Abstract
Productive humoral responses require that naïve B cells and their differentiated progeny move among distinct micro-environments. In this review, we discuss how studies are beginning to address the nature of these niches as well as the interplay between cellular signaling, metabolic programming, and adaptation to the locale. Recent work adds evidence to the expectation that B cells at distinct stages of development or functional subsets are influenced by the altered profiles of nutrients and metabolic by-products that distinguish these sites. Moreover, emerging findings reveal a cross-talk among the external milieu, signal transduction pathways, and transcription factors that direct B cell fate in the periphery.
eTOC
The metabolic needs of cells of the B lineage differ dramatically, spanning the quiescent naive and memory states, antigen-driven germinal center B cell stages and terminal differentiation state as antibody-producing cells. Boothby and Rickert outline the metabolic inputs and corresponding pathways that impact peripheral B cell differentiation in distinct microenvironments.
The B cell life cycle and changing microenvironments
B lymphocytes lead a semi-nomadic lifestyle due to their inherent role in surveying the body for foreign antigens and potential pathogens. However, activation and differentiation cues lead to the temporary or permanent retention of B lineage cells in anatomically and functionally distinct microenvironments that differ greatly in nutrient availability, oxygen and redox species. These components of the extracellular milieu impact B cell fate in the context of other signaling events by cytokines, growth factors and antigen. Here we focus on the metabolic control of peripheral B cell fate as defined by the breakdown (catabolism) of carbon sources (e.g. amino acids, nucleotides, lipids and carbohydrates) and the synthesis (anabolism) of cellular constituents (e.g. proteins, nucleic acids and fatty acids). Unlike T cells, relatively little has been established regarding B cell metabolism, placing this nascent field as fertile ground for exploration and discovery.
B cell generation in the bone marrow (BM) involves the rapid differentiation of B cell precursors from common lymphoid progenitors. Expansion at these early stages is cytokine-driven. Interleukin-7 (IL-7), stem cell factor (SCF) and FLT3 ligand enable B cell progenitors to undergo immunoglobulin gene V(D)J recombination and express the “pre-B cell receptor (BCR)” (immunoglobulin (Ig) μ heavy chain paired with surrogate light chains) (Melchers, 2015). Autonomous signaling by the pre-BCR drives subsequent proliferation to produce a large pool of resting pre-B cells. Ig light chain gene rearrangement among pre-B cells results in the expression of a functional BCR on immature B cells that egress to the periphery to become mature recirculating B cells (Melchers, 2015). Subsequent B cell proliferation and differentiation is antigen-driven and co-stimulated by toll-like receptor (TLR) ligands and T cell help to generate antibody-producing cells. In addition, a cohort of B cells receiving T cell help can undergo further differentiation in the germinal center (GC). This process results in the production of recirculating memory B cells, or plasmablasts that can become long-lived plasma cells in the BM (Corcoran and Tarlinton, 2016; Victora and Nussenzweig, 2012). Thus, the metabolic demands are high in the proliferative early B cell stages in the BM and lessen in the pre-B, immature, and transitional stages of the BM and spleen. After generation of the mature B cell repertoires, energy needs would seem to be relatively low in quiescent blood-borne naïve and memory B cells, but elevated during antigen-driven proliferation accompanied by differentiation in the secondary lymphoid tissues. Because of the energy and nutrient mass needed to secrete large amounts of glycosylated antibody molecules, metabolic demands remain high in sessile plasma cells (Aronov and Tirosh, 2016).
In summary, the demands of adaptive immunity require that the lymphoid lineages use cells that can transition between exceptionally rapid proliferation and non-cycling quiescent states. The resting cells (naïve B; memory B; and long-lived plasma cells) persist stably for periods up to years or decades and, in the case of memory B cells, may recirculate. A corollary is that while exposed to a variety of nutritional environments, metabolic programming needs to generate sufficient energy in concert with appropriately balancing anabolism, catabolism, and a sustainably stable economy of essential molecules of intermediary metabolism. Overall, signaling and transcription regulation are harnessed to assure that the demands of activation, differentiation, and locale can be met while balancing usage of molecules in energy generation and anabolism.
Basics of the energy economy
Glucose, glutamine, and fatty acids are three potential sources of carbon for growth and energy (Figure 1A). Glucose uptake, which increases dramatically after B cell activation (Caro-Maldonado et al., 2014; Cho et al., 2011; Dufort et al., 2007), provides the substrate for glycolysis and use of the two resultant pyruvate molecules for Krebs (citric acid) cycle entry. However, glucose-derived carbon is likely also needed for anabolism and a net increase in the mass of molecules (e.g., lipids, nucleic acids) synthesized during G1 and S phases. These anabolic requirements can be met in part by flux of carbon into the pentose phosphate pathway after glucose import. In parallel, glutamine uptake provides another potential source of carbons that can be used either for oxidative metabolism or anabolism after conversion into nucleic acid precursors and other amino acids. Thus, glutamine can enter mitochondria and feed the Krebs Cycle after anaplerotic conversion into α-ketoglutarate (αKG also known as 2-oxoglutarate) via glutaminase-mediated generation of glutamate (Figure 1B). Alpha-ketoglutarate and its product succinate also have the potential to influence DNA and protein modification by methylation and demethylation events. At present, the balances among these varied processes (net contributions of glucose versus glutamine to ATP generation or to increased biomass) have not yet been quantitated for B lineage cells, for instance by metabolic tracing in B cell subsets ex vivo or in vivo.
Figure 1. The carbon economy – fuel and build.
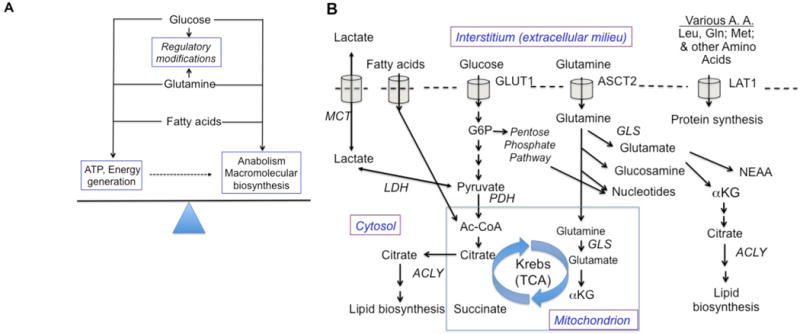
A schematic of the concepts and salient details outlined in the main text. (A) Both during quiescence and after activation and during growth, B cells need to balance the utilization of nutrients so as to generate ATP while also using portions of the uptake supply to provide building blocks for new molecule synthesis. As discussed in the text, the energy and atoms also get used for regulatory modifications such as histone post-translational modification by acetylation or methylation, or as cofactors of dioxygenases involved in HIF stabilization or the editing of chromatin methylation. Anabolic processes require both energy (ATP and GTP used in glycosylation as well as protein, lipid, and nucleic acid synthesis and the importation of sufficient building blocks. (B) Three major categories of fuel source are shown, along with some (but not all) of the branch points in their utilization. The plasma membrane is symbolized by an intermittent dotted line, in which nutrient importers are shown in simplified form (For instance, many can operate bi-directionally; some operate with export coupled to the import process shown). The mitochondrion is shown as a blue-bordered box, with only the Krebs cycle shown from among its many functions. Abbreviations: MCT, monocarboxylate transporter; LDH, lactate dehydrogenase; G6P, glucose-6-phosphate; ACLY, ATP-citrate lyase; PDH, pyruvate dehydrogenase; Ac-CoA, acetyl-Coenzyme A; αKG, alpha-ketoglutarate, also known as 2-oxoglutarate; TCA, tricarboxylic acid; GLS, glutaminase; ASCT2, Alanine, serine, cysteine-preferring transporter 2 (SLC1A5); LAT1, L-type amino acid transporter 1 (SLC7A5); NEAA, non-essential amino acids.
In addition to the carbon economy, which is the main center of research focus, several other metabolic demands need to be met. First, in the phases during which B cells proliferate and undergo population expansion, net import of all other essential atoms and molecules (N, PO4, S) needs to be met to support the increase in biomass (Figure 1B). Some of these factors may derive from the import and subsequent conversion of amino acids (e.g., glutamine, asparagine & cysteine), but this part of the blueprint for building new B cells also awaits analysis, especially in vivo. Another feature that is critical is the capacity to balance both anabolic and bioenergetic processes so that sufficient cytosolic and mitochondrial pools of essential co-factors (e.g., NAD+ and NADH; NADP+ and NADPH) and intermediates (e.g., acetyl coenzyme A) are maintained. In the extremes, sustained failures to maintain the redox balance within viable limits lead to pruning via cell death.
Metabolic checkpoints in B cell quiescence and homeostasis
Naïve recirculating B cells are small non-cycling cells that are poised to respond to antigen, and are maintained by continual signaling via the BCR and the receptor for B-cell activating factor (BAFF)R – both of which employ the phosphatidyl inositol 3’OH kinase (PI3K) pathway (Henley et al., 2008; Jellusova et al., 2013; Srinivasan et al., 2009) (Figure 2A). There is considerable evidence indicating crosstalk between these receptors, including recent data showing that BAFFR may engage the PI3K pathway via CD19 (Hobeika et al., 2015; Jellusova et al., 2013). Consistent with these findings, inactivation of Akt1 and Akt2, or of phosphoinositide-dependent kinase 1 (PDK1), (Figure 2B) results in a prominent loss of recirculating B cells (Baracho et al., 2014; Calamito et al., 2010). While soluble BAFF is non-mitogenic, it is an essential survival factor and also primes B cells for glycolytic growth and proliferation via PKCβ and Akt kinase activation (Figure 2B) (Patke et al., 2006; Woodland et al., 2008).
Figure 2. (A) A simplified view of cell surface receptors mediating B cell homeostasis and activation.
