term medications. However, to date, there is limited evidence that available medication reminder apps are effective. This study aims to determine the impact of medication reminder apps on adherence to cardiovascular medication when compared with usual care for people with coronary heart disease (CHD) and to determine whether an advanced app compared with a basic app is associated with higher adherence.
Methods and analysis
Randomised controlled trial with follow-up at 3 months to evaluate the feasibility and effectiveness of medication reminder apps on medication adherence compared with usual care. An estimated sample size of 156 patients with CHD will be randomised to one of three groups (usual care group, basic medication reminder app group and advanced medication reminder app group). The usual care group will receive standard care for CHD with no access to a medication reminder app. The basic medication reminder app group will have access to a medication reminder app with a basic feature of providing simple daily reminders with no interactivity. The advanced medication reminder app group will have access to a medication reminder app with additional interactive and customisable features. The primary outcome is medication adherence measured by the eight-item Morisky Medication Adherence Scale at 3 months. Secondary outcomes include clinical measurements of blood pressure and cholesterol levels, and medication knowledge. A process evaluation will also be performed to assess the feasibility of the intervention by evaluating the acceptability, utility and engagement with the apps.
Ethics and dissemination
Ethical approval has been obtained from the Western Sydney Local Health Network Human Research Ethics Committee (AU/RED/HREC/1/WMEAD/3). Study findings will be disseminated via usual scientific forums.
Trial registration number
ACTRN12616000661471; Pre-results
Keywords: Medication adherence, medication compliance, mobile phone, smartphone, apps, applications, mHealth, eHealth, coronary heart disease, randomised controlled trial
Strengths and limitations of this study.
Recently smartphone apps have been proposed as potential tools to improve adherence; therefore, in this study, an innovative strategy using medication reminder apps to improve the global problem of medication non-adherence will be investigated in a population with coronary heart disease.
To design this study, a systematic search and a stepwise process to identify high-quality medication reminder apps was conducted, in which the apps were classified into basic medication reminder apps with no interactivity and advanced medication reminder apps with additional interactive and customisable features.
A basic and an advanced medication reminder apps will be used in the study, and a process evaluation will provide insights on which characteristics and features of medication reminder apps are more likely to be useful, increase patient engagement and optimise effects.
Medication adherence will be measured using a validated self-report questionnaire; however, more objective outcomes, such as prescription refills data and clinical measurements of blood pressure and cholesterol levels, will also be measured.
This study is limited by its small sample size and short-term follow-up; however, the results of this study will provide preliminary data essential to design a bigger trial with a longer term follow-up.
Introduction
Cardiovascular disease (CVD) and medication adherence
Coronary heart disease (CHD) is the leading cause of death worldwide, accounting for more than eight million deaths in 2015.1 After an acute coronary event, about half of patients have recurrent events, which can be reduced by adhering to a healthy lifestyle and to evidence-based cardiovascular medication.2 However, non-adherence to long-term therapies is a global concern especially in chronic diseases,3 4 such as CVDs,5 6 as highlighted by the WHO report in 2003.7 In Australia, it is estimated that 14%–43% of the patients do not adhere to their cardiovascular medication after 12–24 months.8
Given this suboptimal adherence to cardiovascular medication, a growing body of research has been investigating different interventions to improve medication adherence. Recently, a meta-analysis of randomised controlled trials (RCTs) investigating interventions to improve adherence to multiple cardiovascular medication in a CHD population found that interventions significantly improved the odds of being adherent; however, the interventions varied widely, and the majority of the interventions were complex, comprising several components.9 When compared with simple one-component interventions, such as a text message intervention, the complex interventions were found to have similar results.
