Abstract
Purpose
To examine the definition, rationale, and effects of thresholding in OCT angiography (OCTA).
Design
A theoretical description of OCTA thresholding in combination with qualitative and quantitative analysis of the effects of OCTA thresholding in eyes from a retrospective case series.
Participants
Four eyes were qualitatively examined: 1 from a 27-year-old control, 1 from a 78-year-old exudative age-related macular degeneration (AMD) patient, 1 from a 58-year-old myopic patient, and 1 from a 77-year-old nonexudative AMD patient with geographic atrophy (GA). One eye from a 75-year-old nonexudative AMD patient with GA was quantitatively analyzed.
Main Outcome Measures
A theoretical thresholding model and a qualitative and quantitative description of the dependency of OCTA on thresholding level.
Results
Due to the presence of system noise, OCTA thresholding is a necessary step in forming OCTA images; however, thresholding can complicate the relationship between blood flow and OCTA signal.
Conclusions
Thresholding in OCTA can cause significant artifacts, which should be considered when interpreting and quantifying OCTA images.
OCT has established itself as an invaluable modality in ophthalmology, where it is routinely used to noninvasively visualize ocular tissues in 3-D, at micron-scale resolutions. However, within clinical ophthalmology it has not been until recently, with the development of OCT angiography (OCTA), that OCT has been extended to visualize ocular blood flow.1–5 Because of the importance of blood flow in a variety of ocular diseases, and because of the introduction of several commercial OCTA instruments, OCTA imaging has enjoyed rapid and widespread adoption. Despite the underlying similarities with structural OCT, OCTA is a fundamentally different modality, having unique capabilities but also unique limitations.
Importantly, OCTA images are generated by processing OCT signals using relatively complex algorithms6–8 that can cause artifacts not existing in standard OCT imaging. One such artifact, unique to OCTA, is caused by the so-called “thresholding” or “masking” step9 and, as outlined in this paper, can have a dramatic effect on the appearance of OCTA images. As OCTA becomes incorporated into clinical decision making, the ability to understand the thresholding process, and the artifacts that this process introduces, is of utmost importance. With this in mind, the aim of this paper is to provide an overview of the definition, rationale, and effects of thresholding in OCTA. We approach the topic in 2 ways: first, in Part I, by presenting a theoretical description of the thresholding process, and second, in Part II, by presenting a qualitative and quantitative analysis of OCTA thresholding through a short case series.
Part I. Theoretical Description of Thresholding in OCT Angiography
In this section we provide a systems-view of the rationale for OCTA thresholding, and then proceed to a discussion about some subtleties related to choosing threshold levels, and interpreting both thresholded and unthresholded OCTA data.
A Model of OCT Angiography Image Formation
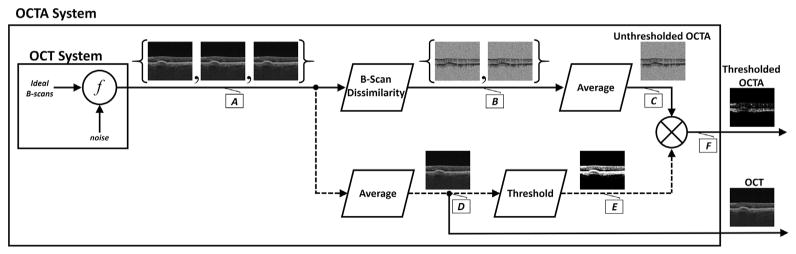
To understand the thresholding process, as well as the artifacts that thresholding introduces, it is essential to understand the processing steps used to generate OCTA data. These steps are schematically shown in the signal flow diagram in Figure 1; the lettered callouts, A through F, denote the signal at different processing steps. Though the exact arrangement and implementation of the processing blocks may vary from system to system, the overarching structure is functionally similar. The first step in OCTA generation is to acquire repeated OCT B-scans, A, from the same tissue location. Typically the number of repeated B-scans ranges between 2 and 5; here, for concreteness, a 3 repeated B-scan protocol is shown. Note that the OCT B-scans, A, are modeled as being formed by mixing “ideal,” noise-free OCT B-scans with a noise signal.† As explained later, it is the presence of system noise that underlies the rationale for thresholding in OCTA. In the next processing step, the repeated OCT B-scans are compared to one another, generating a set of unthresholded, unaveraged OCTA signals, B. Although the dissimilarity block is system dependent, in all OCTA systems motion contrast images are generated by extracting time variations in the repeated B-scans, which correspond to erythrocyte flux. Importantly, in this paper it is assumed that the output of the dissimilarity block is multiplicatively invariant—that is, a constant scaling of the signal does not change the output. Although it is possible to perform OCTA using dissimilarity blocks that do not have this invariance, such implementations typically have the disadvantage that OCTA signals are strongly dependent on OCT signal levels (there are exceptions: recently, Makita et al.10 presented a statistical framework for eliminating the signal-to-noise dependence of the OCTA signal; however, such a discussion is beyond the scope of the current paper). Once the dissimilarity B-scans, B, are generated, they are averaged, C, to increase the signal-to-noise ratio. The B-scan at C is referred to as the unthresholded OCTA B-scan. Separately, shown with dashed lines, the thresholding masks, E, are computed by averaging the repeated OCT B-scans and then comparing, on a pixel-by-pixel basis, the averaged B-scan to a predefined threshold. Specifically, for each pixel, the masking B-scan takes a value of 1 when the pixel in the averaged OCT B-scan, D, is above the threshold level, and 0 when the pixel is below the threshold level. In the final step the masking B-scan is multiplied with the unthresholded B-scan to form the thresholded OCTA B-scan, F. Note that the thresholded OCTA B-scan is identical to the unthresholded OCTA B-scan at the pixels at which the averaged OCT B-scan, D, is above the threshold; the thresholded OCTA B-scan is 0 at the pixels at which the averaged OCT B-scan, D, is below the threshold. Here we want to emphasize that in thresholding the OCTA data, the determination of which pixels are set to 0 and which pixels are left unaltered is based upon the OCT data, not the OCTA data. Although Figure 1 shows the intermediate B-scan outputs, the effects of the different processing steps can be more clearly visualized on a pixel-by-pixel basis (Fig 2).
Figure 1.
