Abstract
Objectives
Immune recovery following highly active antiretroviral therapy (HAART) is commonly assessed by the degree of CD4 reconstitution alone. In this study, we aimed to assess immune recovery by incorporating both CD4 count and CD4:CD8 ratio.
Design
Observational cohort study
Setting and participants
Clinical data from Chinese HIV-positive patients attending the largest HIV service in Hong Kong and who had been on HAART for ≥4 years were accessed.
Main outcome measures
Optimal immune outcome was defined as a combination of a CD4 count ≥500/μL and a CD4:CD8 ratio ≥0.8.
Results
A total of 718 patients were included for analysis (6353 person-years). At the end of year 4, 318 out of 715 patients achieved CD4 ≥500/μL, of which only 33% (105 out of 318) concurrently achieved CD4:CD8 ratio ≥0.8. Patients with a pre-HAART CD8 ≤800/μL (428 out of 704) were more likely to be optimal immune outcome achievers with CD4 ≥500/μL and CD4:CD8 ratio ≥0.8, the association of which was stronger after adjusting for pre-HAART CD4 counts. In a multivariable logistic model, optimal immune outcome was positively associated with male gender, younger pre-HAART age and higher pre-HAART CD4 count, longer duration of HAART and pre-HAART CD8 ≤800/μL. Treatment regimen and cumulative viral loads played no significant role in the pattern of immune recovery.
Conclusions
A combination of CD4 count and CD4:CD8 ratio could be a useful approach for the characterisation of treatment outcome over time, on top of monitoring CD4 count alone.
Keywords: antiretroviral therapy, CD4, CD8, CD4:CD8 ratio, immune outcome
Strengths and limitations of this study.
The combined use of CD4 and CD4:CD8 ratio as an outcome measure offers a new perspective for measuring immune recovery following antiretroviral therapy.
The combined marker could avoid overestimation of treatment performance in patients with CD4 count but low CD4:CD8 ratio.
The study was limited by not having included clinical events in the analysis, a gap which should be filled in larger scale cohort studies.
Introduction
Highly active antiretroviral therapy (HAART) forms the cornerstone of modern-day treatment of HIV infection. In monitoring treatment outcome, peripheral blood CD4+ lymphocyte (hereafter referred as CD4) count measurement is widely used, the results of which feature a rapid rise in the first 3–6 months followed by a second phase of gradual increase, plateauing 4–6 years afterwards.1 Nadir CD4 counts and advanced age are associated with poorer CD4 recovery, a well-reported phenomenon that has been reviewed in the literature.1 2 While high and persistently elevated CD8+ lymphocytes (hereafter referred as CD8) are commonly observed in chronically HIV-infected patients, relatively little attention has been paid to its impact on immunological recovery.3 A large cohort study suggested that markedly elevated CD8 count at HAART initiation was associated with a poor increase in CD4 count.4 Host factors aside, virus burden exerted by HIV could also impact immunological recovery. In the absence of timely and effective HAART, HIV cumulates over time leading to a state of cumulative viraemia, a predictor of suboptimal immunological outcome in primary HIV infection.5 Separate studies have shown that high cumulative viral load was a potential marker for progression to AIDS in chronic HIV infection.6 Despite effective therapy, some 20%–30% of patients were unable to achieve optimal immunological recovery,1 7 an outcome resulting from the interaction of a good range of host and viral factors, as well as coinfection with other pathogens, notably hepatitis C virus (HCV).3
Over the last decade, a CD4-guided approach to treatment initiation has gradually been replaced by early initiation of HAART irrespective of baseline CD4 level.8 Achievement of a high CD4 count of, say, over 500/μL remains a commonly used marker of immune restoration. Knowingly, prompt treatment and full viral suppression do not imply freedom from comorbidities, as HIV disease is also characterised by a state of immune activation, with the emergence of non-AIDS morbidity and mortality.9 This morbid state of immune activation cannot be inferred from the pattern of CD4 recovery alone. Failure of CD4/CD8 normalisation following HAART has however been linked to this scenario of immune activation.10 11 Low CD4:CD8 ratio was observed in patients despite high CD4 level (>500/μL).12 A high CD8 count following HAART was shown to be associated with inflammatory non-AIDS-related clinical events, and in fact a higher risk of myocardial infarction has been reported.4 13 Apparently, a target CD4 count is inadequate for reflecting effective immune recovery. Concurrent rise of the CD4:CD8 ratio is increasingly recognised as an important marker of immune reconstitution.10 14
To better monitor immunological recovery following HAART, new biomarkers are needed, which should preferably be derived from routinely collected laboratory data. Optimal outcome could be founded on CD4/CD8 normalisation on top of the regularly monitored CD4 count. In this study, we define HAART-associated immune recovery by a combination of CD4 outcome and CD4/CD8 restoration. We set out to examine its predictors by analysing regularly collected viral load and immunological data, the latter including CD8 count, in a cohort of patients with HIV following HAART.
Methods
Anonymous clinical data from Integrated Treatment Centre, the largest HIV clinical service in Hong Kong, were accessed for this observational study. Data access approval was granted by the Department of Health, Hong Kong Special Administrative Region Government in compliance with the Personal Data (Privacy) Ordinance. Individual consent for the study was waived following approval of the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee. Patients with HIV aged ≥18 diagnosed in 1985–2012, on HAART continuously for ≥4 years without treatment interruption, with at least one CD4 measurement during treatment and with viral load fully suppressed (without consecutive viral load >500 copies/mL in the first 4 years on treatment) were selected. We included patients who were treatment naïve or have been on non-standard treatment for <1 year before HAART initiation. Data retrieved were: (A) CD4 and CD8 counts at diagnosis, before HAART initiation and 3–4 months subsequently, (B) viral load levels at the respective time points, (C) AIDS diagnosis and the timing, as appropriate, (D) antiretroviral treatment date and regimens, differentiated as protease inhibitor (PI) or non-nucleoside reverse transcriptase inhibitor (NNRTI) based as other regimens were rarely used for treatment-naïve patients.