mHealth interventions
In 2014, there were around 7 billion mobile phone subscriptions globally.10 In addition, mobile subscriptions with internet access were expected to reach 2.3 billion in 2014.10 In Australia, 86% of the household access the internet via mobile or smartphones.11 This growing mobile phone ownership makes it a promising tool to deliver healthcare interventions, so-called mHealth interventions. mHealth interventions can be used to deliver education to patients and health professionals, to collect data, to diagnose, screen and monitor patients, to offer treatment and behavioural change support, as well as to facilitate communication between patients, health professionals and health services.12 Systematic reviews have shown that interventions delivered by mobile phone text messages can improve appointment attendance,13 preventive care14 and smoking cessation.15
mHealth interventions can also be used to improve medication adherence by sending regular reminders to the patients aiming to reduce forgetfulness, a known non-intentional reason for non-adherence. A meta-analysis of text messaging interventions to improve adherence to medication in chronic diseases showed that text message reminders were associated with increased odds of being adherent.16 Although cost-effectiveness analyses of mHealth interventions are limited, a text messaging intervention in a population with CHD has been shown to be cost-saving.17
Smartphone apps for medication adherence
More recently, the use of smartphone apps has been proposed as a potential tool to improve adherence. The increasing popularity of apps is evident as there has been exponential growth in numbers of apps available in online stores. This plethora of health apps has triggered a growing interest in mHealth research; however, the majority of research has focused on designing and testing new mHealth interventions instead of testing what is already available to the general public. There has also been relatively little research that examines which characteristics and features make health apps more effective. To design this study, we first conducted a systematic search and a stepwise process to identify high-quality medication reminder apps.18 We identified around 300 medication adherence apps, of which about half were broadly classified into those with basic features of simple timed medication reminders and the other half into those with more advanced features including interactivity and customisation.
Although these medication reminder apps are readily available to the public and have the potential to improve the effectiveness and reduce the costs of traditional interventions to improve medication adherence,19 there is no published evidence that such apps are effective in improving medication adherence. In addition, it is important to investigate whether having medication information in the app is associated with improvements in medication knowledge, and whether daily reminders will be well accepted by patients. In a population with CHD, who are usually overwhelmed with a high pill burden and complex medication regimens, such apps might be a successful strategy to improve medication adherence and potentially improve clinical outcomes and reduce healthcare costs.
Aims
To determine whether medication reminder apps compared with usual care alone will improve adherence to cardiovascular medications at 3 months;
to determine whether an advanced medication reminder app with additional interactive and customisable features compared with a basic medication reminder app with no interactivity is associated with higher adherence;
to determine the impact of the apps on blood pressure (BP) and cholesterol levels at 3 months;
to determine whether the intervention is associated with higher medication knowledge;
to determine whether the intervention is associated with lower health services utilisation;
to determine feasibility of the intervention by evaluating the acceptability, utility and engagement with the apps.
Methods and analysis
Study design
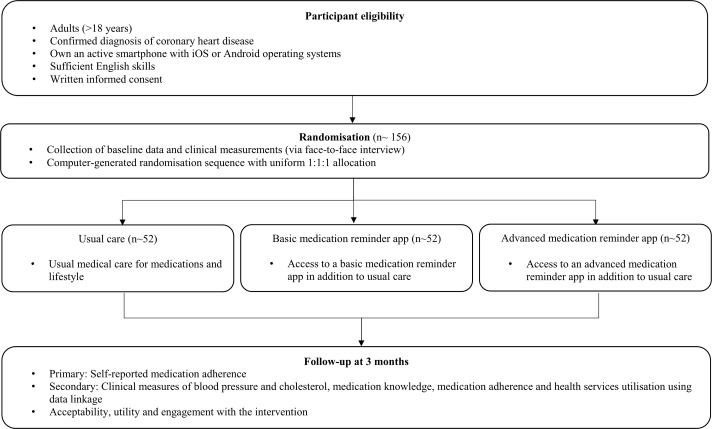
The MEDication reminder APPs to improve medication adherence in Coronary Heart Disease (MedApp-CHD) Study is designed as parallel, prospective, single-blind RCT (figure 1). The study will be conducted at a large urban tertiary public hospital in Sydney, Australia, that serves a culturally and socioeconomically diverse population. A total of 156 patients attending the hospital with a diagnosis of CHD will be randomised to one of three groups (usual care group, basic medication reminder app group and advanced medication reminder app group). The usual care group will receive standard care for CHD with no access to a medication reminder app. The basic medication reminder app group will have access to a medication reminder app with a basic feature of providing simple daily reminders with no interactivity. The advanced medication reminder app group will have access to a medication reminder app with additional interactive and customisable features.
Figure 1.
MedApp-CHD flow diagram. MedApp-CHD, MEDication reminder APPs to improve medication adherence in Coronary Heart Disease.
Randomisation and blinding
Randomisation will be performed using a central password-protected web-based computerised randomisation program with a 1:1:1 allocation ratio. There will be no stratification for the randomisation procedure, and group allocation will be concealed from the study personnel. Due to the nature of the intervention, it is not possible to blind participants to group allocation. However, to minimise bias, at the 3-month follow-up visit, outcome assessors will be blinded to group allocation and objective clinical measures will be measured. In addition, analyses will be conducted by investigators who will be also blinded.