Signal flow diagram of OCT angiography (OCTA) processing steps. Lettered callouts A through F denote the value of the signal at different points in the processing workflow. The repeated OCT B-scans, A, are taken in rapid succession from the same tissue location. Here we are assuming that a 3 repeated B-scan protocol is used. Each of these B-scans is modeled as resulting from the mixing of an “ideal,” noise-free OCT B-scan, combined with a noise signal, where f is the (unspecified) mixing function. The repeated OCT B-scans are then input into the B-Scan Dissimilarity block, generating a set of unthresholded, unaveraged OCTA B-scans, B. Note that if only a single interscan time is used then a set of 3 repeated B-scans generates a set of 2 unaveraged, unthresholded OCTA B-scans. The B-scans at B are then averaged to increase the signal-to-noise ratio, forming C, which is referred to as the unthresholded OCTA B-scan. Separately, shown with dashed lines, the thresholding masks are computed by averaging the repeated OCT B-scans and then comparing, on a pixel-by-pixel basis, this average, D, to a predefined threshold to form the thresholding mask, E. Specifically, a pixel in the threshold mask, E, takes a value of 1 when its corresponding pixel in D is greater than the threshold, and 0 when its corresponding pixel in D is less than the threshold. Finally, the threshold mask, E, is multiplied, on a pixel-by-pixel basis, with the unthresholded OCTA B-scan to form F, which we refer to as the thresholded OCTA B-scan. Typically, the 2 outputs exiting the outer box (the thresholded OCTA B-scan and the OCT B-scan) are the only data that are accessible in commercial systems.
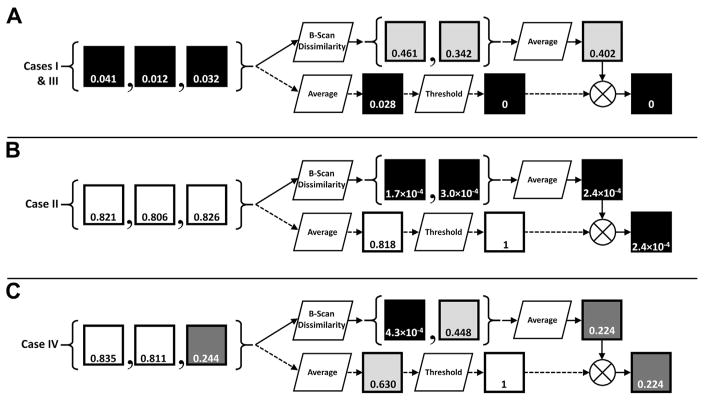
Figure 2.
Pixel-wise OCT angiography processing steps of Figure 1. Each row, A–C, corresponds to a different hypothetical pixel location taken from the B-scans in Figure 1, with the squares representing single pixels. Black pixels correspond to low signals, white pixels correspond to high signals, and the number within each square indicates that pixel’s value (in the normalized range of [0,1]). Cases I–IV, as described in Table 2, are noted. The scenario illustrated in A corresponds to a region of low OCT signal. Note that this scenario may correspond to a region of low/no flow (e.g., vitreous humor), or high flow (e.g., choroid). The scenario illustrated in B corresponds to a region of high, constant OCT signal, typical of highly scattering, avascular layers, such as the retinal pigment epithelium. The scenario illustrated in C corresponds to a region of high, time-varying OCT signal, typical of the retinal vascular layers.
The Effect of Noise on OCT Angiography Images
With the basic OCTA processing steps understood, we now focus our attention on the effect of, and rationale behind, thresholding. However, before proceeding it is worthwhile noting some important distinctions in terminology:
The optical beam that is incident on the tissue is referred to as the incident OCT beam.
The portion of the incident OCT beam backscattered by the tissue is referred to as the backscattered light.
The pixel values that comprise an OCT B-scan are referred to as the OCT signal.
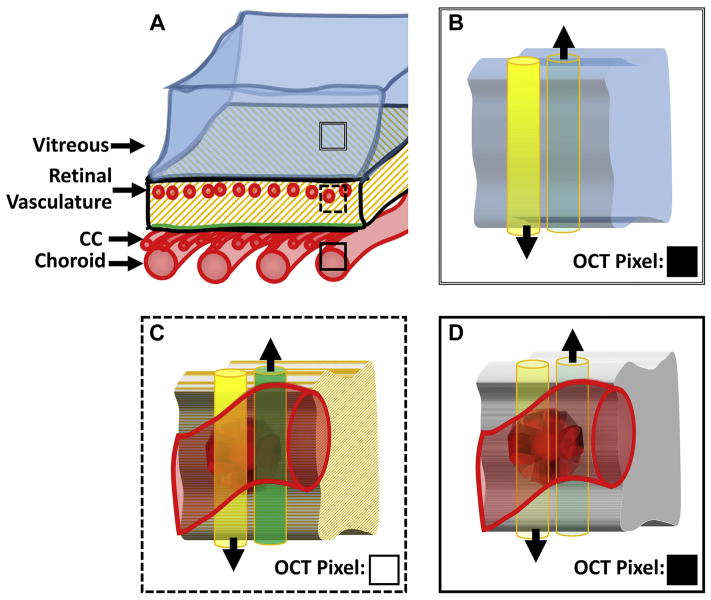
This terminology is important in describing the different causes of low OCT signal (Fig 3): low backscattering, attenuation of the incident OCT beam, or both. As discussed later, these different scenarios affect what conclusions can be drawn from OCTA data.
Figure 3.
Illustration of the different causes of low OCT signal. A, Sketch of the posterior eye, from the vitreous to the choroid. The 3 different boxes— double-lined, dashed, and solid—correspond to panels B, C, and D, respectively. For each of B, C, and D, the yellow (left) cylinder corresponds to the incident OCT beam and the green (right) cylinder corresponds to the backscattered light. These cylinders are shown side-by-side for visual clarity; however, in reality they are superimposed. The black arrows point in the direction of energy (photon) propagation. More opaque yellow coloring (B and C) corresponds to a stronger (i.e., less attenuated) incident OCT beam; more transparent yellow coloring (D) corresponds to a weaker (i.e., more attenuated) incident beam. Similarly, more opaque green coloring, C, corresponds to stronger backscattered light (i.e., more backscattered photons); more transparent green coloring, B and D, corresponds to weaker backscattered light (i.e., less backscattered photons). The squares in the bottom right corner of B–D, labeled “OCT Pixel,” indicate the hypothetical value of the OCT pixel corresponding to that tissue location, with black being low and white being high. CC = choriocapillaris. B, Tissue cube from the vitreous humor. The incident beam is strong, having been minimally attenuated. However, because the vitreous humor does not contain backscatterers, the backscattered light is small. Thus, the corresponding OCT pixel is black. C, Tissue cube from a layer intersecting the retinal vasculature. The incident beam is still strong, and due to the presence of backscattering tissue (in this case an erythrocyte), there is significant backscattered light. Thus, the corresponding OCT pixel is white. D, Tissue cube from the choroid/choriocapillaris. The incident beam is strongly attenuated, having passed through the retinal pigment epithelium and potentially some choroidal vasculature. Thus, even though the tissue at this location contains back-scatterers, the backscattered light is minimal. Thus the corresponding OCT pixel is black.