Estimated cumulative viral load was expressed as years×log10 copies/mL, in accordance with the method reported by Zoufaly et al 15 with modifications. Patients with available negative HIV testing result within 3 years before HIV diagnosis were included, so that one’s seroconversion date could be estimated with confidence.16 In brief, the products of the log10 viral load were summed from estimated seroconversion to subsequent specified time point(s), with the computation of the highest viral load for the undiagnosed interval and an upward adjustment by 1 log10 for the presumed primary infection period. The time of seroconversion was determined as the midpoint between last negative and first positive HIV antibody testing dates. On the other hand, optimal immune outcome was defined as the achievement of a CD4 count of ≥500/μL and a CD4:CD8 ratio ≥0.817 while conventional outcome was defined as achieving CD4 count of ≥500/μL but not CD4:CD8 ratio ≥0.8 within specific time. Late HIV diagnosis was defined as the diagnosis of AIDS within 3 months of HIV diagnosis. The latest CD4 and CD8 measurements ≤30 days before HAART initiation were used as the baseline.
Comparisons between pre-HAART CD8 >800/μL vs ≤800/μL were made by OR and multivariable logistic regression with pre-HAART CD4 as confounder, while correlation coefficients were calculated to test the associations between CD4 and CD8 before and 4 years after HAART. The CD8 threshold was adopted by taking reference from the criteria of high CD8 count (ie, over 800/μL) during primary infection reported in another study.18 CD4 (maximum value), CD8 (minimum value) and CD4:CD8 ratio (maximum value) of patients achieving optimal immune outcome and conventional outcome by year 4 on HAART over time (≤12 months, 12.1–24 months, …, >96 months) were compared in generalised estimating equations (GEE). Multivariable logistic regression model (stepwise) was applied to examine the predictors of optimal immune outcome and conventional outcome. Complete case analysis was performed. Loss to follow-up and death were data end points. Statistical tests were performed in SPSS 21.
Results
As of the end of 2012, data of 2974 diagnosed adults were accessed. Of these, 718 eligible treatment-naïve diagnosed cases who had been on HAART continuously for ≥4 years were included in the study. Their case records contained 18 857 clinical measurements (18 693 CD4, 18 521 CD8 and/or 17 776 viral load measurements) at multiple time points spanning over 6353 person-years’ follow-up. General characteristics of the study population are displayed in table 1. Overall, a majority (84%) were male with a median age at diagnosis of 37 years (IQR: 31–45 years). The median interval from diagnosis to the latest assessment was 100 months (IQR=74–141 months). Most were infected by either HIV-1 subtype B (31%) or CRF01_AE (38%), with men who have sex with men (MSM) accounting for 39% of the study population. The pre-HAART median CD4 and CD8 counts were 109/μL and 673/μL, respectively, which were positively correlated (Pearson correlation coefficient r=0.50, p<0.001) (see online supplementary figure 1d). The distribution of CD4 and CD4:CD8 ratio at baseline before initiation of HAART is shown in online supplementary table 1a. The lifetime estimated cumulative viral load at the last assessment increased with the interval between seroconversion and HAART initiation (r=0.94, p<0.001).
Table 1.
General characteristics of study population (n=718)
| Frequency | % | |
| Median | (IQR) | |
| (a) Demographics | ||
| Male gender | 605 | 84% |
| Ethnicity | ||
| Chinese | 581 | 81% |
| Asian (Asian other than Chinese) | 87 | 12% |
| White | 47 | 7% |
| African | 3 | 0.4% |
| Age (years, at HIV diagnosis) | 37 | (31–45) |
| (b) HIV infection and diagnosis | ||
| Mode of transmission | ||
| Heterosexual | 394 | 55% |
| Man-to-man sex | 280 | 39% |
| Injection drug use | 34 | 5% |
| Contaminated blood transfusion | 6 | 1% |
| Undetermined | 4 | 1% |
| HIV-1 subtype | ||
| CRF01_AE | 270 | 38% |
| B | 224 | 31% |
| C | 8 | 1% |
| Others | 31 | 4% |
| Unavailable | 185 | 26% |
| AIDS diagnosis before treatment | 239 | 33% |
| Late HIV diagnosis* | 192 | 27% |
| Estimated cumulative viral load† from seroconversion to diagnosis (n=199) | 8 | (3–18) |
| (c) Pre-HAART status | ||
| Age (years) | 39 | (33–46) |
| Months from diagnosis to treatment initiation | 8.67 | (2.75–33.13) |
| CD4 count (cells/µL) | 109 | (29–190) |
| CD4:CD8 ratio‡ | 0.14 | (0.06–0.23) |
| CD8 count (cells/µL)‡ | 673 | (441–966) |
| Viral load (log10 copies/mL)§ | 5.15 | (4.62–5.58) |
| Estimated cumulative viral load† from seroconversion to treatment initiation (n=199) | 18 | (11–29) |
| (d) Antiretroviral treatment and clinical outcomes | ||
| First HAART regimen | ||
| NNRTI based | 182 | 25% |
| PI based | 131 | 18% |
| PI based with booster | 397 | 55% |
| Non-standard | 8 | 1% |
| Total treatment duration (months) | 85.38 | (63.39–117.32) |
| AIDS free during treatment (n=479) | 456 | 95% |
| Highest CD4 count within 4 years¶ | 476 | (354–630) |
| Highest CD4:CD8 ratio within 4 years** | 0.55 | (0.39–0.76) |
| CD4 count ≥500/μL within 4 years¶ | 318 | 44% |
| CD4:CD8 ratio ≥0.8 within 4 years†† | 145 | 20% |
| Deceased | 39 | 5% |
*Late HIV diagnosis refers to the diagnosis of AIDS within 3 months of HIV diagnosis.