Study population
Participants will be eligible to participate in the study if they have a confirmed diagnosis of CHD, including a prior diagnosis of myocardial infarction, unstable or stable angina, coronary revascularisation procedure or >50% stenosis in at least one major vessel on a coronary angiogram. In addition, participants must meet the following criteria: (1) be an adult (>18 years), (2) own an active smartphone that operates with iOS or Android operating systems, (3) have sufficient English language skills and (4) provide written informed consent. Patients will be excluded if they already use a medication reminder app or other electronic reminder systems to help them adhere to their medication regimen, for example, calendar alerts in their phones. In addition, smartphones that are not capable of downloading the apps, for example, older Apple iPhones, such as iPhone 4, that operate with older iOS versions will be excluded, as the basic app is only compatible with iOS version 7.0 or later and the advanced app with iOS version 8.0 or later.
Recruitment
Potential participants will be any patient presenting to the hospital with CHD. Participants will be identified by screening daily admissions in the cardiology wards, coronary angiogram case lists and cardiology and cardiac rehabilitation outpatient clinics. Eligible participants will be approached either in person or by phone by the researcher responsible for recruitment while the participant is still in hospital, before or after their outpatient clinics or cardiac rehabilitation appointments. Interested individuals will be invited to an initial face-to-face interview at the hospital. At this initial visit, written informed consent will be obtained, and a baseline assessment will be completed by the research assistant. A record of basic demographic information and reasons for non-participation will be kept for those who are ineligible or who decline to participate in the study.
Interventions
There will be three treatment groups in the study, which are: usual care group, basic medication reminder app group and advanced medication reminder app group. The basic and advanced medication reminder apps being used in this study were selected through a systematic review and stepwise process to identify high-quality apps. This process has been described previously,18 but in brief, it consisted of: (1) a search strategy, (2) assessment of eligibility criteria, (3) app selection process, (4) data extraction to evaluate the presence of features considered important in medication reminder apps, (5) analysis including classification of the apps as basic and advanced medication reminder apps, scoring and ranking and (6) a quality assessment by using the Mobile App Rating Scale.20 Among the 272 medication reminder apps identified in this review, the best basic and advanced apps were selected to be used in this study. The features present in each app are presented in table 1.
Table 1.
Characteristics and features of the basic and advanced medication reminder apps18
| Basic app | Advanced app | |
| Availability in the app stores: | ||
| Available in both iTunes and Google Play app stores | ✓ | ✓ |
| Available for free | ✓ | ✓ |
| App updated in 2016 | ✓ | ✓ |
| Medicine-related features: | ||
| Ability to add a medication list from an in-app medication database | ✓ | ✓ |
| Ability to schedule reminders to occur on a non-daily basis | ✓ | ✓ |
| Ability to track taken or missed doses | ✓ | |
| Ability to snooze the reminder | ✓ | |
| Ability to set refill reminders | ✓ | |
| Consumer medicine information | ✓ | ✓ |
| Customisable reminder alert sounds | ✓ | |
| Availability of icons or a picture to provide visual clues to ensure the correct medication is taken | ✓ | ✓ |
| Ability to change time zones to ensure medication is taken at the right time when travelling | ✓ | |
| No internet connection required for the reminders to function | ✓ | |
| Adherence statistics and charts | ✓ | |
| Ability to export/share the medication information to a third party | ✓ | |
| Ability to generate medication reminders to more than one user | ✓ | |
| Availability of an option to alert other people about when missed doses are registered | ✓ | |
| Other features: | ||
| Languages other than English | ✓ | |
| Password protection | ✓ | |
| Ability to track clinical measurements such as blood pressure and weight | ✓ | ✓ |
| Ability to set appointment reminders | ✓ | |
| Ability to add personal health information | ✓ | |
| Information about general health | ✓ | |
| Information about heart disease | ✓ | |
| Information about healthy diet | ✓ |
Usual care group
Participants allocated to the usual care group will receive standard care for their cardiovascular health as determined by their doctors, including cardiovascular medication prescription and advice, as well as lifestyle counselling. The participants in this group will have no access to a medication reminder app.