Until now, it has not been made clear why the thresholding step is required—especially considering that thresholding is not required for generating standard OCT data. Fundamentally, the need for thresholding arises from the way the OCTA dissimilarity block processes noise. The critically important fact is this: system noise has a tendency to change its values rapidly in time. Thus, if a pure noise signal, of any mean power, were input into the dissimilarity block, the output would be a high-amplitude signal. This is problematic because the central idea in OCTA is to extract the time variation that arises from flowing erythrocytes. The noise, also being a time-varying signal, masquerades as blood flow and is also extracted when the OCTA image is formed. Fortunately, in much of the retina the power of the OCT signal is far greater than that of the noise. In these cases the time variation of the OCT signal, A, relative to its average value, is comparably small if the scanned region is not time-varying (no flowing erythrocytes). Consequentially, in these regions, the output, B, of the dissimilarity block will be a low-amplitude signal, even in the presence of noise. However, in certain areas of the eye, such as the vitreous, or below the retinal pigment epithelium (RPE), there is insufficient OCT signal, and noise causes substantial time variation relative to the mean value of the signal. In these cases, the OCT signal, A, will have a significant time-varying component, irrespective of whether the scanned region is time-varying (contains flowing erythrocytes) or stationary (contains no flowing erythrocytes). Therefore, in both cases the output, B, of the dissimilarity block will be a high-amplitude signal. Because of this, regions with insufficient OCT signal—even if they have no blood flow—have high signals in the unthresholded OCTA B-scans. This is clearly undesirable insomuch as high OCTA signals are typically considered as corresponding to blood flow. To get around this difficultly, thresholding is performed to remove the regions of the image that are significantly influenced by noise. Thus, at its core, thresholding is aimed at suppressing artifacts caused when the OCT signal is at a level similar to that of the system noise.
Sufficient and Insufficient OCT Signals
At this point, it is helpful to clarify what precisely is meant by an “insufficient OCT signal” (Table 1). A region of sufficient OCT signal is a region that, if made stationary (i.e., if all moving scatterers are hypothetically stopped), has an acceptable probability of producing not more than a negligible dissimilarity signal, B. There is some flexibility in what is meant by “acceptable” and “negligible.” Roughly, an “acceptable probability” corresponds to the confidence level at which it is desired to draw conclusions, for example 95%; a “negligible dissimilarity signal” is a dissimilarity signal, B, that is negligible compared with that produced by the blood flow in the smallest ocular capillaries. Equivalently, by contraposition, a sufficient OCT signal is one whose power is sufficiently above the noise level so that the presence of an unthresholded OCTA signal implies, at an acceptable confidence, the presence of blood flow. The lowest sufficient OCT signal is referred to as the sufficient OCT signal level, and the term insufficient OCT signal refers to an OCT signal that is not sufficient. In practice it is hard to actually determine the sufficient OCT signal level, and so the sufficient OCT signal level is used as a conceptual quantity; however, it is useful for understanding how confident one can be that an unthresholded OCTA signal truly corresponds to blood flow.
Table 1.
Summary of Useful Terms for Describing OCT Angiography Thresholding
| Term | Definition |
|---|---|
| Threshold level | The level determining which OCTA pixels are set to 0. OCTA pixels whose corresponding OCT pixels are below the threshold level are set to 0; OCTA pixels whose corresponding OCT pixels are above the threshold level are left unchanged. |
| Noise signal level | A measure of the power of the noise signal. Since the noise signal is random, the noise signal level will be related to parameters of its statistical distribution, such as its variance. |
| Sufficient OCT signal level | The OCT signal level above which we can judge the presence of an OCTA signal as implying, with an acceptable confidence, the presence of blood flow. In general, as the OCT signal level increases relative to the noise signal level, the probability that an OCTA signal is attributable to blood flow, rather than noise, increases. When this probability reaches an acceptable value, for instance 0.95, we say that the OCT signal level is sufficient. Alternatively stated, the sufficient OCT signal level is the highest threshold level that could be sensibly used. Although the sufficient OCT signal level is difficult to compute in practice, it is a useful concept for understanding what conclusions can be drawn from OCTA data. |
OCTA = OCT angiography.
Choosing a Thresholding Level
Because noise signals are stochastic, their values will be governed by some statistical distribution, and they are characterized by parameters of their statistical distributions, such as their variances. Consequentially, it will not be the case that the noise always falls below some constant value. One may be able to pick a value above which the noise signal rarely occupies, but it will, on occasion, still occupy these high values. This randomness makes the choice of the threshold level a more subtle issue. In general, a balance must be struck between excluding noise and including valid signal: if the threshold is set low, then there will be more noise above the threshold (and hence in the resulting thresholded OCTA image), but the subsequent OCTA output will be less likely to have eliminated any true vasculature. Conversely, if the threshold is set high, then there will be less noise above the threshold (and hence in the resulting OCTA image), but the risk of eliminating true vasculature increases.
Summary of Causes of Low OCT Angiography Signals
Although the thresholding step is convenient in that it produces a simpler OCTA B-scan, often corresponding more closely to true ocular blood flow, it also hides some important limitations of OCTA. With reference to Table 2, note that in thresholded OCTA images, low OCTA signal may be the result of low OCT signal, no/low blood flow, or both. Thus, in the absence of additional information, low OCTA signal in a thresholded OCTA image is not conclusive evidence that there is no/low blood flow in that region. The converse artifact is present in unthresholded OCTA images: high OCTA signal may be the result of blood flow, insufficient OCT signal, or both. Thus, in the absence of additional information, high OCTA signal in an unthresholded OCTA image is not conclusive evidence that there is blood flow in that region.
Table 2.
Summary of the OCT Angiography Signal Levels for Thresholded and Unthresholded OCT Angiography Data
| Unthresholded OCTA Signal | Thresholded OCTA Signal | |
|---|---|---|
| Case I: No/low blood flow & below-threshold OCT signal | High | Low |
| Case II: No/low blood flow & sufficient OCT signal | Low | Low |
| Case III: Blood flow & below-threshold OCT signal | High | Low |
| Case IV: Blood flow & above-threshold OCT signal | High | High |
OCTA = OCT angiography.
Typically, high OCTA signals are interpreted as indicating the presence of blood flow and low OCTA signals are interpreted as indicating no/low blood flow. Underlined entries indicate cases where this correspondence does not hold. Case I is typical of the vitreous humor, outer nuclear layer, and nonflow regions beneath the retinal pigment epithelium. Case II is typical of the retinal pigment epithelium. Case III is typical of the deeper choroidal vasculature and, in some cases, the choriocapillaris, particularly beneath highly attenuating drusen. Case IV is typical of the retinal vasculature.