†Estimated cumulative viral load expressed as years×log10 viral load copies/mL.
‡14 missing values.
§18 missing values.
¶2 missing values.
**8 missing values.
††3 missing values.
HAART, highly active antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
bmjopen-2017-016886supp001.pdf (301.4KB, pdf)
bmjopen-2017-016886supp002.pdf (17.3KB, pdf)
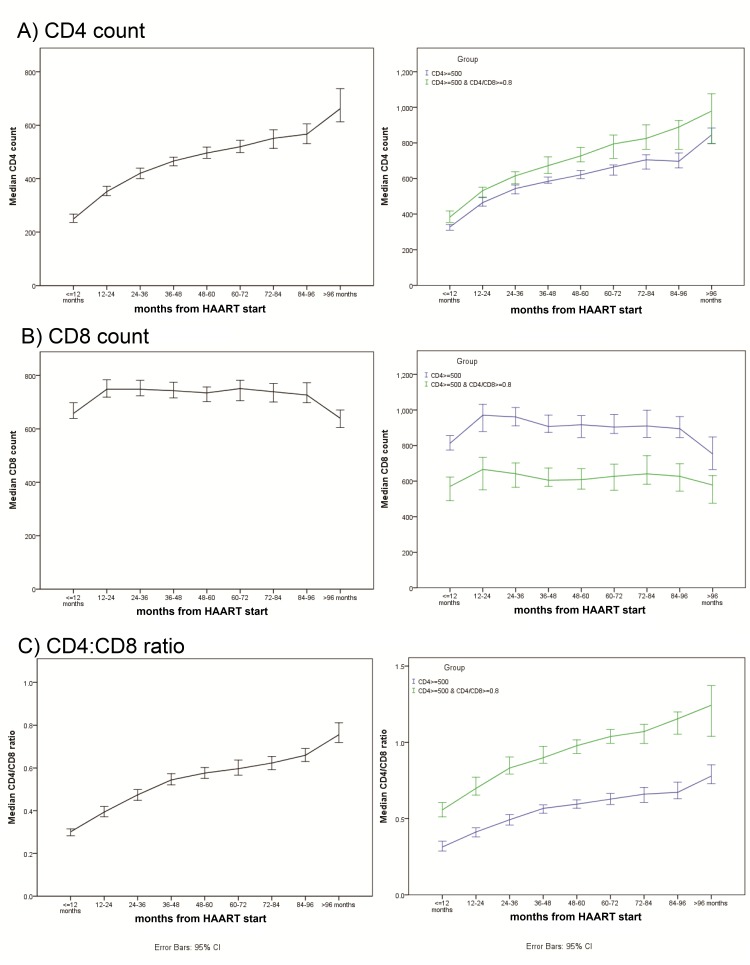
During the study period, a CD4-guided approach was in place, implying that HAART was recommended when one’s CD4 count fell below 350/μL. A majority of the patients (74%) had been started on a PI-based regimen with 25% on NNRTI-based regimen, and 1% had been started on non-standard regimen subsequently changed to HAART within 1 year. Integrase inhibitors (INSTI) had not been used as a component of one’s first regimen, but three patients had changed to raltegravir-based regimen afterwards (table 1). The median treatment duration was 85.38 months (IQR: 63.39–117.32). As of the end of a 4-year observation period, the CD4 count of 318 patients (44%) had reached 500/μL or above, of which 105 (33%) gave a CD4:CD8 ratio of ≥0.8 concurrently, while 205 (64%) patients reached the CD4 target but not the ratio. On the other hand, 145 patients reached the optimal ratio, of which 32 (22%) patients could not reach the CD4 target (table 2). The temporal changes of CD4 count, CD8 count and CD4:CD8 ratio over time are shown in figure 1, while distribution of CD4 and CD4:CD8 ratio at the end of year 4 is shown in online supplementary table 1b. Whereas both CD4 count (figure 1A) and CD4:CD8 ratio (figure 1C) showed a steady rise from the first time point following HAART, the temporal pattern of CD8 counts was inconspicuous (figure 1B). Patients with optimal immune outcome had significantly higher median CD4 and CD4:CD8 ratio but lower CD8 count than those only with satisfactory CD4 recovery (conventional outcome) in all time points (GEE model results in online supplementary table 2 supplementary table 1). The CD4 count at year 4 was positively correlated with pre-HAART CD4 (r=0.38, p<0.001) (see online supplementary figure 1a). Categorised by one’s pre-HAART CD8 count, about half (n=428, 61%) had a lower count of ≤800/μL. The two groups had similar demographic, cumulative viral load levels and had received similar treatment regimens. The CD4 count at year 4 was positively correlated with pre-HAART CD8 count (r=0.18, p<0.001) (see online supplementary figure 1b) whereas the latter was also positively correlated with CD8 at year 4 (r=0.35, p<0.001) (see online supplementary figure 1c). After adjusting for pre-HAART CD4, patients with lower pre-HAART CD8 had a higher chance of achieving a higher CD4:CD8 ratio at year 4 (adjusted OR (aOR)=64.63, 95% CI 23.47 to 177.98) (table 3). Likewise, a low pre-HAART CD8 count of ≤800/μL was associated with the optimal immune outcome at year 4, with an increased odds (aOR=5.07, 95% CI 2.74 to 9.41) after adjusting for pre-HAART CD4. There was no significant correlation between year 4 CD8 and pre-HAART CD4 (see online supplementary figure 1e), but positive association between CD4 and CD8 at year 4 (r=0.33, p<0.001) could be demonstrated (see online supplementary figure 1f).