Basic medication reminder app group
Participants allocated to the basic medication reminder app group will have access to a basic medication reminder app in addition to standard care. The basic medication reminder app has a basic feature, which is the ability to provide simple daily reminders with no interactivity to reinforce correct medication-taking behaviour. The basic app reminders will act similarly to an alarm or text message to remind the patients when it is time to take their medications. The basic app provides a one-time only reminder at each scheduled time, with no ability to snooze or reschedule the reminder. There is also no ability to register whether the medication was taken or not, once the reminder alerts the patient to take the medication. As the basic app was developed by a non-for-profit Australian organisation,18 it has a database of medications available in the Australian market, which includes consumer medicine information for some of the medications, providing additional information on what the medication is used for, how it works and side effects. In addition, the basic app has non-medication-related features (table 1), including: (1) ability to track clinical measurements, in which the patients can, for example, record BP levels in the app whenever they measure their BP either at home or during a medical consultation, (2) information content about heart attack symptoms and action plan and (3) healthy diet information with recipe suggestions. At the initial visit, after the baseline assessment and concealed randomisation, the researcher will provide an initial training session on how to use the basic app as described in the ‘apps training session’ section below.
Advanced medication reminder app group
Participants allocated to the advanced medication adherence app group will have access to an advanced medication reminder app in addition to standard care. The advanced medication reminder app has additional features that make it more interactive and customisable than the basic app. These additional features aim to support prospective memory, which is the ability to remember to do something in the future, by providing daily reminders for medication taking, as well as retrospective memory, which is the ability to remember something that was done in the past, by tracking taken or missed medication doses. This important tracking feature allows the patients to record whether the medication was taken and at what time it was taken, providing a tool for the patients to better control their medication adherence. The advanced app also allows that patients to snooze the reminder, if they cannot take their medication immediately after the reminder alerts them. By default, if the patient does not click that he/she took the medication when the first reminder pops in the smartphone screen, the reminder will be repeated up to three times at 10 min intervals or until the patient registers that the medication was taken, whichever occurs first. However, the snooze time length can be customised according to the patient’s preference, for example, every 5 min. Importantly, if the patient does not confirm that they have ‘taken’ their medication after the three default reminders, the dose is counted as ‘missed’.
Similar to the basic app, the advanced app has a medication database and provides consumer medicine information in a written form or in videos. Other medication-related features are (table 1): (1) refill reminders, in which the patients can set up reminders to alert them when they are running out of pills and prompt them to refill their prescriptions at a pharmacy; (2) medication adherence statistics, where the patients can check their weekly adherence rates based on the number of ‘taken’ and ‘missed’ doses; (3) exporting and sharing data, where the patients can send their medication list and adherence reports to family members or healthcare providers via email and (4) the ability to provide alerts to other people, for example, a family member, when the patient misses a dose, which can provide peer support to encourage medication adherence.
The advanced app also has non-medication-related features (table 1), including: (1) the ability to track clinical measurements, like the basic app; (2) the ability to record doctors and appointment details and set up appointment reminders; (3) the ability to record personal medical information in the ‘diary’ section of the app and (4) information content on general health, provided as health tips that are regularly updated in the app. At the initial visit, after the baseline assessment and concealed randomisation, the researcher will provide an initial training session on how to use the advanced app as described in the ‘apps training session’ section below.
Apps training session
The training session will be provided in the same manner for both the basic and advanced medication reminder app groups. The researcher will help the participant to download the allocated app through the patient’s smartphone app store, for example, the iTunes app store for smartphones that operate with iOS (eg, iPhones) and the Google Play Store for smartphones that operate with Android system (eg, Samsung, HTC, Huawei and Sony). Once the app is downloaded, the researcher will demonstrate to the patient how to input their current list of medications into the app, by searching the medication’s name and dosage in the app’s medication database. Next, the researcher will demonstrate how to select the appropriate dose frequency (eg, once daily) and quantity of pills per dose, as well as how to set the time that the medication is usually taken (eg, 08:00). The researcher will help the participants to enter the indicated cardiovascular medications prescribed by their doctors, including antiplatelets, ACE inhibitor or angiotensin receptor blocker, beta-blocker and statin. Non-cardiovascular medications will also be inserted if the participant wishes. The researcher will always attempt to enter all the prescribed cardiovascular medication; however, if after the demonstration of how to input one medication the patient feels confident, he/she can insert the rest of the medications by himself/herself after the training is finished, he/she will be welcomed to do so. The researcher will also demonstrate how to edit or delete a medication if the medication dosage or timing is changed or suspended by the treating doctor. Finally, the researcher will demonstrate how to access the consumer medicine information available for some of the medications. After entering the medications in the app, the researcher will point out that every day, at the scheduled time, a reminder will pop-up on their smartphone screen, alerting them that it is time to take their medication. It will be emphasised that the reminders are set up by time, meaning that at 08:00, for example, the reminder will alert them to take all the medications scheduled for that time.