Part II. Clinical Illustration of OCT Angiography Thresholding
In this section we use a short case series to examine the qualitative and quantitative effects that thresholding has on OCTA data.
Methodology
Subjects
The study was approved by the institutional review boards at the Massachusetts Institute of Technology (Cambridge, MA) and Tufts Medical Center (Boston, MA). All participants were imaged in the ophthalmology clinic at the New England Eye Center at Tufts Medical Center. Written informed consent was obtained from all subjects before imaging. The research adhered to the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. All subjects underwent a complete ophthalmic examination, including a detailed history, refraction, intraocular pressure measurement, anterior segment examination, and a dilated fundus examination by a general ophthalmologist or a retinal specialist at the New England Eye Center.
Image Acquisition
Patients were imaged on a prototype OCTA system developed at the Massachusetts Institute of Technology. The prototype is an ultrahigh-speed swept-source OCT system operating at a ~1050 nm center wavelength and a 400 kHz A-scan rate. Each B-scan consisted of 500 A-scans; 5 repeated B-scans, corresponding to an ~1.5 ms interscan time, were acquired at each retinal location. Each acquired volume consisted of 500 B-scans, for a total of 5 × 500 × 500 A-scans per volume, and a total acquisition time of ~3.8 seconds.
Image Processing
OCTA images were generated using an intensity-based decorrelation method. To investigate the effects of thresholding, the threshold level was adjusted to 3 different levels: no threshold, 3 standard deviations above the mean of the estimated noise process, and 6 standard deviations above the mean of the estimated noise process. An estimate of the noise process was taken once per acquisition, meaning that it is possible for the threshold levels to change, though typically only by a small amount, from patient to patient, or even from acquisition to acquisition. The threshold at 3 standard deviations above the mean was chosen on the basis of qualitative optimization of the appearance of the choriocapillaris/choroid in OCTA images; we will refer to this threshold level as the choroidal threshold. The threshold at 6 standard deviations above the mean was chosen similarly, on the basis of qualitative optimization of the appearance of the retinal vasculature in OCTA images; we will refer to this threshold level as the retinal threshold. Because the incident beam is less attenuated in the retinal layers than in the choroid and choriocapillaris, which lie beneath the RPE, the retinal threshold is higher (i.e., it sets more pixels to black) than the choroidal threshold. The mean and standard deviation of the noise process was estimated separately for each acquired volume.
Qualitative Analysis
Case 1: 27-Year-Old Normal Patient
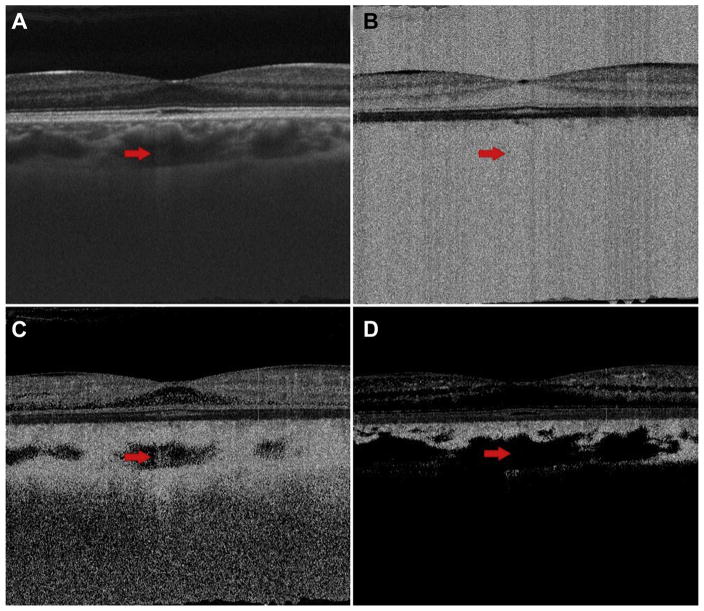
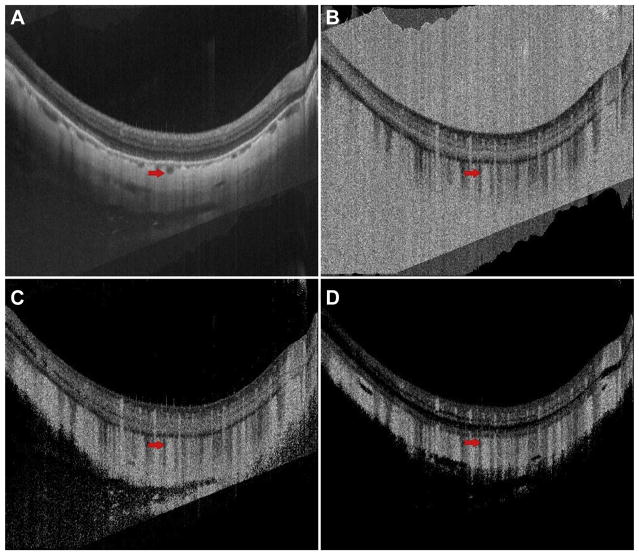
Figure 4 shows the effects of thresholding on OCT and OCTA B-scans in a 27-year-old normal patient. Note how all the areas of low OCT signal, which appear relatively dark in Figure 4A, correspond to high OCTA signal in the unthresholded OCTA B-scan in Figure 4B. In some of these regions, such as the vitreous humor, there is no blood flow, whereas in others, such as the choroid, there is blood flow. Because there is no flow in the vitreous humor and the unthresholded OCTA signal is high, one can conclude that there is insufficient OCT signal in this region. In the choroid, especially at the shallow depths, it is difficult to say what portion of the OCTA signal is due to noise and what portion is due to blood flow; however, as we move deeper into the choroid and the incident OCT beam becomes more attenuated, the effect of noise will become increasingly dominant. Figure 4C and D show the choroidal and retinal thresholded OCTA B-scans, respectively. Note that for both thresholds the OCTA signal in the vitreous humor is removed, and the OCTA signal in some of the less backscattering layers, such as the outer nuclear layer, is reduced. For the choroidal threshold, the larger choroidal vessels show mostly low OCTA signal; however, the area of increased light penetration pointed to by the arrow exhibits an OCTA signal, indicating that the corresponding region in the OCT B-scan takes values above the choroidal threshold. For the retinal threshold, nearly all of the area lying within, or below, the larger choroidal vasculature is thresholded and thus appears dark. Here, in the larger choroidal vasculature, the absence of an OCTA signal in the thresholded OCTA images is not a result of low blood flow, but rather of thresholding regions of low OCT signal. This example indicates the risk of interpreting low OCTA signal on a thresholded OCTA image as low blood flow.
