Abstract
Immune checkpoint blockade including programmed cell death 1 pathway inhibition with agents such as nivolumab is gaining ground in a wide array of malignancies, so far demonstrating significantly improved survival rates even in metastatic, often multiply pretreated settings.
Although targeted in nature and generally well-tolerated compared with conventional anticancer treatments, these agents are often linked to a newly emerged group of adverse reactions, referred to as immune-related adverse events, which can also affect endocrine organs. This is a case report of a patient who received nivolumab for the treatment of recurrent metastatic non-small cell lung cancer and developed primary hypothyroidism and secondary adrenal insufficiency caused by selective pituitary dysfunction (with preservation of all other endocrine functions). After hormone replacement with daily administration of T4, T3 and hydrocortisone, the patient achieved complete recovery.
Adequate characterisation of these rare yet potentially severe entities is essential for prompt diagnostic and therapeutic interventions that will permit us to fully benefit from these new agents’ therapeutic potential.
Keywords: nivolumab, lung cancer, immune-related adverse events
Introduction
Nivolumab is a human IgG4 monoclonal antibody of the family of immune checkpoint inhibitors. It acts by blocking ligand activation of programmed cell death 1 (PD-1) receptor on T cells, thus releasing an otherwise physiological immune brake employed by malignant cells to allow for tumour evasion. Nivolumab has been proven effective in various clinical settings, such as in the management of patients with inoperable or metastatic melanoma, metastatic squamous non-small cell lung carcinoma (NSCLC), renal cell carcinoma, as well as for patients with metastatic urothelial carcinoma. The manufacturers warn against potentially increased risk of severe immune-mediated reactions involving the respiratory and gastrointestinal tracts, the liver, the kidneys, as well as the thyroid gland. Early identification of side effects results in timely management with minimisation of morbidity.
Case report
A 60-year-old male patient presented with soft tissue oedema in the right cervical region at the Ioannina University Hospital in 2014. The patient was a heavy smoker (with an exposure of 86 pack-years) and a social alcohol consumer. A whole-body CT scan identified a 2.6 cm mass in the right upper lobe of the lung along with abnormally enlarged N2 mediastinal lymph nodes without further evidence of distant metastases. Bronchoscopic biopsy established the histological diagnosis of adenocarcinoma, clinically staged as T2N2M0 NSCLC (stage IIIA). Molecular analysis did not identify estimated glomerular filtration rate-activating mutations or anaplastic lymphoma kinase translocation. As the tumour was deemed unresectable, concurrent chemoradiotherapy (54 Gy, 30 fractions) with weekly cisplatin at 40 mg/m2 resulted in partial remission by Response Evaluation Criteria In Solid Tumours version 1.1.
In May 2016, a surveillance CT scan showed progressive disease with enlargement of the tumour and mediastinal lymphadenopathy and a new supraclavicular/cervical lymph node bloc. Second-line therapy with intravenous nivolumab (3 mg/kg once every two weeks) was initiated. The patient was fit (performance status (PS) 1) and tolerated the administration of nivolumab with no adverse effects and resulting stable disease at CT after seven cycles.
Following the 11th cycle of nivolumab, the patient developed symptoms of dizziness, gait instability, fatigue, anorexia, withdrawal, periodic confusion and reduced alertness. Physical examination disclosed ankle oedema and bradycardia with no focal neurological or other organ deficits. A brain CT scan ruled out central nervous system metastases. Worsening of his symptoms over the following 2 weeks prompted us to investigate serum thyroid function tests (TFTs): a markedly high value of thyroid-stimulating hormone (TSH) levels at 188 IU/L (reference range 0.38–5.33 IU/L), as well as low free T4 at 0.58 µg/dL (reference range 6.09–12.23 µg/dL) and T3 levels established the diagnosis of hypothyroidism most probably induced by nivolumab. The patient was commenced on escalating doses of levothyroxine sodium, gradually increasing from T4 50 mg to 100 mg per day within 2 weeks, and liothyronine (T3 25 mg per day). Nivolumab administration was discontinued.