For the advanced reminder app group, after setting up the reminders for the cardiovascular medication, the researcher will demonstrate to the participants how to track if they have taken their daily doses. Every time that a reminder pops-up on their smartphone screen, the participant will be instructed to click on one of the following options: (1) take, (2) snooze or (3) skip the dose. The researcher will also show that a dose is confirmed as ‘taken’ with a green check mark on the top of each pill icon in the app. In addition, it will be shown that the patients can access this information by viewing the medication adherence statistic feature, which will show a percentage of pills taken per week. Other medication-related features, mentioned above in the ‘Advanced medication reminder app group’ section, will also be demonstrated to the participants; however, it will be explained that these are optional features that they can use according to their own needs.
As part of the training, the researcher will also explain that the participants can use the non-medication-related features of the app if they wish; however, they are not required to use these features regularly for the purposes of the study.
Study outcomes
The primary outcome is self-reported medication adherence measured by the eight-item Morisky Medication Adherence Scale21–23 (box 1). Medication adherence will also be a secondary outcome by asking the participants how many pills they have missed in the previous 7 days at 3 months and by calculating the proportion of days covered (PDC)24 using prescription refill data obtained through data linkage with the Australian Pharmaceutical Benefits Scheme (PBS) at 3, 6 and 12 months. In Australia, prescription refills for cardiovascular medications are dispensed every month at the pharmacy as a 28-day or 30-day supply, and a record of each dispensing is included in the PBS data, enabling the calculation of PDC at 3, 6 and 12 months. Other secondary outcomes include clinical measurements of BP and cholesterol levels, and medication knowledge.
Box 1. Primary and secondary outcomes.
Primary
Medication adherence: self-reported eight-item Morisky Medication Adherence Scale.
Secondary
Systolic blood pressure: resting, sitting digital recording, mean of two readings.
Diastolic blood pressure: resting, sitting digital recording, mean of two readings.
Low-density lipoprotein-cholesterol: fasting blood sample.
Total cholesterol: fasting blood sample.
Number of pills missed in the last 7 days: self-reported questionnaire.
Proportion of days covered: prescription refill data through Pharmaceutical Benefits Scheme data linkage.
Health services utilisation: Medicare Benefits Scheme data linkage.
Medication knowledge: self-reported questionnaire.
Acceptability, utility and engagement with the intervention: self-reported questionnaire and focus groups.
Participants will be followed up 3 months after enrolment at a face-to-face interview with a researcher who is blinded to the group allocation. The data collection will include self-reported medication adherence questionnaires, as well as clinical assessments of the BP and heart rate. A medication knowledge questionnaire, adapted from a validated questionnaire,25 asking whether the participants know the names of their prescribed medications and what they are for, will also be administered. Participants will also be asked about the occurrence of any recent cardiovascular clinical events and hospitalisations, and a fasting blood sample will be collected to check the participants’ cholesterol levels. At the end of the interview, after the blinded follow-up assessment, the participants will be asked which group they were assigned to. To avoid cross-group contamination, the researcher will not mention the names of the apps being used in the study during the enrolment visit. This strategy may not ensure that participants allocated to the control group will not download another medication reminder app during the study; however, at the follow-up interview, control patients will be asked whether they downloaded and used any medication reminder app during the study period. Participants allocated to the basic and advanced medication reminder app groups will be asked to complete an additional self-reported questionnaire about the acceptability and utility of the smartphone app. Participants who are unable to attend a face-to-face interview will be asked to answer the questionnaires over the phone, and the clinical information will be obtained through their medical practitioner. At the end of the study, data about prescription refill rates and health services utilisation will also be obtained through data linkage with the Australian Medicare Benefits Scheme (MBS) and PBS. All data will be collected in paper case report forms, and the data will be entered in a secure password-protected server-based database.
Statistical considerations
The primary analysis will be unadjusted, following an intention-to-treat principle, in which the participants will be analysed in the group that they were randomised to. If there are clear differences in baseline characteristics between groups, then sensitivity analyses with additional adjustments for baseline characteristics will be performed. Continuous variables will be analysed using independent t-tests and categorical variables using χ2 tests as appropriate. Mann-Whitney U tests will be used if data are not normally distributed. We will also calculate relative risks, 95% CIs and two-sided p values for both primary and secondary outcomes. We will also perform a sensitivity analysis, in which participants with missing data for medication adherence at the follow-up, for example, patients who withdrew from the study or were loss of follow-up, will be considered non-adherent (non-responder imputation). Prespecified subgroup analyses will be performed for age, sex, level of education, adherent/non-adherent participants at baseline, advanced medication reminder app/basic medication reminder app, advanced medication reminder app/usual care and basic medication reminder app/usual care. The criterion for statistical significance will be set at α 0.05.