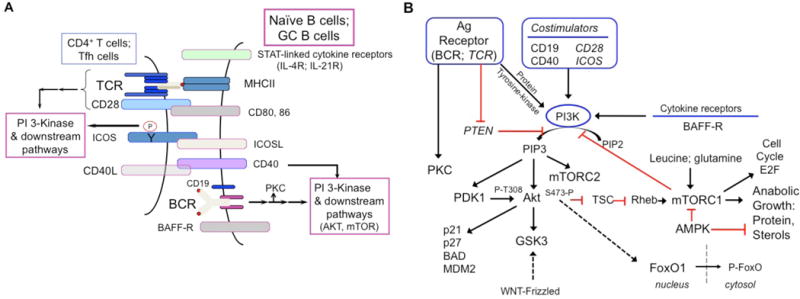
For convenience, the PI 3-kinase activation is highlighted, as naïve & activated B cells are combined, along with helper T cells. Collaboration of BAFF-R, BCR and CD19, as noted in the text, are schematized. (B) A simplified diagram of signaling pathways linking surface receptors and metabolism in B cells. At the top level, multiple inputs into the phosphoinositide 3-kinase (PI3K) pathway from antigen (Ag) receptor, costimulators, and cytokine receptors are shown, along with PTEN conversion of phosphatidylinositol 4, 5-biphosphate (PIP2) to phosphatidylinositol 3, 4, 5-triphosphate (PIP3). The dual lipid-protein phosphatase PTEN (Phosphatase and tensin homolog) restrains the pathway. Antigen receptor signaling also includes activation of protein kinases C (PKC). PDK1, phospho-inositide-dependent kinase 1; TSC, tuberous sclerosis complex; GSK, glycogen synthase kinase; WNT, Wingless-related integration site; AMPK, AMP-activated kinase.
Cell cycle entry is marked by the release of cell cycle inhibitors (Rb, p21, p27) and the activation of cyclin-dependent kinases (CDK) to prepare the cell for mitosis (Figure 2B). Passage through the late G1 stage is a noted restriction point, since subsequent mitotic events can proceed in the absence of growth factor stimulation. Such a checkpoint may also be present to regulate the onset of anabolic growth and the steep demands of cell division in terms of new protein synthesis, lipogenesis and nucleotide synthesis. The late G1 restriction point is characterized by the phosphorylation of Rb by the CDKs, resulting in the release of the E2F1 transcription factor that drives the production of cell cycle proteins as well as mitochondrial proteins to promote oxidative phosphorylation (Blanchet et al., 2011; Lee and Finkel, 2013) (Figure 2B). Constitutively active glycogen synthase kinase-3 (GSK3) has been recently found to be a metabolic checkpoint regulator in resting B cells, promoting cell survival by restricting protein synthesis and cell size in the absence of antigen or growth factor stimulation (Jellusova et al., 2017). Consistent with maintenance of T cell quiescence (Yang et al., 2011), it is possible that GSK3 serves an important role in suppressing mTORC1 via activation of the Tuberous Sclerosisi Complex (TSC) (Inoki et al., 2006), although mTORC1 was not hyperactivated in stimulated GSK3-deficient B cells (Jellusova et al., 2017) (Figure 2B). Alternate explanations include GSK3-dependent modulation of E2F1 activity or c-Myc stability to prevent G1 progression (Garcia-Alvarez et al., 2007; Gregory et al., 2003). Although much remains to be known regarding the maintenance of B cell quiescence in terms of nutrient sensing and the parsing of signals by the BCR and BAFFR, the main attributes appear to be preservation of homeostatic mitochondrial function and harnessed anabolism (Adams et al., 2016).
While memory B cells share the property of quiescence with naïve B cells, important distinctions exist. Memory B cells are antigen-experienced, i.e., have been activated previously, and often express a class-switched BCR. When they arise from the GC reaction, memory B cells have experienced multiple productive encounters with T helper (Th) cells, undergone multiple rounds of cell division, and received sustained or repetitive BCR stimulation. The quiescent state may be imposed by the disruption of these processes upon reaching a tipping point in terms of antigen sequestration via rising antibody titers, altered migration patterns or transcriptional re-programming (see below). The memory B cell population is heterogeneous with respect to Ig class, propensity to form antibody producing cells and dependence on the GC reaction (Tarlinton and Good-Jacobson, 2013). Nonetheless, a fundamental distinction of memory B cells is their ability to persist in a BAFF-independent manner (Scholz et al., 2008). Moreover, studies of Atg7-deficient mice suggest that memory persistence may differ from that of naïve B cells in a requirement for autophagy (Chen et al., 2014; Chen et al., 2015), though evidence from Atg5 loss-of-function studies provides indications that homeostasis and primary humoral responses may be promoted by another essential component of the autophagosome (Arnold et al., 2016; Clarke et al., 2015). There is also evidence suggesting that an IgM receptor may promote more sustainable memory B cell formation and/or survival than IgG (Gitlin et al., 2016; Pape et al., 2011). These differences may be reflective of earlier activation events, BCR (auto)specificity, and/or intrinsic signaling properties of particular Ig isotypes (Davey and Pierce, 2012; Haniuda et al., 2016; Kometani et al., 2013; Laffleur et al., 2015; Pierce and Liu, 2013; Wienands and Engels, 2016; Yang et al., 2016). Likewise, the impact of BCR isotype on cellular bioenergetics, nutrient requirements and priming for anabolic differentiation processes awaits further elucidation.
Metabolic reprogramming upon B cell activation
B cell activation is crucial for antigen-specific clonal expansion and also a precondition to later differentiation. Activation is initiated and then guided by signals initiated at cell surface receptors among which the BCR, CD40, IL-4R, and TLRs are prominent for the naïve B cell (Figure 2A). The mix and balance among signals must be distinct as B cells are directed either to plasma cell fates for antibody secretion or to GC intermediaries that yield memory B cells or a second wave of plasma cells. The BCR complex is the centerpiece of Ag-specific clonal expansion and directs an antibody (Ab) response to the epitopes of a new immune challenge. However, each of these other receptors initiates signaling through pathways that overlap those of the BCR. In vitro, direct engagement of the BCR promotes cell cycle entry, but requires costimulation with CD40, TLR ligands (CpG or LPS) or IL-4 to drive robust proliferation. TLR stimulation alone is sufficient to drive proliferation, class switch recombination (CSR) and differentiation into antibody secreting cells. However, BCR signaling and downstream activation of the PI3K pathway, including both mTORC1 and mTORC2, modulates CSR and the propensity to form plasma cells (Jones et al., 2016; Keating et al., 2013; Limon et al., 2014; Omori et al., 2006). In vivo, the mix, balance, and timing of these signals determines most outcomes such as the extent of clonal expansion, the capacity to breach self-tolerance, and the formation and duration of the GC response. LPS and CpG can drive a hapten-specific humoral response in vivo that is considered largely extra-follicular, consistent with a far higher fraction of progeny directed toward the plasma cell fate than is the case with protein-hapten conjugates. This signaling “code” (nature, magnitude, duration of the combined stimuli) will be further modulated by metabolic adaptations imposed by the microenvironment at the site of activation.
The signal transduction pathways activated by the BCR and initial costimulatory molecules (Figure 2B) promote metabolic reprogramming as resting B cells transition from quiescence to proliferation, but the new biochemical balances can in turn influence signaling. Protein kinase-C beta (PKCβ), which is activated both by the BCR via phospholipase C-γ2 (PLC-γ 2), promotes anti-Ig-induced increases in glycolytic flux (Blair et al., 2012). Intriguingly, PKCδ-deficient B cells exhibit normal BCR-activated glycolysis (Blair et al., 2012), consistent with a number of findings indicating that PKCδ and β have distinct and in some cases opposing roles in B cell activation (Salzer et al., 2016). PKCβ also is downstream from PI3K, which acts through PDK1 and mTOR complex 2 (mTORC2) to enhance PKC as well as AKT (Das et al., 2016; Lee et al., 2010; Lee et al., 2013). mTORC1 activity depends on sensing of amino acid concentrations (and those of other nutrients) but feeds back to inhibit PI3K (Carracedo et al., 2008; Tanaka et al., 2011). Recent findings in B cells provide evidence that among nutrients, oxygen concentrations can modulate mTORC1 activity (Cho et al., 2016). As elaborated upon below, the nutrient-sensing and feedback functions of mTORC1 raise the possibility of a bidirectional cross-talk between nutrients and metabolites on the one hand, and signal transduction on the other.
In other cell types, AMP-activated kinases (AMPK) integrate nutrient and energy status with cell physiology, fate, and function (Figure 2B). For B cells, this potential at present has not yielded evidence of an impact on antibody responses. Thus, although active AMPK inhibits mTORC1 by several mechanisms (Gwinn et al., 2008), and mTORC1 activity is important for later differentiation and Ab production after B cell activation (Aagaard-Tillery and Jelinek, 1994; Cho et al., 2016; Jones et al., 2016), primary antibody responses, even with a booster immunization, are normal in AMPKα1-null mice (Mayer et al., 2008). How much this finding is influenced by the caveat of persistent low-grade inflammation in these mice (Wang et al., 2010), or may parallel analyses of AMPK-deficient cytotoxic T cells (Rolf et al., 2013), will be of interest inasmuch as AMPK is so central to nutrient sensing and energy regulation.