Figure 4.
Case 1: A 27-year-old normal patient. A, OCT B-scan; B, unthresholded OCT angiography (OCTA) B-scan; C, choroidal thresholded OCTA image; and D, retinal thresholded OCTA image. The arrow points to an area of relatively increased OCT signal level on the OCT B-scan.
Case 2: 78-Year-Old Patient with Exudative Age-Related Macular Degeneration
Figure 5 shows the effects of thresholding on both cross-sectional and en face views in a 78-year-old patient with exudative age-related macular degeneration (AMD). To co-locate the same features on both en face and cross-sectional slices, the data were viewed in an orthoplane manner; for each row in this figure, the left panel corresponds to a B-scan along the fast scan axis, the middle panel corresponds to an en face plane, and the right panel corresponds to a B-scan along the slow scan axis, which is orthogonal to the fast scan axis. For each panel, the yellow crosshair indicates the locations of the views in the other 2 panels in that row. The OCT images in row A show several areas of relative low OCT signal that correspond to choroidal vasculature. One of these choroidal vessels is demarcated by the dashed red circle. The unthresholded OCTA images in row B show OCTA signal within this choroidal vessel. Although there is presumably blood flow within this vessel, because there is low OCT signal in this region the high OCTA signal is most likely a result of noise, rather than blood flow. The choroidal thresholded OCTA images in row C also show some OCTA signal within the choroidal vessel, although certain areas within the vessel have been thresholded and thus appear dark. Finally, the retinal thresholded OCT images in row D show no OCTA signal within the choroidal vessel. Again, the absence of an OCTA signal in the thresholded OCTA image is not a result of low blood flow, but rather of thresholding regions of low OCT signal.
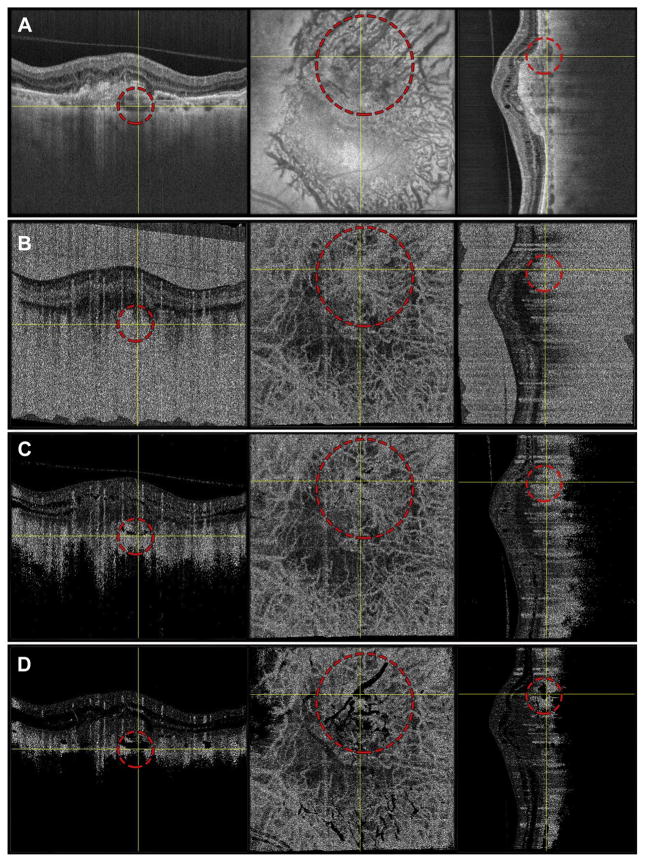
Figure 5.
Case 2: A 78-year-old patient with exudative age-related macular degeneration. To co-locate the same features on both en face and cross-sectional slices, data were viewed in an orthoplane manner; for each row in this figure, the left panel corresponds to a B-scan along the fast scan direction, the middle panel corresponds to an en face plane, and the right panel corresponds to a B-scan along the slow scan direction. For each panel, the yellow crosshairs indicate the locations of the views in the other 2 panels in that row. The red dashed circle demarcates a choroidal vessel. Row A shows the OCT images; row B, the unthresholded OCT angiography (OCTA) images; row C, the choroidal thresholded OCTA data; and row D, the retinal thresholded OCTA data.
Case 3: 58-Year-Old Myopic Patient
Figure 6 shows the effects of thresholding on OCT and OCTA B-scans in a 58-year-old myopic patient. The OCT B-scan (Fig 6A) of this patient shows a thinned choroid with a deep concave OCT B-scan and distorted retinal features at the image periphery; the arrow indicates a choroidal vessel. Note that the OCTA signals at the corresponding positions, marked by arrows, appear high (Fig 6B–D). That the OCTA signal is not affected by either threshold is suggestive that the OCT signal at this location is sufficiently high so as to be able to reliably conclude that the OCTA signal is a result of blood flow. However, it should not be ruled out that some portion of the OCTA signal is due to noise. This possibility could be investigated further by additional adjustments of threshold levels.
Figure 6.
Case 3: A 58-year-old myopic patient. A, OCT B-scan; B, unthresholded OCT angiography (OCTA) B-scan; C, choroidal thresholded OCTA B-scan corresponding; and D, retinal thresholded OCTA B-scan. The arrow indicates a choroidal vessel.
Case 4: 77-Year-Old Nonexudative Age-Related Macular Degeneration Patient with Geographic Atrophy
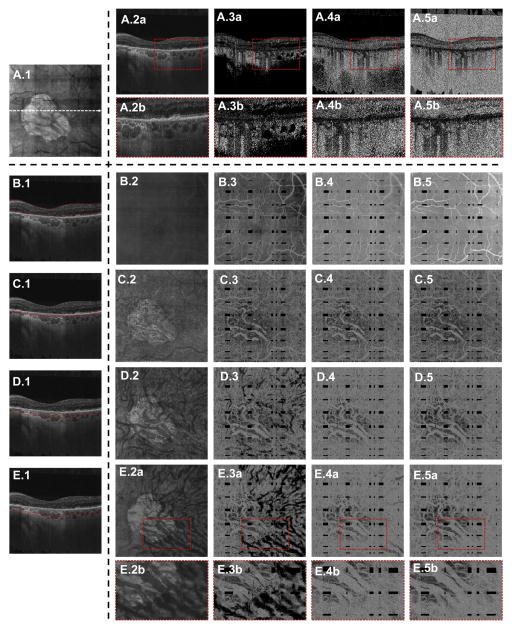
Figure 7 illustrates the effects of thresholding on both cross-sectional and en face views in a 77-year-old patient with nonexudative AMD with geographic atrophy (GA). Eyes with GA offer an interesting view of the effects of thresholding on the visualization of the choroidal vasculature: in the region of atrophy, the OCT beam is not attenuated by the RPE, leaving the choroidal vessels above the thresholding level; however, outside the margins of atrophy, the incident OCT beam is attenuated by the RPE, leaving many of the same choroidal vessels below the threshold level. Thus, by studying the GA margins, we can identify how some choroidal vessels will, in thresholded OCTA images, go from being bright in the region of atrophy to dark outside the region of atrophy (Fig 7E.3a and E.3b).