Sample size calculations are focused on the difference between the proportion of adherent patients when comparing the control group to the intervention groups (basic and advanced medication reminder groups). The calculations assume a ratio of 2:1 for intervention and control subjects and that 50% of patients are fully adherent to all cardiovascular medication. Analysis of pooled data of previous studies that investigated text message reminders in a population with CHD demonstrated significant improvements in adherence with an estimated absolute improvement of 29%.26–28 Therefore, a total sample size of 156 patients is needed to provide 90% power to detect an increase in the proportion of adherent patients from 50% to 79%, with a two-sided α of 0.05 and allowing for a 20% loss of follow-up. Calculations were performed using PASS statistical software V.15.0.2.
Process evaluation
A process evaluation will also be performed to assess the feasibility of the intervention by evaluating the acceptability, utility and engagement with the apps. After the blinded 3-month follow-up assessment, all participants in the basic and advanced medication reminder app groups will be administered an additional study questionnaire, adapted from a previous study (see online supplementary appendix 1).29 30 In addition, a sample of the intervention patients will be invited to participate in focus groups, which will explore in more depth several aspects of the intervention. In this process evaluation, we will examine acceptability and utility of the intervention by asking the participants whether the participants liked or disliked the app, whether the repeated medication reminders were acceptable to them or found to be intrusive, whether they believe the reminders improved their medication-taking behaviour, their opinion on the timing and frequency of the reminders, how was their experience of using the app and which features they used the most, as well as, whether it was easy to use the app, whether there were any technical difficulties while using the app and whether the patients would recommend the app to family and friends. The participants in the advanced app group will also answer additional questions in regards to the additional features of the app, including the ability to track taken and missed doses, snooze the reminders and share medication history with family members and medical practitioners. Engagement with the apps will also be evaluated by analysing the usage patterns data obtained from the app developers, including how often participants used the app and which features were used the most. This qualitative evaluation will give insights into the role of interactivity and customisation, as well as identify the features that may make medication reminder apps more effective.
bmjopen-2017-017540supp001.pdf (698.1KB, pdf)
Ethics and dissemination
The findings of this study will be disseminated via scientific forums including peer-reviewed publications and presentations at international and national conferences. The study will be administered at Westmead Hospital, with design and conduct overseen by a steering committee (authors) from The George Institute for Global Health. This committee has expertise in large-scale clinical trials, qualitative research and clinical CVD management. This study will adhere to the National Health and Medical Research Council ethical guidelines for human research. Ethical approval has been obtained from the Western Sydney Local Health Network Human Research Ethics Committee (AU RED HREC/1/WMEAD/3). Written informed consent will be obtained from all enrolled participants. Additional individual informed consent for data linkage through the Australian MBS and PBS will also be obtained. Clinical trial registration number: ACTRN12616000661471.
Discussion and conclusion
Poor medication adherence is a common and major barrier to achieve improvement in clinical outcomes in patients with CVD. In the last decade, there has been an increasing interest in mHealth research investigating how mHealth interventions, such as text messaging interventions, can help consumers to manage their health and, more specifically, their medications. More recently, the growing market of smartphones has promoted the use of smartphone apps as potential tools to improve medication adherence. Currently, there is a plethora of health apps available to the public aimed at improving medication adherence; however, there is a lack of evidence on whether they are effective and on which apps’ characteristics and features would increase engagement and, potentially, improve effectiveness.
This study will provide new evidence from a RCT on the feasibility and effectiveness of using these commonly available medication reminder apps to improve adherence to cardiovascular medications among patients with CHD. A basic and advanced medication reminder apps, selected through a systematic and stepwise approach to identify high-quality apps, will be tested. Medication adherence will be measured using a validated self-report questionnaire21–23; however, more objective outcomes, such as prescription refills data and clinical measurements of BP and cholesterol levels, will also be measured. The process evaluation of the study will explore the acceptability, utility and engagement with the apps and will potentially give insights into the role of customisation and interactivity, as well as the important features that are essential for medication adherence apps to be effective and how they may be improved.