Although the daunting challenge of testing the relevance and impact of nutrient - signaling crosstalk via more complete and robust experiments in vivo lies ahead, the concept has been supported by recent work both with T and B cells (Cho et al., 2016; Pollizzi et al., 2016; Verbist et al., 2016). Earlier work advocating a unique role for asymmetric partitioning of various cellular proteins between the two daughter cells and nuclei of lymphocytes (Chang et al., 2007) prompted investigations into potential links between this model and the nexus of signaling, gene regulation, and metabolism. One fulcrum in such concepts has been the transcriptional network centered on c-Myc, as this protein and its partners in DNA binding can regulate wide “suites” of metabolic genes that govern nutrient and metabolite flux (Wang et al., 2011). Some evidence indicates that c-Myc operates simply as an amplifier of the expression of any active gene (Nie et al., 2012). Nonetheless, this proto-oncogene promotes glycolysis and glutaminolysis along with increased hexose and amino acid uptake by activated T cells (Wang et al., 2011). Moreover, experiments using gene-targeted replacement alleles encoding GFP-cMyc fusion proteins support the concept that c-Myc partitions unequally in B lymphocytes as well as T cells (Lin et al., 2015; Pollizzi et al., 2016; Verbist et al., 2016). Evidence from T cells in these models offers a mechanism whereby asymmetry of c-Myc protein distribution can potentially be propagated through time, perhaps via a threshold-based mechanism (Heinzel et al., 2017). This crucial aspect of “fate determination” is imputed to a feed-forward model involving increased amino acid import and steady-state concentrations that in turn promoted subtle increases in mTORC1 activity and the previously established capacity of mTORC1 to promote translation of c-Myc mRNA (Kobayashi et al., 2003; Verbist et al., 2016; West et al., 1998). In LPS-activated B lymphoblasts, analogous mTORC1-dependent increases in amino acid uptake and mTORC1 activation have been noted (Cho et al., 2016). Asymmetric partitioning of various constituents including c-Myc as LPS blasts divide has been reported as well (Barnett et al., 2012; Lin et al., 2015). However, the overall role and functional importance of such asymmetries remain open to some question inasmuch as findings about asymmetry specifying outcomes have ranged from no detectable impact, in results of single-cell sorting experiments (Hawkins et al., 2013), to more deterministic assessments.
Ultimately, several adaptations are effected as a result of signaling initiated by the BCR and collaborating cell surface receptors. Glucose uptake increases dramatically, in part via higher amounts of the transporter GLUT1, but likely also because of increased downstream demand (i.e., utilization of glucose-6-phosphate). Thus, IL-4 stimulation alone, or BCR cross-linking, or TLR4 engagement by LPS increase glucose oxidation along with the enhancement of pyruvate generation (Cho et al., 2011; Dufort et al., 2007). As such, the degree of lactate export, as a surrogate for “aerobic glycolysis”, is less prominent than in the central canon of T cells – likely due to proportionately higher activity of pyruvate dehydrogenase (PDH) (Caro-Maldonado et al., 2014; Cho et al., 2011). Paradoxically, the impact of IL-4 is dependent on nuclear induction of the transcription factor STAT6 and a STAT6-interacting ADP-ribosyl transferase rather than on PI3K, and these effects are maintained even in B cells activated by IgM crosslinking (Cho et al., 2011; Dufort et al., 2007). The coupling of glycolytic pyruvate generation to Krebs’ TCA cycle would enhance the efficiency of ATP generation, but perhaps the enhanced glucose uptake is used in part to generate more citrate that can be used by ATP-citrate lyase (ACLY) for intrinsic lipogenesis to supply lipids for the synthesis of new membranes needed as a B cell grows and prepares to divide (Dufort et al., 2014). By analogy with other recent data (Moussaieff et al., 2015; Wellen et al., 2009), the production of acetyl CoA from citrate may also provide for maintenance of protein acetylation, for instance in chromatin. While the quantitative ledger accounting for utilization of glucose-derived carbon remains to be determined, B cell anergy appears to include diminution of GLUT1-mediated glucose uptake (Caro-Maldonado et al., 2014). Moreover, manipulation of the import capacity or glycolytic flux may suffice to facilitate or restrict sustained breaches of peripheral tolerance mechanisms, e.g., with constitutive over-expression of GLUT1 or its elimination, or with 2-deoxy-glucose treatment in vivo (Lee and Finkel, 2013; Yin et al., 2015).
Uptake of amino acids including glutamine also increases dramatically after B cell activation, and is promoted by induction of increased solute transporter proteins among which the accessory chain CD98hc (Slc3a2) has been shown as essential for robust B cell proliferation (Cantor et al., 2009), and is rapidly upregulated in a manner dependent on PDK1 and Akt (Kelly et al., 2007). The increases in a subset of amino acids - leucine, lysine, arginine, and glutamine - helps to maintain optimal capacity to have signal flux through the mTORC1 node of pathways downstream from PI3K (Efeyan et al., 2015; Nakaya et al., 2014; Sancak et al., 2008; Sinclair et al., 2013). Branches of the PI3K pathway that include mTORC1 promote the induction of the accessory chain encoded by Slc3a2 as well as solute transporters that pair with it, such as Slc7a5 (LAT1, for large neutral amino acids) and Slc1a5 (ASCT2, for glutamine and other neutral amino acids) (Cho et al., 2016; Verbist et al., 2016). In addition to supporting mTORC1 activity in a positive feed-forward loop, glutamine can in principle provide citrate for membrane biogenesis after ACLY generation of acetyl-CoA, feed nucleotide synthesis, and generate energy after anaplerosis and entry of αKG into mitochondria (DeBerardinis and Cheng, 2010). However, there are few isotope-tracing experiments with B cells to evaluate the programming of such uses at present (Le et al., 2012). In contrast to these increases, oxidative conversion of palmitate to generate energy appears not to be altered by activation or IL-4 stimulation (Cho et al., 2011). In summary, B cell activation for proliferation establishes a greater coordination of glucose oxidation with glycolysis than is the case for canonical T cell metabolism but appears to be similar to T cells in the dramatic induction of multiple nutrient transporters so that the diverse building materials are available for rapid clonal expansion.
The germinal center microenvironment, metabolism and B cell selection
In contrast to B cells activated by multivalent antigens such as polysaccharides that induce strong BCR cross-linking, B cells activated by proteinaceous low-valency antigens have a substantial capacity to seed and participate in a sustained interplay with CD4+ T cells in GC reactions. After extensive divisions before organization of a GC (Gitlin et al., 2014; Qi et al., 2008), initiation and prolongation of the GC reaction involves BCR-mediated uptake of antigen, processing and presentation of Ag-derived peptides on MHC-II glycoproteins, and stimulation of cognate helper T cells (Shlomchik and Weisel, 2012; Victora and Nussenzweig, 2012). In addition, mutual re-inforcement evolves between B and T cells via CD40 and CD40L, and ICOS cross-stimulation by ICOSL, along with homotypic adhesion molecules of the SLAM receptor family and classical integrins (Cannons et al., 2010). Ultimately, these signals evoke and stabilize high expression of the transcription factor Bcl6 in both GC B cells and follicular helper T cells (Tfh). The micro-architecture of GCs revolves around a restricted zone of mesenchyme-derived (i.e., non-hematopoietic) follicular dendritic cells that capture immune complex-associated antigen and demarcate a Light Zone (LZ) highly enriched for the Bcl6hi “GC-Tfh” cells. B cells in this zone that, by virtue of having a ‘winning BCR affinity’ in the lottery, compete successfully for antigen stimulation and again for a limiting pool of cognate GC-Tfh cells (Allen et al., 2007; Gitlin et al., 2014; Shlomchik and Weisel, 2012; Tas et al., 2016; Victora and Nussenzweig, 2012; Victora et al., 2010), appear transiently to spike heightened PI3K activity and c-Myc protein expression (Calado et al., 2012; Dominguez-Sola et al., 2012). Ag-restimulated B cells then move to a Dark Zone (DZ), so designated originally because of the basophilia of proliferating cells. In the DZ, B cells cycle rapidly, and induced activation-induced cytosine deaminase (AID) introduces somatic point mutations into the rearranged V(D)J elements. While this sketch already underscores substantial non-homogeneity of the cells even within each zone (LZ, DZ), several broad points can be made on the relationships between metabolism and GC B cell differentiaiton.
First, gene expression profile analyses that compare GC-phenotype B cells to naïve follicular counterparts, or separate LZ and DZ cells for the analyses, show broad but not universal increases in mRNA encoding nutrient transporters and enzymes organized to support growth and intermediary metabolism (Calado et al., 2012; Dominguez-Sola et al., 2012; Victora et al., 2010). It might seem paradoxical that the most proliferative B cells, those actually needing to double their mass several times daily in the DZ, have lower mRNA for many of these metabolic suites than their LZ counterparts. However, this effect probably is due to a difference in persistence of the relevant proteins as compared to transcriptional control. In point of fact, much of the discourse centers on c-Myc as the transcriptional organizer of these metabolic transcriptional suites, but fluorescent reporter analyses reveal Myc expression (i.e., an amount above some limit-of-detection cut-off) only in a minority of B cells even in the LZ (Calado et al., 2012; Chou et al., 2016; Dominguez-Sola et al., 2012). Recent findings suggest that GSK3-mediated degradation of c-Myc may be critical for this regulation (Jellusova et al., 2017). Thus, while c-Myc is definitely essential for GC organization and likely initiates an organized increase in key metabolic pathways, it may act only transiently. In line with the basic premise of this model, GC B cells have been found to have greater staining for mitochondrial mass and higher uptake of a glucose analog in vivo (Jellusova et al., 2017). Ultimately, elucidation of the temporal sequences in programming the relays to transcription factors and gene expression through signaling relays awaits the development and use of improved single-cell technologies to be applied in situ.