Table 2.
The profiles of immunological outcomes of patients by achievement of none, one or both of the two target immunological markers (CD4 ≥500, CD4:CD8 ratio ≥0.8) before the end of a 4-year observation period*
| Number | Median peak or highest CD4 count (/μL) (IQR) | Median months to CD4 target (IQR) | Median peak or highest CD4:CD8 ratio (IQR) | Median months to target CD4:CD8 ratio (IQR) | |
| CD4 ≥500/μL and CD4:CD8 ratio ≥0.8 | 105 | 741 (618–876) | 20.63 (12.6–30.53) | 0.98 (0.86–1.2) | 28.90 (14.43–42.95) |
| Concurrent achievement of both targets | 15 | 694 (569–1182) | 20.27 (13.07–28.17) | 1.05 (0.9–1.49) | 20.27 (13.07–28.17) |
| CD4 target before ratio target | 57 | 788 (660–921) | 15.13 (8.7–22.88) | 0.89 (0.83–0.99) | 39.23 (30.78–45.98) |
| Ratio target before CD4 target | 33 | 650 (547–764) | 31.13 (22.3–39.4) | 1.17 (1.02–1.56) | 14.40 (8.68–24.08) |
| CD4 ≥500/μL only | 205 | 622 (552–723) | 29.10 (17.43–38.37) | 0.59 (0.49–0.69) | – |
| Ratio ≥0.8 only | 32 | 431 (369–475) | – | 1.05 (0.89–1.17) | 29.32 (18.48–40.33) |
| CD4 target then changed to ratio target | 4 | 588 (519–660) | 20.02 (12.23–35.36) | 0.86 (0.81–0.95) | 36.83 (20.68–49.72) |
| Ratio target then changed to CD4 target | 4 | 583 (521–636) | 29.68 (20.52–40.38) | 0.87 (0.86–1.01) | 13.87 (5.48–25.45) |
| Failure to achieve both targets | 365 | 362 (253–432) | – | 0.43 (0.31–0.55) | – |
*Equivalent to a maximum of <52 months with the inclusion of a 3-month buffer period.
Figure 1.
Yearly changes of (A) CD4 count, (B) CD8 count and (C) CD4:CD8 ratio from HAART initiation to 6 years afterwards. HAART, highly active antiretroviral therapy.
Table 3.
Comparison between patients with high (>800/μL) and low (≤800/μL) pre-HAART CD8 counts. Variables included in the analyses were (a) general baseline characteristics, (b) pre-HAART virological status, (c) antiretroviral therapy and (d) outcome at year 4
| Pre-HAART CD8 >800 (n=276) | Pre-HAART CD8 ≤800 (n=428) | Univariate analysis | Adjusted by pre-HAART CD4 | |||||
| Median/number | IQR/% | Median/number | IQR/% | OR | 95% CI | aOR | 95% CI | |
| (a) Baseline characteristics | ||||||||
| Male gender | 242 | 87.7% | 352 | 82.2% | 0.65 | 0.42 to 1.01 | 1.82 | 1.12 to 2.96* |
| Chinese ethnicity | 222 | 80.4% | 351 | 82.0% | 1.11 | 0.75 to 1.63 | 1.10 | 0.71 to 1.71 |
| Mode of transmission | (n=372) | (n=427) | ||||||
| MSM | 120 | 44.0% | 153 | 35.8% | Ref | Ref | ||
| Heterosexual | 140 | 51.3% | 249 | 58.3% | 1.39 | 1.02 to 1.91* | 0.93 | 0.65 to 1.33 |
| Injection drug user | 13 | 4.8% | 19 | 4.4% | 1.15 | 0.54 to 2.41 | 0.47 | 0.21 to 1.08 |
| Contaminated blood transfusion | 0 | 0.0% | 6 | 1.4% | – | – | ||
| Subtype | (n=206) | (n=322) | ||||||
| CRF01_AE | 95 | 46.1% | 171 | 53.1% | Ref | Ref | ||
| B | 94 | 45.6% | 129 | 40.1% | 0.76 | 0.53 to 1.1 | 1.35 | 0.88 to 2.06 |
| C | 4 | 1.9% | 4 | 1.2% | 0.56 | 0.14 to 2.27 | 1.13 | 0.25 to 5.07 |
| Others | 13 | 6.3% | 18 | 5.6% | 0.77 | 0.36 to 1.64 | 1.37 | 0.6 to 3.17 |
| Age at diagnosis (years) | 36.80 | 31.74–43.54 | 37.46 | 30.27–45.17 | 1.00 | 0.98 to 1.01 | 0.99 | 0.98 to 1.01 |
| Late HIV diagnosis | 48 | 17.4% | 138 | 32.2% | 2.26 | 1.56 to 3.28* | 0.98 | 0.64 to 1.51 |
| AIDS before treatment | 66 | 23.9% | 168 | 39.3% | 2.06 | 1.47 to 2.88* | 0.94 | 0.63 to 1.41 |
| (b) Pre-HAART virological status | ||||||||
| Viral load (log10 copies/mL) | (n=274) | (n=420) | ||||||
| 5.04 | 4.55–5.52 | 5.20 | 4.69–5.58 | 1.23 | 1.03 to 1.47* | 0.80 | 0.64 to 0.99* | |
| Viral load log10 >5 | 145 | 52.9% | 259 | 61.7% | 1.43 | 1.05 to 1.95* | 0.71 | 0.49 to 1.02 |
| Estimated cumulative viral load† | (n=96) | (n=101) | ||||||
| 17.74 | 10.00–29.61 | 18.53 | 10.88–27.73 | 1.004 | 0.98 to 1.03 | 1.004 | 0.98 to 1.03 | |
| (c) Antiretroviral treatment | ||||||||