This study has some limitations. The study primary outcome is self-reported medication adherence and, although self-reported adherence is associated with overestimation of adherence, it is the simplest, most inexpensive and most useful method of assessing adherence in a clinical setting.3 31 We acknowledge that a 3-month period is a short-term follow-up and that the study has a small sample size; however, our findings will provide preliminary data essential to design a bigger trial with a longer term follow-up, including providing a better estimate of effect size, enabling accurate powering of a large-scale trial. We also acknowledge that the intervention is not likely to change cardiovascular clinical events and hospitalisations, given the short-term follow-up. If this intervention is shown to be feasible and acceptable, a future larger trial with hard outcomes and a cost-effectiveness evaluation can expand the knowledge in this area.
In conclusion, while the use of new technology, such as smartphone apps, is an attractive option, evidence is needed to help guide health providers and health services as to whether they should recommend such technologies to patients. Overall, this study will inform future research on the development of mHealth interventions that are likely to be transferrable across chronic diseases and open up a new approach for reinforcement of medication adherence and healthy behaviours.
Supplementary Material
Acknowledgments
The basic medication reminder app being used in this study is called My Heart My Life and was developed by the National Heart Foundation of Australia. The advanced medication reminder app being used in this study is called Medisafe and was developed by the Medisafe Inc. The authors acknowledge the hospital staff who are fully engaged in helping us achieve our target recruitment number and the patients at Westmead Hospital for their support to the study. Use of the MMAS is protected by US copyright laws. A licence agreement to use the scale was obtained from: Donald E. Morisky, ScD, ScM, MSPH, Professor, Department of Community Health Sciences, UCLA School of Public Health, 650 Charles E. Young Drive South, LosAngeles, CA 90095-1772, section.
Footnotes
Contributors: KS, CKC, JC and JR conceived the original concept of the study and the interventions. KS and KR performed the sample size calculations and will lead analysis of the results. KS, CKC and AT are supporting patient recruitment. KS drafted the protocol. All authors contributed to the scientific design of the study and the protocol development, are involved in the implementation of the project and have read and approved the final manuscript.
Funding: This study is supported by a Vanguard Grant (ID101464) funded by the National Heart Foundation of Australia. KS is funded by a University of Sydney International Postgraduate Research Scholarship. CKC is funded by a Career Development Fellowship cofunded by National Health and Medical Research Council (NHMRC) and National Heart Foundation (APP1105447). JC is a chief investigator on NHMRC programme grant (ID1052555). JR is funded by a Career Development and Future Leader Fellowship cofunded by the NHMRC and the National Heart Foundation (APP1061793).
Competing interests: None declared.
Ethics approval: The study was approved by Western Sydney Local Health Network Human Research Ethics Committee (AU RED HREC/1/WMEAD/3).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. World Health Organisation. The top 10 causes of death. Fact Sheet N° 310. 2017. http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed 21 Apr 2017).
- 2. Mozaffarian D, Benjamin EJ, Go AS, et al. . Heart disease and stroke statistics - 2015 update: a report from the American Heart Association. Circulation 2015;131:e29–322. 10.1161/CIR.0000000000000152 [DOI] [PubMed] [Google Scholar]
- 3. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–97. 10.1056/NEJMra050100 [DOI] [PubMed] [Google Scholar]
- 4. Bosworth HB, Granger BB, Mendys P, et al. . Medication adherence: a call for action. Am Heart J 2011;162:412–24. 10.1016/j.ahj.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choudhry NK, Fischer MA, Avorn J, et al. . The implications of therapeutic complexity on adherence to cardiovascular medications. Arch Intern Med 2011;171:814–22. 10.1001/archinternmed.2010.495 [DOI] [PubMed] [Google Scholar]
- 6. Chowdhury R, Khan H, Heydon E, et al. . Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J 2013;34:2940–8. 10.1093/eurheartj/eht295 [DOI] [PubMed] [Google Scholar]
- 7. World Health Organization. Adherence to long-term therapies: evidence for action. 2003. http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf (accessed 21 Apr 2017).
- 8. McKenzie SJ, McLaughlin D, Clark J, et al. . The burden of non-adherence to cardiovascular medications among the aging population in Australia: a meta-analysis. Drugs Aging 2015;32:217–25. 10.1007/s40266-015-0245-1 [DOI] [PubMed] [Google Scholar]
- 9. Santo K, Kirkendall S, Laba TL, et al. . Interventions to improve medication adherence in coronary disease patients: A systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol 2016;23:1065–76. 10.1177/2047487316638501 [DOI] [PubMed] [Google Scholar]
- 10. International Telecommunication Union. Measuring the Information Society Report. 2014. http://www.itu.int/en/ITU-D/Statistics/Documents/publications/mis2014/MIS2014_without_Annex_4.pdf (accessed 21 Apr 2017).