Second, consideration of the connections between metabolic programming and metabolite influences on cell differentiation and function leads to the fact that the concentrations of nutrients and metabolic byproducts (e.g., lactate) in the interstitial micro-environment are likely to be part of the overall equation. Early low-resolution analyses reported that some portions of the spleen had low oxygen tensions whereas others did not (Caldwell et al., 2001). Consistent with this observation, recent papers have made use of chemical probes to investigate the presence of oxygen tensions well below the standard venous concentration, i.e., an imbalance between supply available for B cells and aggregate demand in and outside of GCs (Abbott et al., 2016; Cho et al., 2016; Jellusova et al., 2017). These analyses uncover evidence that the normal physiology of GCs includes an enrichment of hypoxic B cells localizing to the LZ. This feature of the nutrient landscape of the LZ led to stabilization of hypoxia-induced transcription factors (HIF) (Cho et al., 2016). The low oxygen tension reduced mTORC1 activity in part via HIF-mediated dampening of the PI3K-driven increases in amino acid transport (Cho et al., 2016). Moreover, HIF stabilization could shift metabolism toward increased glycolysis (Cho et al., 2016), which was observed in other systems tied to Myc and aerobic glycolysis (Gordan et al., 2007; Zhang et al., 2007). In addition to the inference that the low oxygen tension of the GC LZ contributes to setting a threshold for selection of B cells (Cho et al., 2016; Jellusova et al., 2017), this external metabolic determinant exerted complex time, isotype, and probably stimulus-dependent effects on activation-induced deaminase (AID) expression and antibody class switching. Moreover, “locked-in” states of HIF stabilization or reduced mTORC1 restricted to B cells interfere with GCs, antibody affinity maturation and the capacity for memory responses (Cho et al., 2016; Jones et al., 2016; Keating et al., 2013). Collectively, these findings suggest that the capacity to cope with challenges posed by low oxygen tension via HIF, toggling of the PI3K pathway (Jellusova and Rickert, 2016), and likely other sensors and signal transducers is a crucial mechanism for optimizing antibody responses.
The asymmetry model is among unsettled issues pertaining to the GC that may play into allocations of potential fates among death, continuation (“self-renewal”), and differentiation into switched or unswitched memory B cells or long-lived plasma cells. Pioneering work in this area observed in situ asymmetric distributions of Bcl6 and IL-21R between GC B cells prior to cytokinesis (Barnett et al., 2012). Investigation of such issues about GC B cells is hampered by the lack of a suitable in vitro model and the time (days) and extensive divisions that take place before Ag-activated naïve B cells are recruited to form a GC. In any case, follow-up work in vitro and in vivo suggests that polarity in contacts and asymmetry in distribution of mitochondria and other cellular constituents may influence mTOR signal strength and the probabilities of different outcomes for B cells (Adams et al., 2016). These findings, taken together with work in CD8+ T cells, suggest that the interplay of nutrient supplies, especially amino acids, mTORC1, and c-Myc may be part of a contribution of asymmetric distribution toward overall regulation of GC outcomes.
Finally, although the principal focus of this review is on the B lineage in humoral immunity, the nature of the GC reaction warrants mention of the follicular T cells – both follicular helpers (conventional Tfh) and FoxP3-expressing CXCR5+ regulator cells in the GC (Chung et al., 2011; Linterman et al., 2011; Wollenberg et al., 2011). To the extent that Tfh cells are essential for supporting strong antibody responses, early work with T lineage-specific loss of function experiments show that mTOR in T cells promotes humoral immunity (Delgoffe et al., 2011; Lee et al., 2010). Intriguingly, the transcriptional repressor Bcl6, stably high expression of which is the hallmark of Tfh and GCB cells, appear directly to inhibit expression of glycolytic genes, but with the potential for distinct programs depending on expression of T-bet and antagonism of the Bcl6 effect (Oestreich et al., 2014). A spate of recent papers has documented requirements for mTORC1 and mTORC2 in the aggregate follicular CD4+ T cell populations (Ray et al., 2015; Zeng et al., 2016). Recent work modeling the impact of T-follicular regulatory (Tfr) cells on Tfh cells and B cells in vitro suggests that Tfr cells may suppress IgG1 production stemming from activated B cells by altering Myc expression and the mTOR pathway (Sage et al., 2016). How these will connect to emerging aspects of the nutrient milieu as it impacts co-localized B and T cells in the hypoxic Light Zone remains to be determined. Moreover, perhaps in part due to the technical challenges posed by the inability to propagate follicular dendritic cells in vitro, little is known about the relationship between metabolism and these organizers of the GC and its Light Zone.
Transcriptional regulation of B cell metabolism
Transcriptional regulation of early B cell differentiation is relatively well understood, whereas our understanding of the genetic programs that direct peripheral B cell fate decisions is a work in progress and often involves relationships of reciprocal antagonism. Initial B cell activation results in cell cycle entry, migration to T cell-rich regions and the induction of co-stimulatory molecules to recruit T cell help and undergo clonal expansion. Thus, the stages of B cell activation and differentiation in T cell-dependent responses are temporally and spatially distinct and involve the outcome of multiple receptor-ligand interactions. These stages also reflect metabolic needs that evolve depending on proliferation status and cell fate commitment (e.g. memory B versus plasma cell). Initial recognition of antigen by the BCR promotes cell cycle entry and an increase in cell biomass. Efficient progression through the cell cycle to drive clonal expansion in the context of the GC reaction or extrafollicular antibody response requires costimulation via TLRs, CD40 or cytokine receptors (e.g. IL-4, IL-21). The individual contributions of critical B cell transcription factors to metabolic regulation of peripheral B cell differentiation is described below and represented in Table I and Figure 3.
Table 1.
Transcriptional Regulation of B Cell Metabolism.
| Cellular Function | Transcription Factor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Irf4 | c-Rel | E2A/E2-2 | Pax5 | Blimp1 | Bcl6 | Myc | Ap4 | Foxol | Hif | |
| Initial B cell activation | + | + | ± | ± | ± | ± | + | ± | ± | ± |
| Proliferation | + | + | + | ± | ± | ± | + | + | + | + |
| Survival | + | + | ± | ± | ± | ± | − | ± | ± | − |
| Plasma cell formation | + | + | + | − | + | − | + | + | − | + |
| Class switch recombination | + | + | + | + | ± | ± | ± | ± | + | − |
| Germinal center (overall) | + | + | + | + | ± | + | + | + | ± | − |
| Germinal center light zone function | ± | + | + | ± | − | + | + | ± | ± | − |
| Germinal center dark zone function | ± | + | + | ± | − | + | ± | + | + | ± |
| Memory B cell persistence | − | + | ± | ± | − | + | ± | ± | ± | ± |
Symbols indicate the following: +, promotes: −, inhibits; ±, no known direct effect.
Figure 3. Transcriptional regulation of B cell metabolism.
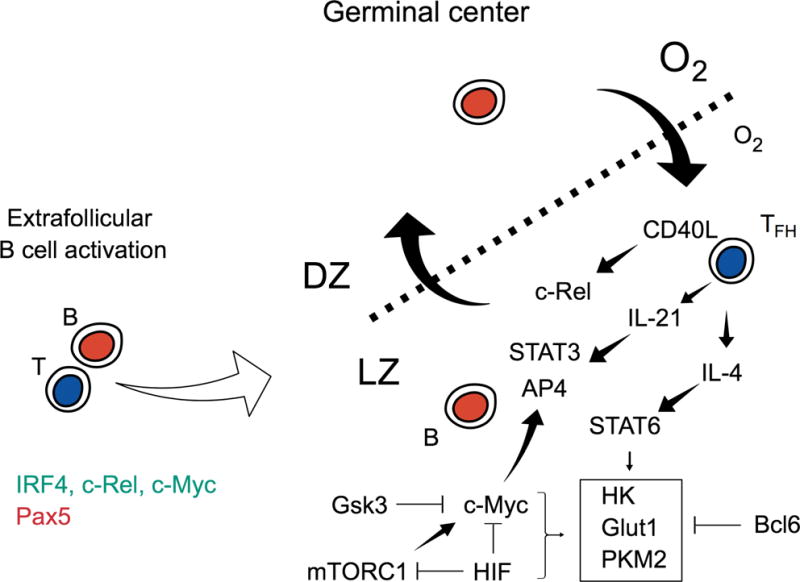
Schematic shows initial B cell activation by antigen and T cell help leading to the upregulation (green) and downregulation of key transcription factors. Events in the germinal center are dynamic as B cell traverse between selection in the hypoxic light zone (LZ) and proliferation in the dark zone (DZ). In the LZ, B cells retrieve antigen from follicular dendritic cell-bound antigen for presentation to TFH cells that reciprocate by providing cytokine support in the form of CD40L, IL-4 and IL-21. These cytokines induce the expression of select transcription factors that drive aspects of the metabolic program. The transcription factors c-Myc, HIF and STAT6 promote expression of glycolytic genes, whereas Bcl6 suppressed the transcription of some of the same target genes. HIF inhibits c-Myc activity which, among other events, would impair transcription of AP4 target genes.
c-Myc is a master regulator of cell proliferation and growth, promoting transcription and translation of key effectors in response to nutrient uptake and mTOR activation (Stine et al., 2015). Consistently, c-Myc is essential for B cell growth and proliferation (de Alboran et al., 2001), including positive selection of B cells in the light zone of the GC (Calado et al., 2012; Dominguez-Sola et al., 2012). c-Myc-expressing cells in the GC upregulate metabolic genes associated with nutrient sensing and glycolysis (Dominguez-Sola et al., 2012). c-Myc is only expressed in a small fraction of light zone B cells, and apparently must overcome or avoid repression by Bcl6 (Calado et al., 2012; Dominguez-Sola et al., 2012; Shaffer et al., 2000). Interestingly, c-Myc induces the transcription factor Activator Protein 4 (AP4) to maintain IL-21 signaling and transit to the dark zone of the GC (Chou et al., 2016). Underscoring the impact and regulation of c-Myc, threshold-based signaling confers c-Myc function during clonal expansion and fate determination (Heinzel et al., 2017).
The IRF4 transcription factor is expressed at low concentrations in resting B cells where it promotes survival, and is rapidly upregulated in accordance with the strength of signal provided by cytokines, TLR ligands or antigen stimulation. IRF4 is essential for plasma cell differentiation and commitment to the GC response (De Silva and Klein, 2015). While IRF4-dependent metabolic regulation has not been directly addressed in B cells, in T cells IRF4 has been shown to be repressed by rapamycin treatment and regulate genes that promote glucose uptake and the glycolytic response, while oxidative phosphorylation is not directly affected (Man et al., 2013; Yao et al., 2013). Many of the IRF4 target genes in this context bear AP1–IRF4 composite sites and thus may be co-regulated by c-Myc (Man et al., 2013). While IRF4 is not widely expressed in the GC, a small fraction of IRF4+ cells co-expressing c-Myc are present and may represent progeny of asymmetric division destined to become plasmablasts (Calado et al., 2012; Dominguez-Sola et al., 2012; Lin et al., 2015; Ochiai et al., 2013).