| Months from diagnosis to HAART initiation | 12.80 | 3.87–35.52 | 5.60 | 2.44–30.58 | 1.00 | 0.99 to 1 | 1.01 | 1 to 1.01* |
| NNRTI-based initial regimen | 70 | 25.4% | 105 | 24.5% | 0.96 | 0.67 to 1.36 | 1.84 | 1.22 to 2.78* |
| (d) Outcome at year 4 | ||||||||
| CD4 count/μL | (n=246) | (n=370) | ||||||
| 488 | 386–625 | 437 | 332–589 | 0.999 | 0.998 to 1 | 1.001 | 0.9997 to 1.002 | |
| CD4 >500/μL | 117 | 47.6% | 141 | 38.1% | 0.68 | 0.49 to 0.94* | 1.29 | 0.88 to 1.91 |
| CD4:CD8 ratio | (n=246) | (n=370) | ||||||
| 0.49 | 0.36–0.68 | 0.57 | 0.41–0.79 | 3.61 | 1.93 to 6.75* | 64.63 | 23.47 to 177.98* | |
| Viral load (log10 copies/mL) | (n=245) | (n=366) | ||||||
| 1.88 | 1.88–2.6 | 1.88 | 1.88–2.6 | 1.18 | 0.73 to 1.91 | 0.83 | 0.48 to 1.44 | |
| Suppressed viral load (≤500 copies/mL) | 245 | 100.0% | 364 | 99.5% | – | – | ||
| CD4 >500/μL and CD4:CD8 ratio >0.8 | (n=243) | (n=370) | ||||||
| 24 | 9.9% | 59 | 15.9% | 1.73 | 1.04 to 2.87* | 5.07 | 2.74 to 9.41* | |
| Treatment (months) | 83.83 | 62.13–117.42 | 85.05 | 64.17–116.75 | 1.000 | 0.997 to 1.004 | 0.999 | 0.99 to 1.003 |
All analyses were performed in logistic regression: simple logistic regression for univariate analyses and multivariable logistic regression with selected confounders for multivariable analyses.
*p<0.05.
†Estimated cumulative viral load from seroconversion expressed as years×log10 viral load copies/mL.
aOR, adjusted OR; HAART, highly active antiretroviral therapy; MSM, men who have sex with men; NNRTI, non-nucleoside reverse transcriptase inhibitor.
bmjopen-2017-016886supp003.pdf (218.2KB, pdf)
The following independent variables were then tested for their prediction of optimal immune outcome and conventional outcome achieved since treatment initiation throughout the observation period: pre-HAART CD4, pre-HAART CD8, pre-HAART age, treatment duration and male gender. In the final model, both high pre-HAART CD4 and low pre-HAART CD8 were significant predictors of optimal immune outcome, while only the former was a significant predictor of conventional outcome (table 4). Patients who were male and started HAART at younger age were more likely to achieve both outcomes. Patients on treatment for longer time (≥97 months) had higher odds to achieve optimal immune outcome (aOR=3.34, 95% CI 2.17 to 5.15, 49–72 months as reference) than conventional outcome (aOR=2.78, 95% CI 1.89 to 4.09, 49–72 months as reference). As a substudy (results not shown), we have performed another set of GEE models with cumulative viral load as an independent variable (results not shown). The results did not support it as a significant predictor of an optimal immune outcome both in CD4 count and CD4:CD8 ratio, though the number of patients eligible for the analyses was only 187.
Table 4.
Multivariable logistic regression for evaluating variables associated with an optimal immunological outcome and conventional outcome
| Optimal immune outcome | Conventional outcome | |||
| aOR | 95% CI | aOR | 95% CI | |
| Male gender | 2.23 | 1.4 to 3.53* | 1.81 | 1.11 to 2.96* |
| Age at HAART initiation | 0.98 | 0.97 to 0.9996* | 0.96 | 0.94 to 0.97* |
| Pre-HAART CD4 (/μL) | ||||
| ≤100 | Ref | Ref | ||
| 101–200 | 2.91 | 1.83 to 4.62* | 2.30 | 1.57 to 3.37* |
| 201–300 | 4.61 | 2.53 to 8.39* | 3.52 | 2.1 to 5.9* |
| >300 | 20.36 | 7.51 to 55.17* | 12.84 | 3.6 to 45.75* |
| Months on treatment | ||||
| 49–72 | Ref | Ref | ||
| 73–96 | 1.58 | 0.93 to 2.67 | 1.67 | 1.08 to 2.57* |
| ≥97 | 3.34 | 2.17 to 5.15* | 2.78 | 1.89 to 4.09* |
| Pre-HAART CD8 ≤800/μL | 0.998 | 0.998 to 0.999* | ||
| Constant | 0.48 | 3.30 | ||
An optimal immunological outcome was defined as achieved CD4 count ≥500/μL and a CD4:CD8 ratio ≥0.8, and conventional outcome was defined as only achieved CD4 count ≥500/μL by study end point.
