Abstract
Like falling asleep and waking up, many biological processes in mammals cycle in a diurnal fashion. Now, Sinturel et al. demonstrate that diurnal size changes in the liver require eating during a mouse’s normal awake time and that these size changes are controlled by a nuclear mechanism that modulates ribosome production.
Starting in the 1960s, the liver was the focus of foundational studies that provided an understanding of the basic composition of ribosomes, the cellular machines that synthesize proteins (Attardi and Amaldi, 1970). Ribosome biogenesis, the process of making ribosomes in cells, initiates in the nucleolus. Indeed, nucleoli were first successfully isolated from liver cells (Monty et al., 1956). Additionally, rat liver cells were used to demonstrate a physiological connection between nucleolar structure and diet, providing one of the first links between diet and ribosome biogenesis (Stenram, 1963). While subsequent decades have provided an understanding of the biochemical reactions that synthesize and process pre-ribosomal RNA (rRNA) (reviewed in Woolford and Baserga, 2013), we still lack an understanding of how ribosome biogenesis is controlled on a physiological level. In this issue of Cell, Sinturel et al. (2017) report a fascinating finding that the size of the liver cycles in a diurnal fashion due to the accumulation or depletion of ribosomes and that this physiological phenomenon is controlled by when a mouse eats (Figure 1).
Figure 1.
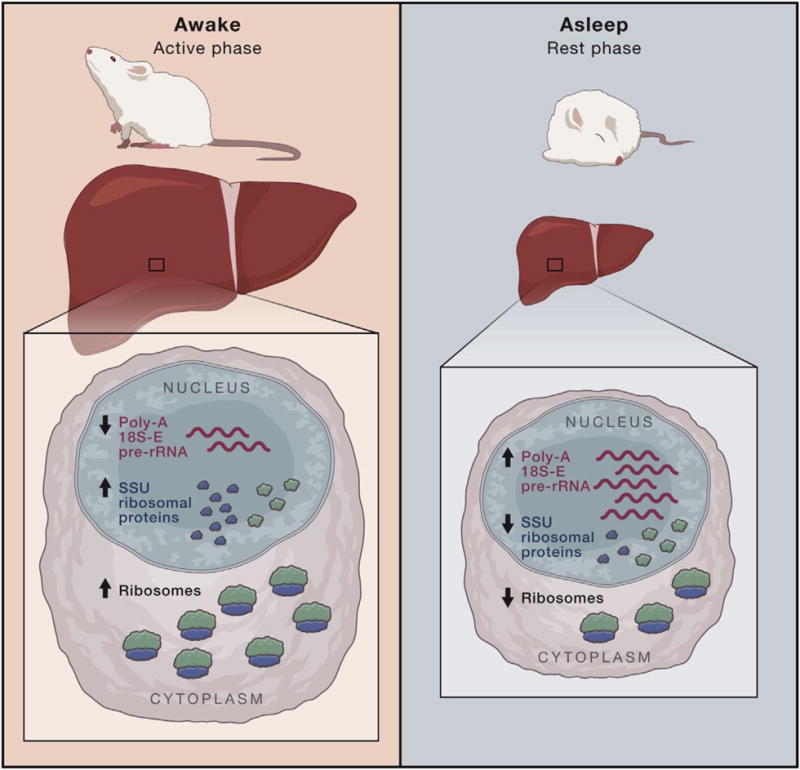
Left: Mice, being nocturnal, are at their most active during the night, which is when they typically eat. During the active phase in night-time or ad libitum fed mice, the liver enlarges, accompanied by an increase in cellular volume caused by increased levels of mature ribosomes. Levels of small ribosomal subunit (SSU) ribosomal proteins are increased, and there is less excess 18S-E pre-rRNA, the last nuclear precursor to mature SSUs, relative to SSU ribosomal protein levels. A mechanism for clearing pre-rRNA that is not stoichiometrically associated with ribosomal proteins occurs via polyadenylation and degradation. Since there is less excess pre-rRNA relative to ribosomal proteins, the 18S-E pre-rRNA is polyadenylated and degraded less frequently.
Right: During the rest phase, there are fewer SSU ribosomal proteins leading to increased excess 18S-E pre-rRNA that is then polyadenylated and degraded. This, in turn, decreases levels of mature ribosomes, shrinking the liver at the organ and cellular levels.
Diurnal rhythms for factors involved in ribosome biogenesis were reported first in the 1970s. Using rat livers as a model system, Glasser and Spelsberg (1972) found that the activity of RNA polymerase I (RNAPI), the nucleolar RNA polymerase that synthesizes pre-rRNA, decreases when rats are asleep (rest phase) and increases while awake (active phase). A recent proteomics study in mouse liver supports this connection: the levels of 13% of nuclear proteins cycle in a diurnal rhythm (Wang et al., 2017), including those involved in ribosome biogenesis. Together, these studies lay the groundwork for current mechanistic investigations into the physiological regulation and consequences of diurnal cycling of ribosome biogenesis.
Sinturel et al. (2017) began their investigations with the observation that murine liver size fluctuates dramatically over a twenty-four hour period. Surprisingly, diurnal changes in liver mass only occurred in mice fed at night, the normal feeding time for mice, or ad libitum, and were not observed in mice fed only during the day. Looking for the source of these daily oscillations in liver biomass in night-time or ad libitum fed mice, they found that the number of ribosomes decreased in mouse liver during the rest phase and accumulated during the active phase. However, probing for a mechanism, they did not observe any differences in the amount of chromatin-associated RNAPI or in steady-state levels of the primary pre-rRNA transcript between the active and rest phases. Thus, even though RNAPI biochemical activity fluctuates in a diurnal rhythm (Glasser and Spelsberg, 1972), it is not likely to be the root cause for the accumulation of ribosomes in the liver.