In M2 macrophages, IL-4 also promotes Irf4 expression and glycolysis but in an mTORC2-dependent manner, acting in parallel with STAT6-dependent induction of Irf4 (Huang et al., 2016). By contrast, IL-4 induces Glut1 upregulation and glycolysis in a STAT6-dependent but PI3K-independent manner (Baracho et al., 2014; Dufort et al., 2007). The glycolytic STAT6 target genes remain to be identified but could include Pkm2 (Sajic et al., 2013), which catalyzes the final and irreversible step of glycolysis in the production of pyruvate and ATP. IRF4 also collaborates with STAT3 to drive IL-21 responses and plasma cell formation via Prdm1 (Kwon et al., 2009), but regulation of metabolic genes has not been addressed.
Similar to IRF4, c-Rel is rapidly upregulated upon activation and in fact drives Irf4 expression (Grumont and Gerondakis, 2000). Translocation of c-Rel is PI3K-dependent and forced expression of c-Rel can compensate for impaired PI3K signaling (Matsuda et al., 2009). Recent studies have shown that c-Rel drives a broad metabolic program that is required to support the energetic and biosynthetic needs of rapidly proliferating GC B cells (Heise et al., 2014). c-Rel translocation only occurs is a small fraction of GC B cells, likely reflecting those receiving T cell help in the form of CD40 stimulation (Basso et al., 2004).
The recent findings that the light zone of the GC is a hypoxic environment introduces a new contributor to B cell selection and metabolism (Abbott et al., 2016; Cho et al., 2016; Jellusova et al., 2017). Hypoxia inducible factors 1 and 2, regulated by stabilization of HIF1α or HIF2α, respectively, drive the glycolytic program through the induction of aldolase A (ALDA), phosphoglycerate kinase 1 (PGK1), and the M2 isoform of pyruvate kinase (PKM2) (Semenza et al., 1994). Moreover, PKM2 also functions as a co-activator of HIF1α to effect a positive feedback loop that amplifies the glycolytic program (Luo et al., 2011). Anabolic growth and respiration may be restrained given that HIF expression inhibits mTORC1 and c-Myc activity (Cho et al., 2016; Zhang et al., 2007). With respect to the latter, c-Myc and HIF both drive glycolysis (Gordan et al., 2007), but HIF represses the Krebs cycle and respiration by inducing the expression of pyruvate dehydrogenase kinase 1 (Kim et al., 2006), and c-Myc has the unique property of promoting mitochondrial biogenesis (Li et al., 2005). Hypoxia may also promote plasma cell differentiation (Abbott et al., 2016), which would be consistent with Irf4 being a HIF-regulated gene (Man et al., 2013; Yao et al., 2013). These collective findings provide the first insights into the complexities and likely dynamic regulation of GC B cell metabolism by hypoxic versus normoxic conditions.
The Bcl6 transcription factor is associated with and required for GC B cell identity and the unique processes therein, consistent with its induction by IL-21 (Linterman et al., 2010; Zotos et al., 2010). It is also an obligatory target of downregulation by the transcription factor Blimp1 to effect the plasma cell differentiation program (Mendez and Mendoza, 2016). Bcl6 has been noted to suppress the glycolysis pathway in macrophages (Oestreich et al., 2014), and thus may counter HIF activity in addition to c-Myc in the GC.
The transcription factors Bach2, Pax5 and Foxo1 act in concert with Bcl6 and antagonize plasma cell differentiation (Recaldin and Fear, 2016), whereas E2A and E2-2 transcription factors are required to drive the GC reaction as well as plasma cell differentiation (Gloury et al., 2016; Quong et al., 1999; Wohner et al., 2016) (Table 1). The contribution of these factors to cellular metabolism in B cells remains to be elucidated. That said, Pax5 has recently been shown to repress energy metabolism in early B cells (Chan et al., 2017), and Foxo1 is known to counter Myc activity in other cell types (Wilhelm et al., 2016), so extrapolation from these studies may be helpful in future studies of mature B cell metabolism.
Metabolic reprogramming of antibody producing cells
Unlike T cells, the end of the line(age) for B cells involves a fundamental self-reinvention from which non-cycling plasma cells arise after a transitional period as plasmablasts. This terminal differentiation event, programmed by the transcription factors Blimp-1 and IRF4 along with silencing of the B cell-defining Pax5 transcription factor (Table I), restructures the cells and their economy for the single-minded purpose of industrial-scale secretion of glycosylated antibodies. With IgH and IgL transcripts constituting ~95% of their mRNA, these cells rich in rough endoplasmic reticulum need a reprogrammed endoplasmic reticulum (ER) stress response, in part through Blimp1-regulated XBP-1, a component of one of the trio of pathways sensing and dealing with unfolded protein. It should be noted that there are probably multiple programs and types of plasma cells, and the needs of plasmablasts (still cycling and dividing, and yet already secreting antibodies) will differ from those of plasma cells. Thus, beyond the challenge of elucidating what differs between the short- and long-lived plasma cell (SLPC and LLPC, respectively), there likely will be differences based on anatomic site (e.g., intestinal vs nasal or BALT vs marrow), and the anatomic sites or plasma cell niches may differ in their concentrations of oxygen tension and other nutrients. Whatever the site, the nature of the plasma cell requires both substantial energy for all the glycoprotein synthesis and large supplies of carbon and amines for the export economy. Nonetheless, the potential implications are vast should it be that aspects of programming the LLPCs devoted to persistent production of pathological auto-antibodies differ from those that are protective against pathogens or promote a healthy relationship with one’s microbiome.
Inasmuch as the longevity of LLPC and the persistence of their production of protective antibodies are essential for the efficacy of most vaccines, what supports these qualities is a vital issue. In an approach analogous to work on memory lymphocytes, a complex role for autophagy has been inferred from loss-of-function analyses with the mouse Atg5 gene (Pengo et al., 2013). In the short run, this means of crippling autophagy leads to increased rates of antibody section from plasma cell populations, but over a longer period ATG5-deficient LLPC exhibit a progressive decline. If analogous to findings with B cell memory, reduced lifespan might be attributable to a weaker capacity to perform quality-control on mitochondria (Chen et al., 2014; Chen et al., 2015). The involvement of autophagy in lysosomal generation of amino acids to provide for Rheb and mTORC1 activity might represent an alternative mechanism, but evidence for this possibility is lacking. A second mechanism, which may be metabolism-related, emerged from conditional loss-of-function experiments with the pro-survival Bcl2 superfamily member Mcl-1 (Peperzak et al., 2013; Vikstrom et al., 2010). Inasmuch as there are biochemical studies that point to regulation of Mcl-1 amounts by GSK3 and in turn by nutrient supply (Maurer et al., 2006; Zhao et al., 2007), it is tempting to extrapolate a connection between Mcl-1 and the findings with GSK3 depletion (Jellusova et al., 2017). Finally, recent work indicates that continued expression of Blimp-1 and IRF4 is vital for maintenance of plasma cells and Ig secretion that evolved in a setting devoid of T cells (Tellier et al., 2016). Intriguingly, the plasma cells remaining after loss of Blimp-1 expressed lower concentrations of mRNA encoding several amino acid transporters and less mTORC1 activity.
Finally, recent work in which mitochondrial uptake of pyruvate is crippled by inactivation of the Mpc2 gene encoding pyruvate carrier protein subunit also launches some metabolic tracing insights pertaining to durable plasma cell survival. While technical issues pose big challenges in the area, a new line of work provided evidence that LLPC have both greater mitochondrial respiration and reserve capacity than SLPC (Lam et al., 2016). LLPC also had higher labeling with the glucose analogue than SLPC, but metabolic tracing analyses indicated that most of the carbon from glucose was devoted to synthesis of the sugars needed for glycosylation of the antibodies produced by these cells rather than to feeding Krebs TCA Cycle. While glutamine utilization will be important to understand in this setting, it appears that fatty acid oxidation is a major component of the respiratory needs (ibid), which is not apparent in B cells. In summary, systematic analyses in this area are at an early part of dawn.
Challenges & future prospects
This review and its companion articles in this Issue of Immunity highlight the substantial evidence that external nutrient and micro-environmental conditions in concert with cell-intrinsic (internal) reorganization of metabolism and sensor pathways condition immunity. In the case of humoral immunity, the body of work with B cells is as yet far less extensive or developed than that with T cells. Accordingly, the simplest prospect for the future is for the extent of analyses with B lineage cells to be filled in as much as for T cells. First steps toward filling this need include tracing the flows of carbon and nitrogen in the anabolic and energetic economies of these cells that ultimately yield protective and pathological antibodies. However, the findings underscore a number of areas in which fundamental insights and even the tools needed to start to investigate key questions are all lacking. Among the foremost of the challenges is that although T cell help and GC processes are so powerful as determinants of the vigor of humoral immunity and of pathogenic autoantibodies, there is no in vitro means of truly approximating key aspects of the GC.
Following from studies in stem and cancer cells (Carey et al., 2015; Oldham et al., 2015; Thienpont et al., 2016), the potential for mechanistic links between intracellular metabolites to effect meaningful, stable, and heritable (as cells divide) changes in the transcriptional networks that create (meta)stable epigenetic states needs to be elucidated. Initial efforts in this general area are already stirring but will only intensify and become more rigorous. The actual (not just relative) concentrations of αKG - a cofactor enhancing activity of histone lysine demethylases and TET enzymes that can drive loss of CpG methylation – and the inhibitors succinate and stereo-isomers of 2-hydroxyglutarate (Oldham et al., 2015; Tannahill et al., 2013; Tyrakis et al., 2016) need to be determined. Their subcellular localization and net effect on the various dioxygenase enzymes that can impact immunity – among them, prolyl hydroxyl dioxygenases that regulate HIF stabilization as well as the Jumonji lysine demethylases and TET proteins – are as yet not known. Examples of the possibilities also include pools of acetyl-CoA for histone acetylation. For all of these processes, finally, it will be essential to measure the rate at which change is effected under physiological conditions. As noted in the previous paragraph, an added challenge is posed by the inability of in vitro approaches to model the cycling and regulation of GC B cells, since such experiments provide crucial control over environmental conditions.