Figure 7.
Case 4: A 77-year-old patient with nonexudative age-related macular degeneration with geographic atrophy (GA). A.1, En face mean projection through the entire OCT volume. The cross-sectional OCT/OCT angiography (OCTA) B-scans in A.2, A.3, A.4, and A.5, as well as in B.1, C.1, D.1, and E.1, are extracted from the dashed white arrow in A.1. A.2a, Extracted OCT B-scan. A.3a, Extracted choroidal thresholded OCTA B-scan. A.4a, Extracted retinal thresholded OCTA B-scan. A.5a, Extracted unthresholded OCTA B-scan. The enlargements A.2b, A.3b, A.4b, and A.5b correspond to the dashed red boxes in A.2a, A.3a, A.4a, and A.5a, respectively. The enlargement A.3b shows circular areas of low OCTA signal corresponding to the choroidal vasculatures; these features are not present in A.4b and A.5b. B–E, En face illustration of thresholding. The red lines in B.1, C.1, D.1, and E.1 correspond to the contours between which the OCT(A) signal was projected to form the en face images in B.2–B.5, C.2–C.5, D.2–D.5, and E.2–E.5, respectively. The en face projections B.2, C.2, D.2, and E.2 are projections of the OCT volume; the en face projections B.3, C.3, D.3, and E.3 are projections of the choroidal thresholded OCTA volume; the en face projections B.4, C.4, D.4, and E.4 are projections of the retinal thresholded OCTA volume; and the en face projections B.5, C.5, D.5, and E.5 are projections of the unthresholded OCTA volume. The enlargements E.2b, E.3b, E.4b, and E.5b are taken from the dashed red boxes in E.2a, E.3a, E.4a, and E.5a, respectively. The enlargement E.3b shows how the larger choroidal vasculature has high OCTA signal within the margin of GA, but has low OCTA signal outside of the GA margin; this is because in the region of the GA the retinal pigment epithelium is absent, allowing more light to be incident on the choroid.
Quantitative Analysis
Case 5: 75-Year-Old Nonexudative Age-Related Macular Degeneration Patient with Geographic Atrophy
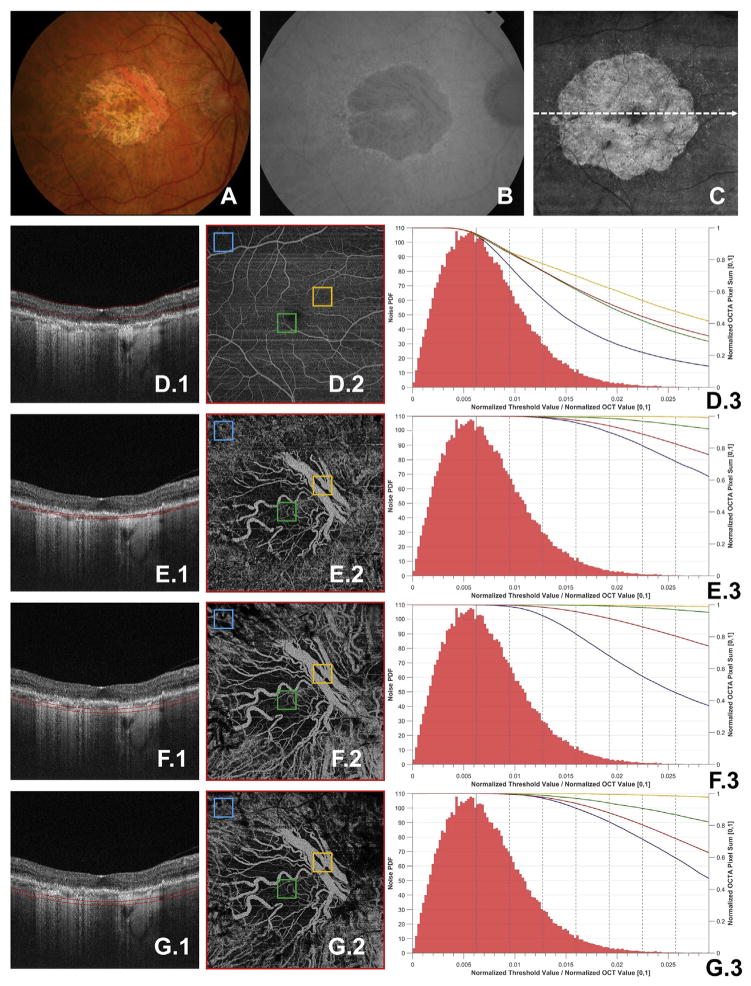
As shown in the preceding section, variations in thresholding levels can cause qualitative variations in the resulting OCTA signal. It is not surprising, then, that variations in threshold levels also affect the quantitative analysis of OCTA signals. However, the dependency of the quantitative OCTA metrics on the thresholding level can be more subtle. To understand this behavior it is helpful to study how a simple OCTA metric changes as a function of threshold level. This is done in Figure 8, which investigates the dependency of the (normalized) sum of the OCTA signal as a function of thresholding level in an eye with GA. From the figure we can see that this OCTA metric has not only a dependency on threshold values, but also a dependency on the layer of interest (e.g., retinal vasculature vs. choroidal vasculature) and on the region of interest (e.g., entire field of view vs. sub-fields). As explored more in the Discussion section, this dependency complicates the quantification of OCTA signals.
Figure 8.