- 11. Australia Bureau of Statistics. Household Use of Information Technology, Australia, 2014-15. 2016. http://www.abs.gov.au/ausstats/abs@.nsf/mf/8146.0 (accessed 12 April 2017).
- 12. Santo K, Chalmers J, Chow CK, et al. . m-Health in Coronary Disease Preventive Care. Journal of Cardiology and Therapy 2015;2:459–64. 10.17554/j.issn.2309-6861.2015.02.98 [DOI] [Google Scholar]
- 13. Free C, Phillips G, Watson L, et al. . The effectiveness of mobile-health technologies to improve health care service delivery processes: a systematic review and meta-analysis. PLoS Med 2013;10:e1001363 10.1371/journal.pmed.1001363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vodopivec-Jamsek V, de Jongh T, Gurol-Urganci I, et al. . Mobile phone messaging for preventive health care. Cochrane Database Syst Rev 2012;12:CD007457 10.1002/14651858.CD007457.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whittaker R, Borland R, Bullen C, et al. . Mobile phone-based interventions for smoking cessation. Cochrane Database Syst Rev 2009;4:Cd006611. [DOI] [PubMed] [Google Scholar]
- 16. Thakkar J, Kurup R, Laba TL, et al. . Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med 2016;176:340–9. 10.1001/jamainternmed.2015.7667 [DOI] [PubMed] [Google Scholar]
- 17. Burn E, Nghiem S, Jan S, et al. . Cost-effectiveness of a text message programme for the prevention of recurrent cardiovascular events. Heart 2017;103:893–4. 10.1136/heartjnl-2016-310195 [DOI] [PubMed] [Google Scholar]
- 18. Santo K, Richtering SS, Chalmers J, et al. . Mobile Phone Apps to Improve Medication Adherence: A Systematic Stepwise Process to Identify High-Quality Apps. JMIR Mhealth Uhealth 2016;4:e132 10.2196/mhealth.6742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dayer L, Heldenbrand S, Anderson P, et al. . Smartphone medication adherence apps: potential benefits to patients and providers. J Am Pharm Assoc 2013;53:172–81. 10.1331/JAPhA.2013.12202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoyanov SR, Hides L, Kavanagh DJ, et al. . Mobile app rating scale: a new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth 2015;3:e27 10.2196/mhealth.3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morisky DE, Ang A, Krousel-Wood M, et al. . Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens 2008;10:348–54. 10.1111/j.1751-7176.2008.07572.x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Krousel-Wood M, Islam T, Webber LS, et al. . New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care 2009;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 23. Morisky DE, DiMatteo MR. Improving the measurement of self-reported medication nonadherence: response to authors. J Clin Epidemiol 2011;64:255–7. 10.1016/j.jclinepi.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pharmacy Quality Alliance. Update on medication quality measures in medicare part D plan star ratings-2016. http://pqaalliance.org/measures/cms.asp (accessed 12 Apr 2017).
- 25. Okere AN, Renier CM, Morse J. Development and Validation of a Survey to Assess Patient-Perceived Medication Knowledge and Confidence in Medication Use. J Nurs Meas 2014;22:120–34. 10.1891/1061-3749.22.1.120 [DOI] [PubMed] [Google Scholar]
- 26. Quilici J, Fugon L, Beguin S, et al. . Effect of motivational mobile phone short message service on aspirin adherence after coronary stenting for acute coronary syndrome. Int J Cardiol 2013;168:568–9. 10.1016/j.ijcard.2013.01.252 [DOI] [PubMed] [Google Scholar]
- 27. Khonsari S, Subramanian P, Chinna K, et al. . Effect of a reminder system using an automated short message service on medication adherence following acute coronary syndrome. European Journal of Cardiovascular Nursing 2015;14:170–9. 10.1177/1474515114521910 [DOI] [PubMed] [Google Scholar]
- 28. Park LG, Howie-Esquivel J, Chung ML, et al. . A text messaging intervention to promote medication adherence for patients with coronary heart disease: A randomized controlled trial. Patient Educ Couns 2014;94:261–8. 10.1016/j.pec.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 29. Chow CK, Redfern J, Hillis GS, et al. . Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 2015;314:1255–63. 10.1001/jama.2015.10945 [DOI] [PubMed] [Google Scholar]
- 30. Redfern J, Santo K, Coorey G, et al. . Factors Influencing Engagement, Perceived Usefulness and Behavioral Mechanisms Associated with a Text Message Support Program. PLoS One 2016;11:e0163929 10.1371/journal.pone.0163929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-017540supp001.pdf (698.1KB, pdf)