As such, a second pressing need on the frontier is the unmet need to be able to measure what actually is the composition of extra-cellular (interstitial) and single-cell micro-environments. At present, single-cell technologies for analysis of nucleic acids after amplification permit bioinformatic estimates to correlate DNA and histone modifications with concentrations of RNA expressed in cells of defined phenotype. However, technical capabilities are needed for measurements of metabolically relevant molecules outside cells (e.g. glucose, glutamine, lactate, et alia) and to distinguish various micro-anatomic niches (e.g., LZ vs DZ). Moreover, phenotypic lag and post-translational regulation of enzyme activities highlight the desirability of measurements of such proteins’ functions in cells. Ironically, work far ahead of its time had applied cyclical amplification to measurements of enzyme activities or post-translational modifications (Henriksson et al., 1986; Henriksson et al., 1988; Manchester et al., 1994). Moreover, the virtues of 2-deoxyglucose as a clinically useful probe to estimate regions of high glucose utilization point to the value of having further in vivo probes, eventually to be applied even in real time. This incomplete set of brushstrokes hints at a picture whose development will require substantial technological advances and high spatial resolution. Excitingly, though, the fact that manipulations of these processes can alter immune function or pathology indicates that we are only at the beginnings of the story even after starting the second hundred years of immunology as an organized discipline whose findings illuminate work in most areas of human health and disease.
Acknowledgments
The authors apologize in advance for and regret any omissions, either unintended or due to space limitations or a chosen focus on events after B cell maturation and exclusion of the complex topics involving malignant B lineage cells. We gratefully acknowledge a critical reading of drafts by A. Raybuck and J. Jellusova. Preparation of this review was supported by NIH grants AI113292 (M.B.), HL106812 (M.B.) and AI41649 (R.C.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aagaard-Tillery KM, Jelinek DF. Inhibition of human B lymphocyte cell cycle progression and differentiation by rapamycin. Cell Immunol. 1994;156:493–507. doi: 10.1006/cimm.1994.1193. [DOI] [PubMed] [Google Scholar]
- Abbott RK, Thayer M, Labuda J, Silva M, Philbrook P, Cain DW, Kojima H, Hatfield S, Sethumadhavan S, Ohta A, et al. Germinal Center Hypoxia Potentiates Immunoglobulin Class Switch Recombination. J Immunol. 2016;197:4014–4020. doi: 10.4049/jimmunol.1601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams WC, Chen YH, Kratchmarov R, Yen B, Nish SA, Lin WW, Rothman NJ, Luchsinger LL, Klein U, Busslinger M, et al. Anabolism-Associated Mitochondrial Stasis Driving Lymphocyte Differentiation over Self-Renewal. Cell Rep. 2016;17:3142–3152. doi: 10.1016/j.celrep.2016.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
- Arnold J, Murera D, Arbogast F, Fauny JD, Muller S, Gros F. Autophagy is dispensable for B-cell development but essential for humoral autoimmune responses. Cell Death Differ. 2016;23:853–864. doi: 10.1038/cdd.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov M, Tirosh B. Metabolic Control of Plasma Cell Differentiation- What We Know and What We Don’t Know. J Clin Immunol. 2016;36(Suppl 1):12–17. doi: 10.1007/s10875-016-0246-9. [DOI] [PubMed] [Google Scholar]
- Baracho GV, Cato MH, Zhu Z, Jaren OR, Hobeika E, Reth M, Rickert RC. PDK1 regulates B cell differentiation and homeostasis. Proc Natl Acad Sci U S A. 2014;111:9573–9578. doi: 10.1073/pnas.1314562111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett BE, Ciocca ML, Goenka R, Barnett LG, Wu J, Laufer TM, Burkhardt JK, Cancro MP, Reiner SL. Asymmetric B cell division in the germinal center reaction. Science. 2012;335:342–344. doi: 10.1126/science.1213495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso K, Klein U, Niu H, Stolovitzky GA, Tu Y, Califano A, Cattoretti G, Dalla-Favera R. Tracking CD40 signaling during germinal center development. Blood. 2004;104:4088–4096. doi: 10.1182/blood-2003-12-4291. [DOI] [PubMed] [Google Scholar]
- Blair D, Dufort FJ, Chiles TC. Protein kinase Cbeta is critical for the metabolic switch to glycolysis following B-cell antigen receptor engagement. Biochem J. 2012;448:165–169. doi: 10.1042/BJ20121225. [DOI] [PubMed] [Google Scholar]
- Blanchet E, Annicotte JS, Lagarrigue S, Aguilar V, Clape C, Chavey C, Fritz V, Casas F, Apparailly F, Auwerx J, Fajas L. E2F transcription factor-1 regulates oxidative metabolism. Nat Cell Biol. 2011;13:1146–1152. doi: 10.1038/ncb2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado DP, Sasaki Y, Godinho SA, Pellerin A, Kochert K, Sleckman BP, de Alboran IM, Janz M, Rodig S, Rajewsky K. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol. 2012;13:1092–1100. doi: 10.1038/ni.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamito M, Juntilla MM, Thomas M, Northrup DL, Rathmell J, Birnbaum MJ, Koretzky G, Allman D. Akt1 and Akt2 promote peripheral B-cell maturation and survival. Blood. 2010;115:4043–4050. doi: 10.1182/blood-2009-09-241638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell CC, Kojima H, Lukashev D, Armstrong J, Farber M, Apasov SG, Sitkovsky MV. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J, Browne CD, Ruppert R, Feral CC, Fassler R, Rickert RC, Ginsberg MH. CD98hc facilitates B cell proliferation and adaptive humoral immunity. Nat Immunol. 2009;10:412–419. doi: 10.1038/ni.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey BW, Finley LWS, Cross JR, Allis CD, Thompson CB. Intracellular [agr]-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green DR, Rathmell JC. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol. 2014;192:3626–3636. doi: 10.4049/jimmunol.1302062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LN, Chen Z, Braas D, Lee JW, Xiao G, Geng H, Cosgun KN, Hurtz C, Shojaee S, Cazzaniga V, et al. Metabolic gatekeeper function of B-lymphoid transcription factors. Nature. 2017;542:479–483. doi: 10.1038/nature21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Chen M, Hong MJ, Sun H, Wang L, Shi X, Gilbert BE, Corry DB, Kheradmand F, Wang J. Essential role for autophagy in the maintenance of immunological memory against influenza infection. Nat Med. 2014;20:503–510. doi: 10.1038/nm.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Kodali S, Jang A, Kuai L, Wang J. Requirement for autophagy in the long-term persistence but not initial formation of memory B cells. J Immunol. 2015;194:2607–2615. doi: 10.4049/jimmunol.1403001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Ahn AK, Bhargava P, Lee CH, Eischen CM, McGuinness O, Boothby M. Glycolytic rate and lymphomagenesis depend on PARP14, an ADP ribosyltransferase of the B aggressive lymphoma (BAL) family. Proc Natl Acad Sci U S A. 2011;108:15972–15977. doi: 10.1073/pnas.1017082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Raybuck AL, Stengel K, Wei M, Beck TC, Volanakis E, Thomas JW, Hiebert S, Haase VH, Boothby MR. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature. 2016;537:234–238. doi: 10.1038/nature19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C, Verbaro DJ, Tonc E, Holmgren M, Cella M, Colonna M, Bhattacharya D, Egawa T. The Transcription Factor AP4 Mediates Resolution of Chronic Viral Infection through Amplification of Germinal Center B Cell Responses. Immunity. 2016;45:570–582. doi: 10.1016/j.immuni.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AJ, Ellinghaus U, Cortini A, Stranks A, Simon AK, Botto M, Vyse TJ. Autophagy is activated in systemic lupus erythematosus and required for plasmablast development. Annals of the Rheumatic Diseases. 2015;74:912. doi: 10.1136/annrheumdis-2013-204343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran LM, Tarlinton DM. Regulation of germinal center responses, memory B cells and plasma cell formation-an update. Curr Opin Immunol. 2016;39:59–67. doi: 10.1016/j.coi.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Das F, Ghosh-Choudhury N, Mariappan MM, Kasinath BS, Choudhury GG. Hydrophobic motif site-phosphorylated protein kinase CβII between mTORC2 and Akt regulates high glucose-induced mesangial cell hypertrophy. American Journal of Physiology - Cell Physiology. 2016;310:C583. doi: 10.1152/ajpcell.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey AM, Pierce SK. Intrinsic differences in the initiation of B cell receptor signaling favor responses of human IgG(+) memory B cells over IgM(+) naive B cells. J Immunol. 2012;188:3332–3341. doi: 10.4049/jimmunol.1102322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Alboran IM, O’Hagan RC, Gartner F, Malynn B, Davidson L, Rickert R, Rajewsky K, DePinho RA, Alt FW. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity. 2001;14:45–55. doi: 10.1016/s1074-7613(01)00088-7. [DOI] [PubMed] [Google Scholar]
- De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Sola D, Victora GD, Ying CY, Phan RT, Saito M, Nussenzweig MC, Dalla-Favera R. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol. 2012;13:1083–1091. doi: 10.1038/ni.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufort FJ, Bleiman BF, Gumina MR, Blair D, Wagner DJ, Roberts MF, Abu-Amer Y, Chiles TC. Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J Immunol. 2007;179:4953–4957. doi: 10.4049/jimmunol.179.8.4953. [DOI] [PubMed] [Google Scholar]