Six weeks later, despite a slow and relative biochemical improvement of the patient’s TFTs (serum TSH at 20 IU/L), no clinical improvement was discernible. In fact, his symptoms deteriorated, as he developed marked fatigue, loss of appetite, joint stiffness, nausea and periodic abdominal pain. On physical examination, the patient was found to have hypotension, dehydration and skin exfoliative dermatitis. As his symptoms and signs were compatible with adrenocortical insufficiency, further hormonal investigation revealed low levels of plasma cortisol 42.3 nmol/L coupled to low levels of adrenocorticotrophic hormone (ACTH) 1.4 pg/mL (7.2–63.6 pg/mL). All other biochemical and haematological serum examinations of the patient were normal. Brain MRI was normal (see figure 1), excluding metastatic involvement of the pituitary gland but not hypophysitis, which has also been described in the absence of pituitary gland enlargement.
Figure 1.
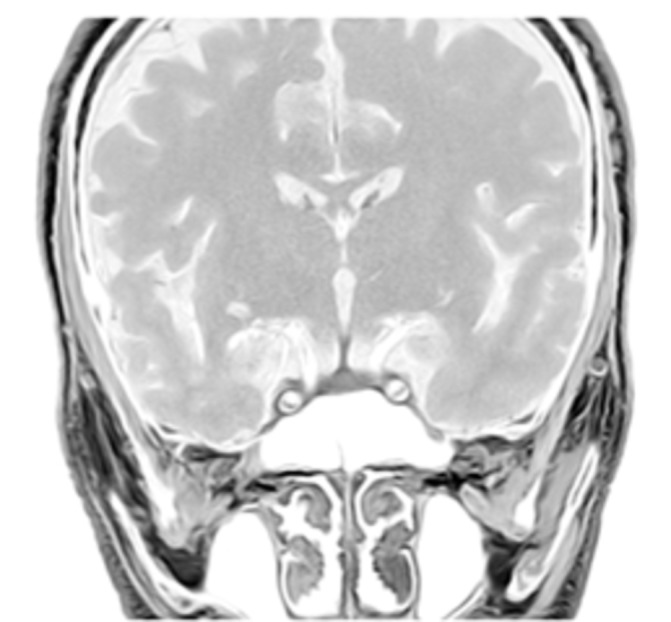
Brain MRI (sagittal plane) showing normal size of the pituitary.
The results confirmed the clinically suspected adrenal insufficiency most likely caused by selective hypopituitarism induced by the therapeutic anti-PD1 blockade. Pituitary secretion of gonadotropins (luteinizing lormone (LH) and follicular stimulating hormone (FSH)) and serum testosterone were normal. The patient was commenced on hydrocortisone replacement therapy orally at 20 mg in the morning and 10 mg in the afternoon, while continuing levothyroxine sodium (175 mg/day) and liothyronine (25 mg/day). Therapy resulted in a gradual resolution of all symptoms and signs related to hypothyroidism and adrenal failure. At physical examination in March 2017, the patient had a PS of 0, was alert, afebrile, with normal blood pressure, good appetite and disappearance of skin exfoliation (see figure 2). Regarding serum biochemistry, the TSH value was 0.94 IU/L, cortisol and ACTH within normal range (table 1, figure 3). A CT scan of the neck, chest and upper abdomen performed in April 2017 revealed stable disease in the neck, lung and mediastinum (Figure 4).
Figure 2.