Case 5: A 75-year-old nonexudative age-related macular degeneration with geographic atrophy (GA). A, Fundus photograph, B, fundus auto-fluorescence, and C, en face projection of the entire OCT volume. D.1, E.1, F.1, G.1, OCT B-scans taken from the dashed white arrow in C; the red contours in these B-scans correspond to the depths through which the corresponding OCT angiography (OCTA) volumes were mean projected to form D.2, E.2, F.2, and G.2. Each of D.2, E.2, F.2, and G.2 were thresholded using the retinal threshold. The 4 squares—blue, green, orange, and red, the last of which encompasses the entire field of view—correspond to the similarly colored curves in plots D.3, E.3, F.3, and G.3. For each of these regions of the interest, the threshold was varied from 0 (i.e., unthresholded) to 7 standard deviations above the estimated mean of the noise. For each analyzed threshold value, the sum of the OCTA signal over the boxes shown in D.2, E.2, F.2, and G.2 was taken. This sum was then normalized by the sum of the unthresholded OCTA signal, summed over the region of interest. The results are plotted in D.3, E.3, F.3, and G.3, where the x-axis corresponds to the threshold value, which has been normalized to the maximum OCT B-scan value, averaged over all B-scans in the volume; the right y-axis corresponds to the normalized OCTA sum. Also plotted in D.3, E.3, F.3, and G.3 is the histogram of the measured noise, normalized to have unity area, so that it corresponds to a valid probability density function. Note that the noise is measured once per OCT acquisition, so the same noise histogram appears in D.3, E.3, F.3, and G.3. When reading the histogram, the x-axis corresponds to the pixel value of the noise, and the left y-axis corresponds to the value of the histogram at that pixel value. Bin sizes for the histogram were calculated using Freedman-Diaconis rule. For reference, the mean of the value of the noise distribution is shown as the solid vertical gray line, with each of the dotted gray lines to the right of the mean corresponding to an additional standard deviation above the mean. The field of view for all OCT(A) images is 6 mm × 6 mm.
Discussion
Thresholded vs. Unthresholded OCT Angiography Data
The preceding sections suggest that when it comes to inferring information about blood flow, unthresholded and thresholded OCTA images both have their advantages and disadvantages. Thresholded OCTA images are particularly useful for inferring the presence of blood flow on the basis of the presence of an OCTA signal. The absence of a thresholded OCTA signal can be used to infer the absence of blood flow only if the incident OCT beam does not fall below the thresholding level due to attenuation. Because, in general, the incident beam is only weakly attenuated by the retinal layers, thresholded OCTA images are particularly useful for visualizing the retinal vasculature. However, in the choriocapillaris, where the incident OCT beam is attenuated by the RPE, the OCT signal level may in some regions fall below the thresholding level, causing these regions to be thresholded out. In these cases, the absence of an OCTA signal is not conclusive evidence for the absence of blood flow. Conversely, unthresholded OCTA images are most useful for inferring the absence of blood flow on the basis of the absence of an OCTA signal: absence of an unthresholded OCTA signal definitively implies the absence of blood flow. The presence of an unthresholded OCTA signal can be used to infer the presence of blood flow only if there is sufficient OCT signal. Unthresholded OCTA images have significant disadvantages when used for viewing the retinal vasculature because of the presence of noise in regions of low OCT signal and no/low blood flow.
Threshold Level: Set It and Forget It?
For the reasons discussed above, we believe that thresholding can hide some important subtleties of OCTA data. Although thresholding makes for simpler OCTA data, the simplicity may ultimately be deceptive, and may lead to misinterpretations. Exacerbating the problem is the fact that only the thresholded images, in addition to the standard OCT images, are accessible to clinicians. We believe that it may be beneficial if commercial systems allow threshold levels to be accessible, or ultimately adjustable, so as to empower clinicians to more confidently and flexibly interpret their OCTA images. Admittedly, allowing thresholds to be adjusted increases the complexity of the analysis, and, if done improperly, could itself cause misinterpretations. Furthermore, adjustable thresholds add another variable that must be controlled for in studies, which would be particularly important for studies using quantitative metrics. That said, even if the threshold level is kept constant, the variation in threshold levels among different OCTA systems can similarly complicate comparison of data.
Threshold Level: One Size Fits All?
In this study, in addition to presenting unthresholded OCTA images, we showed OCTA images corresponding to different threshold levels. In particular, we chose one threshold optimized for visualizing the choriocapillaris and choroid, and another optimized for visualizing the retinal vasculature. In general, there are 2 risks that need to be balanced when choosing a threshold: eliminating true flow in vasculature (false-negative), and including noise (false-positive). If the threshold is set higher, then OCTA devices may threshold out vasculature in low-signal areas. If the threshold is set lower, then there may be excess noise in the OCTA image. In the retinal vasculature, where the OCT signal is relatively high, a more stringent threshold can be used with little risk of eliminating vasculature; however, in the choriocapillaris and choroid, where the OCT signal is lower due to RPE attenuation, a less stringent threshold may be desirable. However, it is unfortunate that in the choriocapillaris noise is especially problematic: because the vascular features of the choriocapillaris are smaller than the transverse resolution of standard OCT systems, they cannot be resolved. Thus, in the choriocapillaris, unlike in the retinal vasculature, vascular continuity cannot be used to distinguish noise from blood flow, and we should be especially cautious in our interpretation.
There is also the question of whether threshold levels should be computed adaptively, depending on the particular acquisition, or be set to a fixed level. In this study, for each acquisition we estimated the mean and standard deviation of the noise process and set the threshold levels based on these estimates. This adaptive approach has the advantage that if the noise levels increase, the threshold levels increase as well; the converse is also true. Roughly, this means that the strengths of the inferences, such as thresholded OCTA signal implies blood flow, are fixed, irrespective of the noise level. However, it has the downside that certain regions risk being thresholded out if the noise level rises. Of course, the ability to adjust the threshold level would allow raw data to be processed retrospectively, and therefore the choice of adaptive-level vs. fixed-level thresholding could be made on a study-by-study basis.
Minimizing the Risks of Threshold-Related Misinterpretation
There are other solutions to the thresholding problem besides the adjustable-threshold approach. The simplest, which is immediately implementable, is to consider thresholded OCTA images alongside their corresponding OCT images: if a region of low OCTA signal in the thresholded OCTA image corresponds to a region of low OCT signal, then caution should be taken when interpreting the blood flow status. Another simple, more quantitative solution would be to mark, for example with a colored overlay, areas of the thresholded OCTA image that correspond to low OCT signal. Clinicians could then be advised to interpret these areas with caution. Unfortunately, this simple approach has the drawback of treating all low-OCT-signal areas the same—both the vitreous humor and the deep choroid would be marked as being unreliable. From Figure 3, and the preceding discussion, we know that these 2 regions, though both generating low OCT signals, correspond to 2 very different physical scenarios. This suggests a need to distinguish low OCT signals caused by an absence of backscattering tissue from low OCT signals caused by attenuation of the incident beam. In fact, there are some simple approaches to doing just this: suppose that there is a pixel, pA, in the OCT B-scan that has a value below the threshold. If there is another pixel, pB, along the same A-scan, but at a deeper depth, having a value above the threshold, then we can conclude that pA has a low OCT signal as a result of an absence of backscatters, rather than attenuation of the incident beam. Thus, the OCTA signal at pA is, relatively speaking, trustworthy. With this approach, regions such as the vitreous humor would be marked as reliable, whereas regions such as the deep choroid would be marked as unreliable.