- Dufort FJ, Gumina MR, Ta NL, Tao Y, Heyse SA, Scott DA, Richardson AD, Seyfried TN, Chiles TC. Glucose-dependent de novo lipogenesis in B lymphocytes: a requirement for atp-citrate lyase in lipopolysaccharide-induced differentiation. J Biol Chem. 2014;289:7011–7024. doi: 10.1074/jbc.M114.551051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alvarez G, Ventura V, Ros O, Aligue R, Gil J, Tauler A. Glycogen synthase kinase-3beta binds to E2F1 and regulates its transcriptional activity. Biochim Biophys Acta. 2007;1773:375–382. doi: 10.1016/j.bbamcr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature. 2014;509:637–640. doi: 10.1038/nature13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin AD, von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent Roles of Switching and Hypermutation in the Development and Persistence of B Lymphocyte Memory. Immunity. 2016;44:769–781. doi: 10.1016/j.immuni.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloury R, Zotos D, Zuidscherwoude M, Masson F, Liao Y, Hasbold J, Corcoran LM, Hodgkin PD, Belz GT, Shi W, et al. Dynamic changes in Id3 and E-protein activity orchestrate germinal center and plasma cell development. J Exp Med. 2016;213:1095–1111. doi: 10.1084/jem.20152003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- Grumont RJ, Gerondakis S. Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor kappaB. J Exp Med. 2000;191:1281–1292. doi: 10.1084/jem.191.8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniuda K, Fukao S, Kodama T, Hasegawa H, Kitamura D. Autonomous membrane IgE signaling prevents IgE-memory formation. Nat Immunol. 2016;17:1109–1117. doi: 10.1038/ni.3508. [DOI] [PubMed] [Google Scholar]
- Hawkins ED, Oliaro J, Kallies A, Belz GT, Filby A, Hogan T, Haynes N, Ramsbottom KM, Van Ham V, Kinwell T, et al. Regulation of asymmetric cell division and polarity by Scribble is not required for humoral immunity. Nat Commun. 2013;4:1801. doi: 10.1038/ncomms2796. [DOI] [PubMed] [Google Scholar]
- Heinzel S, Binh Giang T, Kan A, Marchingo JM, Lye BK, Corcoran LM, Hodgkin PD. A Myc-dependent division timer complements a cell-death timer to regulate T cell and B cell responses. Nat Immunol. 2017;18:96–103. doi: 10.1038/ni.3598. [DOI] [PubMed] [Google Scholar]
- Heise N, De Silva NS, Silva K, Carette A, Simonetti G, Pasparakis M, Klein U. Germinal center B cell maintenance and differentiation are controlled by distinct NF-kappaB transcription factor subunits. J Exp Med. 2014;211:2103–2118. doi: 10.1084/jem.20132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley T, Kovesdi D, Turner M. B-cell responses to B-cell activation factor of the TNF family (BAFF) are impaired in the absence of PI3K delta. Eur J Immunol. 2008;38:3543–3548. doi: 10.1002/eji.200838618. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Chi MM, Hintz CS, Young DA, Kaiser KK, Salmons S, Lowry OH. Chronic stimulation of mammalian muscle: changes in enzymes of six metabolic pathways. Am J Physiol. 1986;251:C614–632. doi: 10.1152/ajpcell.1986.251.4.C614. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Salmons S, Chi MY, Hintz CS, Lowry OH. Chronic stimulation of mammalian muscle: changes in metabolite concentrations in individual fibers. Am J Physiol. 1988;255:C543–551. doi: 10.1152/ajpcell.1988.255.4.C543. [DOI] [PubMed] [Google Scholar]
- Hobeika E, Levit-Zerdoun E, Anastasopoulou V, Pohlmeyer R, Altmeier S, Alsadeq A, Dobenecker MW, Pelanda R, Reth M. CD19 and BAFF-R can signal to promote B-cell survival in the absence of Syk. EMBO J. 2015;34:925–939. doi: 10.15252/embj.201489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, Pearce EJ. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity. 2016;45:817–830. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- Jellusova J, Cato MH, Apgar JR, Ramezani-Rad P, Leung CR, Chen C, Richardson AD, Conner EM, Benschop RJ, Woodgett JR, Rickert RC. Gsk3 is a metabolic checkpoint regulator in B cells. Nat Immunol advance online publication. 2017 doi: 10.1038/ni.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellusova J, Miletic AV, Cato MH, Lin WW, Hu Y, Bishop GA, Shlomchik MJ, Rickert RC. Context-specific BAFF-R signaling by the NF-kappaB and PI3K pathways. Cell Rep. 2013;5:1022–1035. doi: 10.1016/j.celrep.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellusova J, Rickert RC. The PI3K pathway in B cell metabolism. Crit Rev Biochem Mol Biol. 2016;51:359–378. doi: 10.1080/10409238.2016.1215288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DD, Gaudette BT, Wilmore JR, Chernova I, Bortnick A, Weiss BM, Allman D. mTOR has distinct functions in generating versus sustaining humoral immunity. J Clin Invest. 2016;126:4250–4261. doi: 10.1172/JCI86504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating R, Hertz T, Wehenkel M, Harris TL, Edwards BA, McClaren JL, Brown SA, Surman S, Wilson ZS, Bradley P, et al. The kinase mTOR modulates the antibody response to provide cross-protective immunity to lethal infection with influenza virus. Nat Immunol. 2013;14:1266–1276. doi: 10.1038/ni.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AP, Finlay DK, Hinton HJ, Clarke RG, Fiorini E, Radtke F, Cantrell DA. Notch-induced T cell development requires phosphoinositide-dependent kinase 1. EMBO J. 2007;26:3441–3450. doi: 10.1038/sj.emboj.7601761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Saeki K, Yuo A. Granulocyte-macrophage colony-stimulating factor and interleukin-3 induce cell cycle progression through the synthesis of c-Myc protein by internal ribosome entry site–mediated translation via phosphatidylinositol 3-kinase pathway in human factor–dependent leukemic cells. Blood. 2003;102:3186. doi: 10.1182/blood-2003-02-0567. [DOI] [PubMed] [Google Scholar]
- Kometani K, Nakagawa R, Shinnakasu R, Kaji T, Rybouchkin A, Moriyama S, Furukawa K, Koseki H, Takemori T, Kurosaki T. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity. 2013;39:136–147. doi: 10.1016/j.immuni.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, Carotta S, Donovan CE, Goldman ML, Tailor P, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffleur B, Duchez S, Tarte K, Denis-Lagache N, Peron S, Carrion C, Denizot Y, Cogne M. Self-Restrained B Cells Arise following Membrane IgE Expression. Cell Rep. 2015;10:900–909. doi: 10.1016/j.celrep.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Lam WY, Becker AM, Kennerly KM, Wong R, Curtis JD, Llufrio EM, McCommis KS, Fahrmann J, Pizzato HA, Nunley RM, et al. Mitochondrial Pyruvate Import Promotes Long-Term Survival of Antibody-Secreting Plasma Cells. Immunity. 2016;45:60–73. doi: 10.1016/j.immuni.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–121. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IH, Finkel T. Metabolic regulation of the cell cycle. Curr Opin Cell Biol. 2013;25:724–729. doi: 10.1016/j.ceb.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Heffington L, Jellusova J, Nam KT, Raybuck A, Cho SH, Thomas JW, Rickert RC, Boothby M. Requirement for Rictor in homeostasis and function of mature B lymphoid cells. Blood. 2013;122:2369–2379. doi: 10.1182/blood-2013-01-477505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, Kim JW, Yustein JT, Lee LA, Dang CV. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limon JJ, So L, Jellbauer S, Chiu H, Corado J, Sykes SM, Raffatellu M, Fruman DA. mTOR kinase inhibitors promote antibody class switching via mTORC2 inhibition. Proc Natl Acad Sci U S A. 2014;111:E5076–5085. doi: 10.1073/pnas.1407104111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WH, Adams WC, Nish SA, Chen YH, Yen B, Rothman NJ, Kratchmarov R, Okada T, Klein U, Reiner SL. Asymmetric PI3K Signaling Driving Developmental and Regenerative Cell Fate Bifurcation. Cell Rep. 2015;13:2203–2218. doi: 10.1016/j.celrep.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man K, Miasari M, Shi W, Xin A, Henstridge DC, Preston S, Pellegrini M, Belz GT, Smyth GK, Febbraio MA, et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat Immunol. 2013;14:1155–1165. doi: 10.1038/ni.2710. [DOI] [PubMed] [Google Scholar]
- Manchester J, Kong X, Nerbonne J, Lowry OH, Lawrence JC., Jr Glucose transport and phosphorylation in single cardiac myocytes: rate-limiting steps in glucose metabolism. Am J Physiol. 1994;266:E326–333. doi: 10.1152/ajpendo.1994.266.3.E326. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Mikami Y, Ohtani M, Fujiwara M, Hirata Y, Minowa A, Terauchi Y, Kadowaki T, Koyasu S. Critical role of class IA PI3K for c-Rel expression in B lymphocytes. Blood. 2009;113:1037–1044. doi: 10.1182/blood-2008-06-163725. [DOI] [PubMed] [Google Scholar]
- Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Mayer A, Denanglaire S, Viollet B, Leo O, Andris F. AMP-activated protein kinase regulates lymphocyte responses to metabolic stress but is largely dispensable for immune cell development and function. Eur J Immunol. 2008;38:948–956. doi: 10.1002/eji.200738045. [DOI] [PubMed] [Google Scholar]