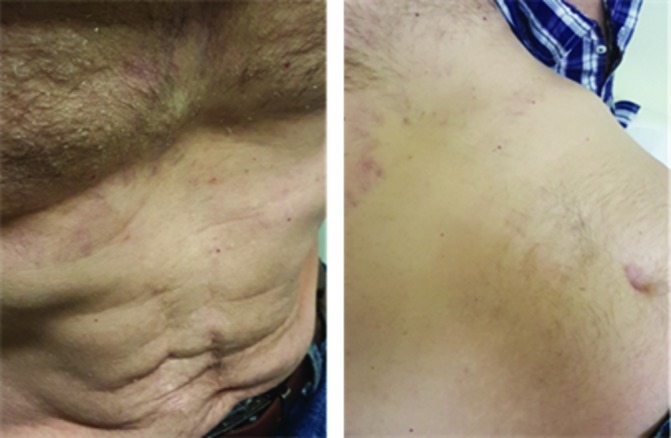
The patient’s exfoliative dermatitis at baseline (left) and in March 2017 (right).
Table 1.
Clinical and laboratory evolution over time.
| August 2016 | December 2016 | January 2017 | February 2017 | March 2017 | |
| Nivolumab administration (yes/no) | Yes | Yes | Yes | No | No |
| Performance status | 1 | 2 | 3 | 0 | 0 |
| Clinical picture | None | Dizziness, gait instability, anorexia, withdrawal, periodic confusion and reduced alertness. | Symptoms of fatigue, anorexia, joint stiffness, nausea and periodic abdominal pain; signs of hypotension, dehydration and skin exfoliative dermatitis. | Alert, afebrile, with normal blood pressure, good appetite and disappearance of skin exfoliation. | |
| TSH (IU/L) | 1.16 | 188 | 20.47 | 61.62 | 0.94 |
| T3 | ? | ||||
| fT4 (μg/dL) | 0.46 | 0.58 | |||
| Cortisol (nmol/L) | 42.3 | 32.3 | N | ||
| ACTH (pg/mL) | ?1.4 | N | |||
| T4 therapy (mg/d) | – | – | 50–100 | 175 | |
| T3 therapy (mg/d) | – | – | 25 | 25 | |
| Hydrocortisone (mg/d) | – | – | – | 20+10 | |
| Overall response | At baseline | No improvement | Improvement | Marked improvement |
TSH, thyroid-stimulating hormone; fT4, free T4.
Figure 3.
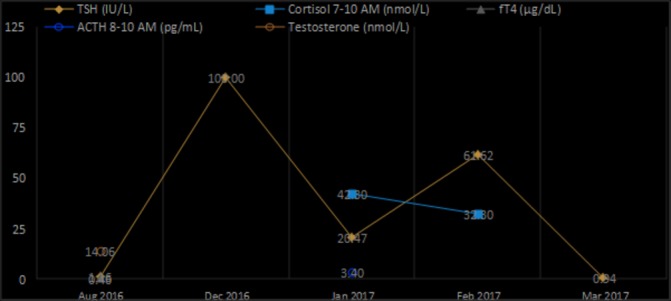
Serum hormone levels over time.
Figure 4.
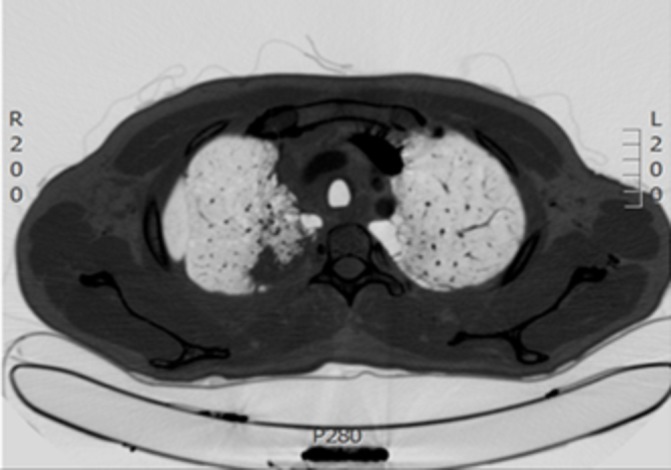
CT scan of the thorax performed in April 2017 after nivolumab therapyshowing prolonged stability of malignancy
Discussion and literature review
Immune checkpoint blockade agents have long been incriminated for opening the autoimmune Pandora’s box, with severity of the reported complications ranging from mild to fatal. We herein report a case primary hypothyroidism followed by secondary adrenal insufficiency in a patient receiving nivolumab for relapsed metastatic NSCLC. Unlike hypothyroidism, which occurs more frequently with anti-PD-1 antibodies than with ipilimumab 3 mg/kg (4%–10% vs 2%–4%, respectively, and up to 9% with ipilimumab 10 mg/kg, although never severe),1 hypophysitis has rarely been associated with anti-PD-1 agents (less than 1%, contrary to up to 4% at 3 mg/kg and 13% at 10 mg/kg of ipilimumab1). However, it has been claimed that the non-specific nature of its manifestations, many of which can often be interchangeably attributed to cancer, may account for underdiagnosis of hypophysitis.1 2
Intriguingly, a Japanese group recently described the same pattern of selective pituitary dysfunction leading to adrenal insufficiency in two patients receiving nivolumab for the treatment of metastatic melanoma.3 The researchers stressed out the absence of pituitary enlargement in brain MRI, contrary to what is usually seen in classic autoimmune and anti-CTLA4-associated hypophysitides.4 It would be interesting to examine whether selective hypopituitarism in the form of isolated ACTH deficiency is characteristic of PD-1 inhibition, as this could further imply the existence of a distinct pathogenetic mechanism from that of anti-CTLA4-related hypophysitis.