*p<0.05.
aOR, adjusted OR; HAART, highly active antiretroviral therapy.
Discussion
Pre-HAART CD4 count has long been shown to be a predictor of immunological outcome 3–5 years following antiretroviral therapy.1 Our previous longitudinal studies in a cohort of Chinese patients with HIV have demonstrated positive associations between nidus and maximum CD4 count over 5 years irrespective of the causative virus subtype or the regimens prescribed.19 20 In assessing antiretroviral treatment response, however, CD4 count alone appeared to add little to viral load monitoring.21 To account for the potential risk of non-AIDS-related comorbidities including metabolic complications,9 parallel CD4:CD8 ratio testing is gaining popularity as it reflects also the intensity of chronic inflammation implicated.9 10 In this study, a CD4 count ≥500/μL in conjunction with a ratio of ≥0.8 was examined as a target outcome indicator for chronically infected patients on continued antiretroviral therapy. This target was achieved in 15% (105 out of 715) of our patients at the end of a 4-year treatment period. The association of pre-HAART CD8 with optimal immune outcome was stronger with a cut-off ratio of ≥1 but the proportion of patients achieving the target outcome would be very low at 6% (46 out of 718). Both pre-HAART CD4 and CD8 counts, as well as the treatment interval, were independent predictors of this new outcome target. While CD4 remained a useful prognostic marker, using it as the sole marker might overestimate treatment performance by including patients with high CD4 count but high CD8 count and low CD4:CD8 ratio as achiever (205 out of 715 achieved CD4 target only).
In this study, we have shown that 44% of patients on HAART achieved a CD4 count ≥500/μL at the end of 4 years, an outcome slightly poorer than that of 59% reported by the Swiss HIV Cohort Study, a discrepancy which could be attributed to our shorter observation period (4 instead of 5 years) and the lower median pre-HAART CD4 count (158/μL compared with 180/μL).22 As concluded in the recently published ‘START’ study examining the benefits of the initiation of antiretroviral therapy in HIV-positive adults with a CD4 count >500/μL, CD4 count per se could not capture all outcome effects arising from immediate HAART in chronic HIV infection.23 Our study confirmed that CD4:CD8 ratio could be a readily available supplementary marker to monitor immune recovery. Evidently, the ratio may vary with lengths of observations, demographics and/or even HAART regimens.17 24 25 As the CD4:CD8 ratio tended to rise more slowly than CD4 recovery, we have chosen an interim ratio of 0.817 to assess the state of immune recovery at 4 years after HAART initiation. Normalisation to a ratio of 1 could in fact be demonstrated in 13% of patients within 7 years, the median observation interval of our cohort.
Pre-HAART CD8 count and its normalisation following antiretroviral treatment are relatively underinvestigated.26 27 In our study, pre-HAART CD4 and pre-HAART CD8 counts were positively correlated. It was noted that heterosexuals gave a lower pre-HAART CD8 (table 3) compared with MSM but the difference became insignificant after the adjustment of pre-HAART CD4. Over time, CD4 rise went in parallel with slowly falling CD8 until reaching an optimal CD4 level of ≥500/μL with a near-normalised CD4:CD8 ratio ≥0.8 at year 4. Pre-HAART CD8 was a significant predictor of optimal immune outcome but not conventional outcome. The median CD8 count of former group was lower than latter group of patients in all time points since HAART initiation. Significant expansion of CD8 is known to occur soon after infection and the phenomenon might persist throughout the course of HIV infection. Recent studies suggested that CD8 normalisation was associated with early initiation of HAART during acute infection.18 HIV-specific CD8 has been proposed to play an important role in effecting functional cure of HIV infection.28 Its relationship with the absolute count of CD8 before and after HAART has not been established. With the growing evidence of the role of CD4:CD8 ratio as a new biomarker for non-AIDS morbidity and chronic inflammation,9 10 29 30 it is possible that HIV-positive patients’ clinical outcome could be better explained from both the ratio and CD4 count rather than from the latter alone. From a virological perspective, the estimated cumulative viral load can be viewed as a surrogate of prolonged non-suppression of virus load. It does not however independently predict CD4 or CD4:CD8 ratio outcomes. Apparently, its immunological impacts could be overtaken by a long interval of HAART, if the pre-HAART CD4 and CD8 status were optimal. Overall, our results lent support to early initiation of HAART in chronic HIV infection to avoid temporal accumulation of virus, a conclusion similar to that for primary HIV infection.5
We acknowledge that our study carries a number of limitations. Foremost, all patients had been on HAART during the time when a CD4-guided approach to treatment initiation was enforced. As the patients had been started on either a PI-based or NNRTI-based regimen, the possible impacts of newer generations of antiretroviral like INSTI could not be ascertained. The results should therefore be interpreted with caution, especially that strong association between INSTI-based regimen and CD4/CD8 normalisation has recently been reported.31 These was selection bias which might have limited the extrapolation of results to the entire HIV population. In addition, our dataset did not include other inflammatory or infectious outcomes (eg, HCV and/or cytomegalovirus coinfections32–35) and therefore these could not be analysed in perspective. As the main comparative period was 4 years, the minimum treatment duration of study population, the immunological recovery achieved by patients in this study may not necessarily be reflecting the ultimate response to HAART. We have nevertheless evaluated the outcome (comprising both CD4 count and CD4:CD8 ratio) of all enrolled patients with a median duration of treatment of over 6 years in the final analysis. Theoretically, cohorts with patients observed throughout their lifetime would be invaluable to determine the health benefits of HAART. Analyses from such lifelong cohorts should become a reality in the coming years or decades.