- Melchers F. Checkpoints that control B cell development. J Clin Invest. 2015;125:2203–2210. doi: 10.1172/JCI78083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez A, Mendoza L. A Network Model to Describe the Terminal Differentiation of B Cells. PLoS Comput Biol. 2016;12:e1004696. doi: 10.1371/journal.pcbi.1004696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, Nemirovski A, Shen-Orr S, Laevsky I, Amit M, et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21:392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Xiao Y, Zhou X, Chang JH, Chang M, Cheng X, Blonska M, Lin X, Sun SC. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Hu G, Wei G, Cui K, Yamane A, Resch W, Wang R, Green DR, Tessarollo L, Casellas R, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai K, Maienschein-Cline M, Simonetti G, Chen J, Rosenthal R, Brink R, Chong AS, Klein U, Dinner AR, Singh H, Sciammas R. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity. 2013;38:918–929. doi: 10.1016/j.immuni.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, Krishnamoorthy V, Weinmann AS. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat Immunol. 2014;15:957–964. doi: 10.1038/ni.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham WM, Clish CB, Yang Y, Loscalzo J. Hypoxia-Mediated Increases in L-2-hydroxyglutarate Coordinate the Metabolic Response to Reductive Stress. Cell Metab. 2015;22:291–303. doi: 10.1016/j.cmet.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori SA, Cato MH, Anzelon-Mills A, Puri KD, Shapiro-Shelef M, Calame K, Rickert RC. Regulation of class-switch recombination and plasma cell differentiation by phosphatidylinositol 3-kinase signaling. Immunity. 2006;25:545–557. doi: 10.1016/j.immuni.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331:1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med. 2006;203:2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pengo N, Scolari M, Oliva L, Milan E, Mainoldi F, Raimondi A, Fagioli C, Merlini A, Mariani E, Pasqualetto E, et al. Plasma cells require autophagy for sustainable immunoglobulin production. Nat Immunol. 2013;14:298–305. doi: 10.1038/ni.2524. [DOI] [PubMed] [Google Scholar]
- Peperzak V, Vikstrom I, Walker J, Glaser SP, LePage M, Coquery CM, Erickson LD, Fairfax K, Mackay F, Strasser A, et al. Mcl-1 is essential for the survival of plasma cells. Nat Immunol. 2013;14:290–297. doi: 10.1038/ni.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SK, Liu W. Encoding immunological memory in the initiation of B-cell receptor signaling. Cold Spring Harb Symp Quant Biol. 2013;78:231–237. doi: 10.1101/sqb.2013.78.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi KN, Sun IH, Patel CH, Lo YC, Oh MH, Waickman AT, Tam AJ, Blosser RL, Wen J, Delgoffe GM, Powell JD. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat Immunol. 2016;17:704–711. doi: 10.1038/ni.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quong MW, Harris DP, Swain SL, Murre C. E2A activity is induced during B-cell activation to promote immunoglobulin class switch recombination. EMBO J. 1999;18:6307–6318. doi: 10.1093/emboj/18.22.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, Laidlaw BJ, Araki K, Ahmed R, Kaech SM, Craft J. The Interleukin-2-mTORc1 Kinase Axis Defines the Signaling, Differentiation, and Metabolism of T Helper 1 and Follicular B Helper T Cells. Immunity. 2015;43:690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recaldin T, Fear DJ. Transcription factors regulating B cell fate in the germinal centre. Clin Exp Immunol. 2016;183:65–75. doi: 10.1111/cei.12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA. AMPKalpha1: a glucose sensor that controls CD8 T-cell memory. Eur J Immunol. 2013;43:889–896. doi: 10.1002/eji.201243008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage PT, Ron-Harel N, Juneja VR, Sen DR, Maleri S, Sungnak W, Kuchroo VK, Haining WN, Chevrier N, Haigis M, Sharpe AH. Suppression by TFR cells leads to durable and selective inhibition of B cell effector function. Nat Immunol. 2016;17:1436–1446. doi: 10.1038/ni.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajic T, Hainard A, Scherl A, Wohlwend A, Negro F, Sanchez JC, Szanto I. STAT6 promotes bi-directional modulation of PKM2 in liver and adipose inflammatory cells in rosiglitazone-treated mice. Scientific reports. 2013;3:2350. doi: 10.1038/srep02350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer E, Santos-Valente E, Keller B, Warnatz K, Boztug K. Protein Kinase C delta: a Gatekeeper of Immune Homeostasis. J Clin Immunol. 2016;36:631–640. doi: 10.1007/s10875-016-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz JL, Crowley JE, Tomayko MM, Steinel N, O’Neill PJ, Quinn WJ, 3rd, Goenka R, Miller JP, Cho YH, Long V, et al. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A. 2008;105:15517–15522. doi: 10.1073/pnas.0807841105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- Shaffer AL, Yu X, He Y, Boldrick J, Chan EP, Staudt LM. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity. 2000;13:199–212. doi: 10.1016/s1074-7613(00)00020-0. [DOI] [PubMed] [Google Scholar]
- Shlomchik MJ, Weisel F. Germinal center selection and the development of memory B and plasma cells. Immunol Rev. 2012;247:52–63. doi: 10.1111/j.1600-065X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14:500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine ZE, Walton ZE, Altman BJ, Hsieh AL, Dang CV. MYC, Metabolism, and Cancer. Cancer Discov. 2015;5:1024–1039. doi: 10.1158/2159-8290.CD-15-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Babic I, Nathanson D, Akhavan D, Guo D, Gini B, Dang J, Zhu S, Yang H, De Jesus J, et al. Oncogenic EGFR Signaling Activates an mTORC2–NF-κB Pathway That Promotes Chemotherapy Resistance. Cancer Discovery. 2011;1:524. doi: 10.1158/2159-8290.CD-11-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1[bgr] through HIF-1[agr] Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlinton D, Good-Jacobson K. Diversity Among Memory B Cells: Origin, Consequences, and Utility. Science. 2013;341:1205. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- Tas JM, Mesin L, Pasqual G, Targ S, Jacobsen JT, Mano YM, Chen CS, Weill JC, Reynaud CA, Browne EP, et al. Visualizing antibody affinity maturation in germinal centers. Science. 2016;351:1048–1054. doi: 10.1126/science.aad3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellier J, Shi W, Minnich M, Liao Y, Crawford S, Smyth GK, Kallies A, Busslinger M, Nutt SL. Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat Immunol. 2016;17:323–330. doi: 10.1038/ni.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thienpont B, Steinbacher J, Zhao H, D’Anna F, Kuchnio A, Ploumakis A, Ghesquiere B, Van Dyck L, Boeckx B, Schoonjans L, et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature. 2016;537:63–68. doi: 10.1038/nature19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrakis PA, Palazon A, Macias D, Lee KL, Phan AT, Velica P, You J, Chia GS, Sim J, Doedens A, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. 2016;540:236–241. doi: 10.1038/nature20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist KC, Guy CS, Milasta S, Liedmann S, Kaminski MM, Wang R, Green DR. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532:389–393. doi: 10.1038/nature17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- Victora GD, Schwickert TA, Fooksman DR, Kamphorst AO, Meyer-Hermann M, Dustin ML, Nussenzweig MC. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter. Cell. 2010;143:592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikstrom I, Carotta S, Lüthje K, Peperzak V, Jost PJ, Glaser S, Busslinger M, Bouillet P, Strasser A, Nutt SL, Tarlinton DM. Mcl-1 Is Essential for Germinal Center Formation and B Cell Memory. Science. 2010;330:1095. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Dale GL, Song P, Viollet B, Zou MH. AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem. 2010;285:19976–19985. doi: 10.1074/jbc.M110.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Stoneley M, Willis AE. Translational induction of the c-myc oncogene via activation of the FRAP/TOR signalling pathway. Oncogene. 1998;17:769–780. doi: 10.1038/sj.onc.1201990. [DOI] [PubMed] [Google Scholar]
- Wienands J, Engels N. The Memory Function of the B Cell Antigen Receptor. In: Kurosaki T, Wienands J, editors. B Cell Receptor Signaling. Berlin: Springer-Verlag Berlin; 2016. pp. 107–121. [Google Scholar]
- Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger T, et al. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529:216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohner M, Tagoh H, Bilic I, Jaritz M, Poliakova DK, Fischer M, Busslinger M. Molecular functions of the transcription factors E2A and E2-2 in controlling germinal center B cell and plasma cell development. J Exp Med. 2016;213:1201–1221. doi: 10.1084/jem.20152002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- Woodland RT, Fox CJ, Schmidt MR, Hammerman PS, Opferman JT, Korsmeyer SJ, Hilbert DM, Thompson CB. Multiple signaling pathways promote B lymphocyte stimulator dependent B-cell growth and survival. Blood. 2008;111:750–760. doi: 10.1182/blood-2007-03-077222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Robinson MJ, Chen X, Smith GA, Taunton J, Liu W, Allen CD. Regulation of B cell fate by chronic activity of the IgE B cell receptor. Elife. 2016;5:e21238. doi: 10.7554/eLife.21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Buzo BF, Pham D, Jiang L, Taparowsky EJ, Kaplan MH, Sun J. Interferon regulatory factor 4 sustains CD8(+) T cell expansion and effector differentiation. Immunity. 2013;39:833–845. doi: 10.1016/j.immuni.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Choi SC, Xu Z, Perry DJ, Seay H, Croker BP, Sobel ES, Brusko TM, Morel L. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra218. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, Cloer C, Kishton RJ, Gao X, Youngblood B, et al. mTORC1 and mTORC2 Kinase Signaling and Glucose Metabolism Drive Follicular Helper T Cell Differentiation. Immunity. 2016;45:540–554. doi: 10.1016/j.immuni.2016.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Altman BJ, Coloff JL, Herman CE, Jacobs SR, Wieman HL, Wofford JA, Dimascio LN, Ilkayeva O, Kelekar A, et al. Glycogen synthase kinase 3alpha and 3beta mediate a glucose-sensitive antiapoptotic signaling pathway to stabilize Mcl-1. Mol Cell Biol. 2007;27:4328–4339. doi: 10.1128/MCB.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotos D, Coquet JM, Zhang Y, Light A, Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, et al. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell–intrinsic mechanism. The Journal of Experimental Medicine. 2010;207:365. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]