Given the high incidence of hypothyroidism in the course of anti-PD-1 treatment (around 5.9%5), monitoring TFTs in these patients is a reasonable recommendation. With primary thyroid dysfunction presenting from 3 weeks after treatment initiation to 3 years after treatment completion,1 TFTs should be monitored on a monthly basis for the first 6 months of treatment.5 However, despite its fatal potential, adrenal insufficiency is so uncommon with PD-1 antibodies, which we would not recommend routine monitoring during treatment other than a baseline measurement before treatment initiation. Evaluation should however be prompted on clinical suspicion, with early-morning cortisol, ACTH, TSH, LH and FSH, oestradiol in women and testosterone in men, in addition to prolactin. Of note, a drop in TSH may occur prior to symptom development, and the hormone should therefore be regularly assessed.1 SynACTHen test is not diagnostically useful during the early stages of pituitary damage, while the adrenals have not yet atrophied and are still capable of adequately responding to external stimulation.5 Importantly, as the role of pituitary MRI in the diagnosis of immune-related hypophysitis remains unclear, central endocrinopathies cannot be excluded on the basis of a normal-sized pituitary gland, especially in the context of anti-PD-1 treatment.
Several guidelines have been proposed for the management of immune-related endocrinopathies. Hypothyroidism is usually managed with thyroid hormone replacement with levothyroxine at 1–1.5 µg/kg and does not usually require treatment interruption, as severity grades 3–4 have never been reported. In the case of hypophysitis, all data come from ipilimumab studies; nobody would however argue that immunotherapy should be withheld. Hypophysitis not constituting an adverse event as per Common Terminology Criteria for Adverse Events version 4.0, some groups adopt a dualistic approach based on the presence of symptomatology as a synonym to mass effect. In this light, hormone replacement is favoured over high-dose corticosteroids in the absence of compression symptoms (with cortisol replacement preceding levothyroxine initiation for 1 week), so as to reserve prednisolone 1–2 mg/kg or intravenous equivalent for cases where headaches, visual disturbances or hypotension are present. In the latter case, a taper of at least 4 weeks’ duration is necessary followed by hormone replacement as needed. The same therapeutic protocol also applies to adrenal insufficiency (as was the case with our patient).