If it is not RNAPI driving diurnal accumulation of ribosome levels, what is? Diurnal reduction in the number of ribosomes would be one mechanism (Figure 1). Nuclear polyadenylation of pre-rRNAs by PAPD5 leads to their degradation (Shcherbik et al., 2010), which is a possible means to reduce the number of ribosomes in the liver for the resting phase. Polyadenylation and degradation of pre-18S rRNAs would lead to depletion of the small ribosomal subunit (SSU). Indeed, in murine liver, polyadenylation of the 18S-E pre-rRNA, the last nuclear precursor to the mature SSU 18S rRNA, increased during the resting phase, resulting in decreased levels of the SSU. Likewise, polyadenylation of the 18S-E pre-rRNA decreased during the active phase, resulting in increased levels of the SSU (Sinturel et al., 2017). However, the levels of the two enzymes required for polyadenylation and degradation of the 18S-E pre-rRNA are PAPD5 and the RNA exosome, respectively (Sinturel et al., 2017 and Shcherbik et al., 2010), do not cycle diurnally. The authors hypothesized that there must be an additional regulatory event that leads to the increase in polyadenylated 18S-E pre-rRNA levels and consequent loss of mature ribosomes.
The regulatory event lies in a principal of introductory chemistry: the stoichiometry of association of two components. For ribosomes, this is the association of the pre-rRNA with ribosomal proteins. The authors found that an increase in SSU ribosomal protein levels occurred concurrently with decreased polyadenylation and degradation of the 18S-E pre-rRNA. This led to the intriguing hypothesis that fewer ribosomal proteins available for ribosome assembly at the beginning of the rest period resulted in polyadenylation and degradation of excess 18S-E pre-rRNA (Figure 1). In support of this hypothesis, depletion of ribosomal proteins of the SSU led to a contemporaneous increase in polyadenylated 18S-E pre-rRNA (Sinturel et al., 2017). Additionally, ribosome profiling has indicated that proteins involved in SSU biogenesis and ribosome function are translated more efficiently in mouse liver at the beginning of the active phase (Janich et al., 2015) than at the beginning of the rest phase. Taken together, these results provide a model in which more ribosomal proteins are made at the beginning of the active phase, increasing cellular and liver volume; conversely, fewer ribosomal proteins are synthesized during the rest phase, resulting in polyadenylation and degradation of pre-rRNA, which in turn decreases cellular and liver volume (Figure 1). Thus, “straight A’s” in the form of a poly-A tail allows ribosome levels to be reduced during sleep.
Although the mechanisms governing diurnal fluctuations in liver biomass have revealed themselves, the regulation of one of the initial observations by Sinturel et al. (2017) remains a mystery. Why does the mass of the liver not diurnally fluctuate in mice when they are fed during the day? Despite no mechanistic explanation for this phenomenon, it raises a crucial point: what we eat is not only important; when we eat matters too. The questions raised by this study are far-reaching. Evolutionarily, why is it advantageous to prevent diurnal liver cycling when an animal is eating during its normal resting phase? As obesity is a major public health concern caused by over-eating and results in nonalcoholic fatty liver disease, should we be considering when people eat in addition to what they eat? Finally, for diseases of ribosome biogenesis known as ribosomopathies that affect the liver, such as North American Indian Childhood cirrhosis (reviewed in Sondalle and Baserga, 2014), do diurnal rhythms play a role in pathogenesis? Sinturel et al. (2017) have solidified the liver as an invaluable model going forward as we strive to understand the human physiology of ribosome biogenesis.
Acknowledgments
We would like the thank the members of the Baserga laboratory for critically reading this Preview. The authors of this work are supported by NIH grant R01GM115710 (SJB) and NIH grants T32GM007205, T32HD007149, and F30DK109582 (SBS).
References
- Attardi G, Amaldi F. Annual review of biochemistry. 1970;39:183–226. doi: 10.1146/annurev.bi.39.070170.001151. [DOI] [PubMed] [Google Scholar]
- Glasser SR, Spelsberg TC. Biochemical and biophysical research communications. 1972;47:951–958. doi: 10.1016/0006-291x(72)90585-2. [DOI] [PubMed] [Google Scholar]
- Janich P, Arpat AB, Castelo-Szekely V, Lopes M, Gatfield D. Genome research. 2015;25:1848–1859. doi: 10.1101/gr.195404.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monty KJ, Litt M, Kay ER, Dounce AL. The Journal of biophysical and biochemical cytology. 1956;2:127–145. doi: 10.1083/jcb.2.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbik N, Wang M, Lapik YR, Srivastava L, Pestov DG. EMBO reports. 2010;11:106–111. doi: 10.1038/embor.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinturel F, Gerber A, Mauvoisin D, Wang J, Gatfield D, Stubblefield JJ, Green CB, Gauchon F, Schibler U. 2017 doi: 10.1016/j.cell.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondalle SB, Baserga SJ. Biochimica et biophysica acta. 2014;1842:758–764. doi: 10.1016/j.bbadis.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenram U. Experimental cell research. 1963;24(Suppl9):176–181. doi: 10.1016/0014-4827(63)90258-1. [DOI] [PubMed] [Google Scholar]
- Wang J, Mauvoisin D, Martin E, Atger F, Galindo AN, Dayon L, Sizzano F, Palini A, Kussmann M, Waridel P, et al. Cell metabolism. 2017;25:102–117. doi: 10.1016/j.cmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford JL, Jr, Baserga SJ. Genetics. 2013;195:643–681. doi: 10.1534/genetics.113.153197. [DOI] [PMC free article] [PubMed] [Google Scholar]