Thresholding and the Challenges of Quantitative OCT Angiography
Quantitative OCTA analysis is likely to become increasingly important because of the continued push to develop OCTA-based markers of disease progression. Although OCTA has offered an entirely new framework through which to study the eye, its dependency on a relatively complex series of processing steps also generates new pitfalls. This article has shown that qualitative OCTA assessment can, at times, be challenging, especially if the processing parameters are not known and/or not adjustable; it is our belief that quantitative OCTA assessment is more challenging still. For example, when looking for the presence or absence of a certain feature—for example, a vessel—in an OCTA image, one will, without thinking, use myriad factors that are far more abstract than a simple assessment of pixel value—for example, continuity of vasculature, the surrounding vascular pattern, and so forth. However, when one writes a computer program to quantify an OCTA signal, at least using simple schemes, the quantification is, in essence, blind to all these factors. The outcome of quantification—a number—can be difficult to understand and, as illustrated in Figure 8, dependent on factors that are embedded in the underlying processing steps. In this there lies a risk. It should be noted, however, that it is in no way our intention to suggest that the community should not attempt to use, and develop further, quantitative OCTA schemes; we see great promise in quantitative OCTA, and we praise the previous and current efforts. Rather, it is the authors’ intention to bring attention to one of several complexities that accompanies the development of such schemes.
Conclusion
This paper presents an overview—using a combination of theory and qualitative and quantitative analysis of clinical cases—of the rationale for, and effects of, thresholding in OCTA. Furthermore, several possible solutions for improving current OCTA visualization, and for reducing threshold-related misinterpretations, are proposed. The effects of thresholding can dramatically alter OCTA data and, as such, should be considered in any careful analysis.
Acknowledgments
Financial Support: This work was in part supported by a grant from the Macula Vision Research Foundation, New York, National Institute of Health (NIH R01-EY011289-29A, R44-EY022864, R01-CA075289-16), Air Force Office of Scientific Research (AFOSR FA9550-15-1-0473 and FA9550-12-1-0499), and Thorlabs matching funds to Praevium Research Inc. Additional support came from an unrestricted Research to Prevent Blindness grant and the Massachusetts Lions Clubs, and a Samsung Scholarship. E.A.N. and R.N.L. are researchers supported by CAPES Foundation, Ministry of Education of Brazil, Brasilia, DF, Brazil.
Abbreviations and Acronyms
- AMD
age-related macular degeneration
- GA
geographic atrophy
- OCTA
OCT angiography
- RPE
retinal pigment epithelium
Footnotes
Throughout the text we will make frequent usage of the term “system noise.” For our purposes, the meaning of “noise” may be best understood by negation: if we use a signal to make inferences about some entity, we can divide the signal into 2 parts, 1 of which we deem to carry information about that entity, and 1 of which we deem to not. We refer to the latter as noise. With our definition, noise could refer to a number of corrupting phenomena in OCT(A): eye motion, registration errors, decorrelation tails, or even speckle. Though important in their own right, in this study when we use the term noise we will be referring to the noise introduced during the acquisition, or recording, of the signal; for example, shot noise. To avoid confusion with other types of noise, we will denote this type of noise as “system noise.” Furthermore, we will assume that the system noise is random, which, roughly, means that its values cannot be predicted.
Author Contributions:
Research design: Cole, Moult, Dang, Louzada, Novais, Maier, Fujimoto, Waheed, Duker
Data analysis and/or interpretation: Cole, Moult, Dang, Choi, Ploner, Lee, Schottenhaml, Husvogt, Maier, Fujimoto, Waheed, Duker
Data acquisition and/or research execution: Cole, Moult, Choi, Ploner, Lee, Louzada, Novais, Schottenhaml, Husvogt
Manuscript preparation: Cole, Moult, Dang, Choi, Ploner, Lee, Louzada, Novais, Schottenhaml, Husvogt, Maier, Fujimoto, Waheed, Duker
Conflicts of Interest: J.S.D.: Consultant and research support — Carl Zeiss Meditec, OptoVue, and Topcon Medical Systems Inc; stock — Hemera Biosciences Inc, EyeNetra, and Ophthotech Corp. N.K.W.: Consultant — Iconic Therapeutics; speaker’s bureau —ThromboGenics; research support — Carl Zeiss Meditec, Inc. J.G.F.: Royalties — intellectual property owned by the Massachusetts Institute of Technology and licensed to Carl Zeiss Meditec Inc, Optovue Inc; stock options — Optovue Inc. There are no conflicting relationships for any other author.
References
- 1.Makita S, Hong Y, Yamanari M, et al. Optical coherence angiography. Opt Express. 2006;14(17):7821–7840. doi: 10.1364/oe.14.007821. [DOI] [PubMed] [Google Scholar]
- 2.Mahmud MS, Cadotte DW, Vuong B, et al. Review of speckle and phase variance optical coherence tomography to visualize microvascular networks. J Biomed Opt. 2013;18(5):50901. doi: 10.1117/1.JBO.18.5.050901. [DOI] [PubMed] [Google Scholar]
- 3.Mariampillai A, Standish BA, Moriyama EH, et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt Lett. 2008;33(13):1530–1532. doi: 10.1364/ol.33.001530. [DOI] [PubMed] [Google Scholar]
- 4.An L, Wang RK. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express. 2008;16(15):11438–11452. doi: 10.1364/oe.16.011438. [DOI] [PubMed] [Google Scholar]
- 5.Makita S, Jaillon F, Yamanari M, et al. Comprehensive in vivo micro-vascular imaging of the human eye by dual-beam-scan Doppler optical coherence angiography. Opt Express. 2011;19(2):1271–1283. doi: 10.1364/OE.19.001271. [DOI] [PubMed] [Google Scholar]
- 6.Zhang A, Zhang Q, Chen CL, Wang RK. Methods and algorithms for optical coherence tomography-based angiography: a review and comparison. J Biomed Opt. 2015;20(10):100901. doi: 10.1117/1.JBO.20.10.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang RK, An L, Saunders S, Wilson DJ. Optical microangiography provides depth-resolved images of directional ocular blood perfusion in posterior eye segment. J Biomed Opt. 2010;15(2):020502. doi: 10.1117/1.3353958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35(11):2163–2180. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makita S, Kurokawa K, Hong Y-J, Miura M, Yasuno Y. Noise-immune complex correlation for optical coherence angiography based on standard and Jones matrix optical coherence tomography. Biomed Opt Express. 2016;7:1525–1548. doi: 10.1364/BOE.7.001525. [DOI] [PMC free article] [PubMed] [Google Scholar]