Conclusions
Conventionally, CD4 count has been commonly used as the main outcome marker following HAART. In light of the increasing incidence of comorbidities associated with HIV-related chronic inflammations, CD4 count per se appears to carry little prognostic value in predicting HAART-associated immune recovery. Our results suggested that a combination of CD4 count and CD4:CD8 ratio offers another potentially useful approach to assessing immune outcome, compared with the use of CD4 alone. In evaluating immune recovery following long-term HIV viral suppression, pre-HAART CD8 count could be as important as nidus CD4 count as the independent predictors of the ultimate immune outcome. As both CD4 and CD8 are often routinely collected in the course of HIV management, an assessment of the temporal trends of CD4, CD8 and CD4:CD8 ratio could conveniently predict the immunological outcome without the need for sophisticated immune markers. Virological impact, as inferred from the estimated cumulative viral load after infection, does not however add to the outcome reflected from viral load suppression. The monitoring of the host immunological responses remains the most important approach in assessing treatment outcome following HAART.
Supplementary Material
Acknowledgments
The authors thank all staff of the Integrated Treatment Centre for their contribution to the delivery of quality clinical care to patients whose data were accessed for use in the analyses described in the manuscript. Li Ka Shing Institute for Health Sciences at The Chinese University of Hong Kong is acknowledged for technical support in developing the analyses. NSW was supported by Guangdong Provincial Centers for Skin Diseases and STI Control, the UNC-South China STD Research Training Center (FIC1D43TW009532-01) and NIH Fogarty International Center.
Footnotes
Contributors: SSL motivated and designed the study. KHW, BCKW and KCWC contributed the data and their interpretation. NSW analysed the data. SSL wrote the article. All authors contributed to interpretation of results and critically reviewed and edited the article.
Funding: The study was supported by Health and Medical Research Fund (Project code: CU-15-A15) of the Food and Health Bureau, Hong Kong Special Administrative Region Government. The funder did not have any role in study design, analysis and interpretation of data, and drafting the manuscript.
Disclaimer: The opinions and assertions contained herein are private views of the authors and do not necessarily reflect those of the Centre for Health Protection, Hong Kong Special Administrative Region Government Department of Health, or the other affiliating institutions.
Competing interests: None declared.
Ethics approval: The Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (CREC).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Battegay M, Nüesch R, Hirschel B, et al. . Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis 2006;6:280–7. 10.1016/S1473-3099(06)70463-7 [DOI] [PubMed] [Google Scholar]
- 2. Gazzola L, Tincati C, Bellistrì GM, et al. . The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis 2009;48:328–37. 10.1086/695852 [DOI] [PubMed] [Google Scholar]
- 3. Tsiara CG, Nikolopoulos GK, Dimou NL, et al. . Effect of hepatitis C virus on immunological and virological responses in HIV-infected patients initiating highly active antiretroviral therapy: a meta-analysis. J Viral Hepat 2013;20:715–24. 10.1111/jvh.12101 [DOI] [PubMed] [Google Scholar]
- 4. Helleberg M, Kronborg G, Ullum H, et al. . Course and Clinical Significance of CD8+ T-Cell Counts in a Large Cohort of HIV-Infected Individuals. J Infect Dis 2015;211:1726–34. 10.1093/infdis/jiu669 [DOI] [PubMed] [Google Scholar]
- 5. Seng R, Goujard C, Krastinova E, et al. . Influence of lifelong cumulative HIV viremia on long-term recovery of CD4+ cell count and CD4+/CD8+ ratio among patients on combination antiretroviral therapy. AIDS 2015;29:1–607. 10.1097/QAD.0000000000000571 [DOI] [PubMed] [Google Scholar]
- 6. Marconi VC, Grandits G, Okulicz JF, et al. . Cumulative viral load and virologic decay patterns after antiretroviral therapy in HIV-infected subjects influence CD4 recovery and AIDS. PLoS One 2011;6:e17956 10.1371/journal.pone.0017956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaardbo JC, Hartling HJ, Gerstoft J, et al. . Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol 2012;2012:670957 10.1155/2012/670957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. WHO | Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV 2015, 2015. [PubMed] [Google Scholar]
- 9. Saracino A, Bruno G, Scudeller L, et al. . Chronic inflammation in a long-term cohort of HIV-infected patients according to the normalization of the CD4:CD8 ratio. AIDS Res Hum Retroviruses 2014;30:1178–84. 10.1089/aid.2014.0080 [DOI] [PubMed] [Google Scholar]
- 10. Mussini C, Lorenzini P, Cozzi-Lepri A, et al. . CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015;2:e98–106. 10.1016/S2352-3018(15)00006-5 [DOI] [PubMed] [Google Scholar]
- 11. Lu W, Mehraj V, Vyboh K, et al. . CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc 2015;18:20052 10.7448/IAS.18.1.20052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serrano-Villar S, Sainz T, Lee SA, et al. . HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014;10:e1004078 10.1371/journal.ppat.1004078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Badejo OA, Chang CC, So-Armah KA, et al. . CD8+ T-cells count in acute myocardial infarction in HIV disease in a predominantly male cohort. Biomed Res Int 2015;2015:246870 10.1155/2015/246870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serrano-Villar S, Deeks SG. CD4/CD8 ratio: an emerging biomarker for HIV. Lancet HIV 2015;2:e76–7. 10.1016/S2352-3018(15)00018-1 [DOI] [PubMed] [Google Scholar]