To our knowledge, there are no reports on the use of alternative immunosuppressants for the management of endocrine immune-related adverse events (irAEs), which implies a generally good response to glucocorticoids. Nonetheless, unlike most other irAEs, hypothyroidism and hypophysitis may fail to resolve, often engaging patients in long-term hormone replacement.1 6 Recovery of the pituitary–thyroid axis reportedly occurs in 37%–85% of cases, whereas the pituitary–adrenal axis is unlikely to recover.1 We currently lack data on whether high-dose steroids prevent inflammatory destruction and endocrine impairment. However, with gradual tapering and hormone replacement, immunotherapy can safely be resumed with close monitoring, except in the rare cases of adrenal crisis or other life-threatening conditions.7 Thankfully, the antitumour effect of immunotherapeutic agents seems to last beyond the actual course of treatment, as was hopefully the case with our patient.
The mechanisms underlying immune-related thyroid dysfunction remain far from being fully characterised. In a cohort of 10 patients with painless thyroiditis and hypothyroidism after treatment with a PD-1 antibody, antithyroglobulin and antithyroid peroxidase antibodies were detected in the vast majority of patients (8 out of 10), whereas assays were negative for thyrotropin-binding inhibitory immunoglobulins.8 Based on data from CTLA-4 studies, investigators have proposed that polymorphic variants in the PD1 gene in some subgroup of individuals may act as a predisposing factor for the development of various endocrine disorders.5 8
The rarity of anti-PD-1-related hypophysitis has not yet allowed the establishment of solid theories concerning its pathogenesis. Some researchers compared CTLA4-related hypophysitis with the classic lymphocytic hypophysitis and found that they both share clinical, laboratory and MRI findings (despite differences in the axis involved and the recovery rates6). It would therefore be reasonable to assume that autoimmune-mediated lymphocytic destruction of pituitary cells lies behind both entities.6 However, a distinct mechanism has been proposed to explain anti-CTLA4-related secondary hypophysitis. Iwama et al have demonstrated ectopic expression of the CTLA-4 receptor in a subset (equal to 3% and 1%, respectively) of prolactin-secreting and thyrotropin-secreting pituitary cells, in murine as well as in human tissue, both thus constituting possible targets for ipilimumab.9
In conclusion, we report a case of a patient who developed several combined autoimmune endocrinopathies induced by nivolumab. Such a coexistence of different organ-specific as well as multiple selective endocrine failures harbour the risk of rendering the diagnosis incomplete or erroneous. As improvement of the patient’s condition will only result from appropriate diagnosis and management of all endocrine deficits, a high degree of clinical suspicion is warranted.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
References
- 1.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 2016;44:51–60. 10.1016/j.ctrv.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 2.Ryder M, Callahan M, Postow MA, et al. . Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer 2014;21:371–81. 10.1530/ERC-13-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitajima K, Ashida K, Wada N, et al. . Isolated ACTH deficiency probably induced by autoimmune-related mechanism evoked with nivolumab. Jpn J Clin Oncol 2017;47:463–6. 10.1093/jjco/hyx018 [DOI] [PubMed] [Google Scholar]
- 4.Juszczak A, Gupta A, Karavitaki N, et al. . Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: a case report and review. Eur J Endocrinol 2012;167:1–5. 10.1530/EJE-12-0167 [DOI] [PubMed] [Google Scholar]
- 5.Byun DJ, Wolchok JD, Rosenberg LM, et al. . Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 2017;13:195–207. 10.1038/nrendo.2016.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahzari M, Liu D, Arnaout A, et al. . Immune checkpoint inhibitor therapy associated hypophysitis. Clin Med Insights Endocrinol Diabetes 2015;8:21–8. 10.4137/CMED.S22469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar V, Chaudhary N, Garg M, et al. . Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 2017;8:49 10.3389/fphar.2017.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlov S, Salari F, Kashat L, et al. . Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab 2015;100:1738–41. 10.1210/jc.2014-4560 [DOI] [PubMed] [Google Scholar]
- 9.Iwama S, De Remigis A, Callahan MK, et al. . Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med 2014;6:230ra45 10.1126/scitranslmed.3008002 [DOI] [PubMed] [Google Scholar]