- 15. Zoufaly A, Stellbrink HJ, Heiden MA, et al. . Cumulative HIV viremia during highly active antiretroviral therapy is a strong predictor of AIDS-related lymphoma. J Infect Dis 2009;200:79–87. 10.1086/599313 [DOI] [PubMed] [Google Scholar]
- 16. Pantazis N, Porter K, Costagliola D, et al. . Temporal trends in prognostic markers of HIV-1 virulence and transmissibility: an observational cohort study. Lancet HIV 2014;1:e119–26. 10.1016/S2352-3018(14)00002-2 [DOI] [PubMed] [Google Scholar]
- 17. Menozzi M, Zona S, Santoro A, et al. . CD4/CD8 ratio is not predictive of multi-morbidity prevalence in HIV-infected patients but identify patients with higher CVD risk. J Int AIDS Soc 2014;17:19709 10.7448/IAS.17.4.19709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cao W, Mehraj V, Trottier B, et al. . Early initiation rather than prolonged duration of antiretroviral therapy in HIV infection contributes to the normalization of CD8 T-Cell counts. Clin Infect Dis 2016;62:250–7. 10.1093/cid/civ809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naftalin CM, Wong NS, Chan DP, et al. . Three different patterns of CD4 recovery in a cohort of Chinese HIV patients following antiretroviral therapy - a five-year observational study. Int J STD AIDS 2015;26:803–9. 10.1177/0956462414553826 [DOI] [PubMed] [Google Scholar]
- 20. Wong NS, Reidpath DD, Wong KH, et al. . A multilevel approach to assessing temporal change of CD4 recovery following HAART initiation in a cohort of Chinese HIV positive patients. J Infect 2015;70:676–8. 10.1016/j.jinf.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 21. Ford N, Meintjes G, Pozniak A, et al. . The future role of CD4 cell count for monitoring antiretroviral therapy. Lancet Infect Dis 2015;15:241–7. 10.1016/S1473-3099(14)70896-5 [DOI] [PubMed] [Google Scholar]
- 22. Kaufmann GR, Furrer H, Ledergerber B, et al. . Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis 2005;41:361–72. 10.1086/431484 [DOI] [PubMed] [Google Scholar]
- 23. Lundgren JD, Babiker AG, Gordin F, et al. . Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807. 10.1056/NEJMoa1506816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leung V, Gillis J, Raboud J, et al. . Predictors of CD4:CD8 ratio normalization and its effect on health outcomes in the era of combination antiretroviral therapy. PLoS One 2013;8:e77665 10.1371/journal.pone.0077665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lichtenstein KA, Armon C, Nagabhushanam V, et al. . A pilot study to assess inflammatory biomarker changes when raltegravir is added to a virologically suppressive HAART regimen in HIV-1-infected patients with limited immunological responses. Antivir Ther 2012;17:1301–9. 10.3851/IMP2350 [DOI] [PubMed] [Google Scholar]
- 26. Cao W, Mehraj V, Kaufmann DE, et al. . Elevation and persistence of CD8 T-cells in HIV infection: the Achilles heel in the ART era. J Int AIDS Soc 2016;19:20697 10.7448/IAS.19.1.20697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mudd JC, Lederman MM. CD8 T cell persistence in treated HIV infection. Curr Opin HIV AIDS 2014;9:500–5. 10.1097/COH.0000000000000086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones RB, Walker BD. HIV-specific CD8⁺ T cells and HIV eradication. J Clin Invest 2016;126:455–63. 10.1172/JCI80566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Serrano-Villar S, Pérez-Elías MJ, Dronda F, et al. . Increased risk of serious non-AIDS-related events in HIV-infected subjects on antiretroviral therapy associated with a low CD4/CD8 ratio. PLoS One 2014;9:e85798 10.1371/journal.pone.0085798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sainz T, Serrano-Villar S, Díaz L, et al. . The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS 2013;27:1513–6. 10.1097/QAD.0b013e32835faa72 [DOI] [PubMed] [Google Scholar]
- 31. De Salvador-Guillouët F, Sakarovitch C, Durant J, et al. . Antiretroviral regimens and CD4/CD8 ratio normalization in HIV-Infected patients during the initial year of treatment: a cohort study. PLoS One 2015;10:e0140519 10.1371/journal.pone.0140519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saracino A, Bruno G, Scudeller L, et al. . CD4 and CD4/CD8 ratio progression in HIV-HCV infected patients after achievement of SVR. J Clin Virol 2016;81:94–9. 10.1016/j.jcv.2016.05.019 [DOI] [PubMed] [Google Scholar]
- 33. Brites-Alves C, Netto EM, Brites C. Coinfection by Hepatitis C Is Strongly Associated with Abnormal CD4/CD8 Ratio in HIV Patients under Stable ART in Salvador, Brazil. J Immunol Res 2015;2015:174215 10.1155/2015/174215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Freeman ML, Mudd JC, Shive CL, et al. . CD8 T-Cell Expansion and Inflammation Linked to CMV Coinfection in ART-treated HIV Infection. Clin Infect Dis 2016;62:392–6. 10.1093/cid/civ840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith DM, Nakazawa M, Freeman ML, et al. . Asymptomatic CMV replication during early human immunodeficiency Virus (HIV) infection is associated with lower CD4/CD8 ratio during HIV treatment. Clin Infect Dis 2016;63:1517–24. 10.1093/cid/ciw612 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-016886supp001.pdf (301.4KB, pdf)
bmjopen-2017-016886supp002.pdf (17.3KB, pdf)
bmjopen-2017-016886supp003.pdf (218.2KB, pdf)