Summary
Acute exposure to ionizing radiation induces massive cell death and severe damage to tissues containing actively proliferating cells, including bone marrow and the gastrointestinal tract. However, the cellular and molecular mechanisms underlying this pathology remain controversial. Herein, we show that mice deficient in the double-strand DNA (dsDNA) sensor AIM2 are protected from both subtotal body irradiation-induced gastrointestinal syndrome and total body irradiation-induced hematopoietic failure. AIM2 mediates the caspase-1-dependent death of intestinal epithelial cells and bone marrow cells in response to dsDNA breaks caused by ionizing radiation and chemotherapeutic agents. Mechanistically, we found that AIM2 senses radiation-induced DNA damage in the nucleus to mediate inflammasome activation and cell death. Our results suggest that AIM2 may be a new therapeutic target for ionizing radiation exposure.
Whole-body exposure to 2 Gy or higher radiation can induce hematopoietic syndrome, which might lead to death from infection or hemorrhage within 30 days (1). Higher doses of radiation cause severe damage to the GI tract, resulting in diarrhea, malabsorption and lethality within 10 days(1). However the cellular targets of GI syndrome and the mechanism of radiation-induced cell death remain controversial. Different forms of cell death have been implicated in radiation-induced GI syndrome, including apoptosis (2) or mitotic catastrophe (3) of intestinal epithelial cells (IEC), and apoptosis of endothelial cells in the intestinal vasculature (4). Although GI toxicity is a common complication in cancer patients undergoing radiation or chemotherapy with DNA-damaging agents, there are currently no effective medical treatments to prevent or ameliorate GI syndrome. It is therefore important to gain a better understanding of the underlying mechanisms of cell death and tissue injury in response to double-strand DNA damage.
Several innate pattern recognition receptors (PRR), including NLRP1, NLRP3, NLRC4, NLRP6 and Absent in melanoma 2 (AIM2) can drive the assembly of multi-protein complexes named inflammasomes to govern caspase-1 activation (5). Inflammasomes are critical regulators of intestinal tissue homeostasis through modulating intestinal microbial ecology, inflammation and tissue repair(6). However, the role of inflammasomes in radiation-induced intestinal damage is unknown.
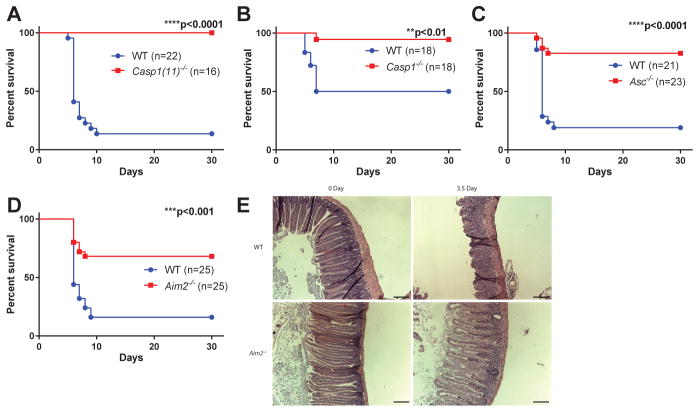
To investigate this, we used an established mouse model of radiation-induced small intestine syndrome in which mice are exposed to a lethal dose of subtotal body irradiation (SBI) with their limbs and head shielded to avoid hematopoietic syndrome(3). In this model, most wildtype (WT) mice died from severe intestinal damage within 10 days of radiation exposure. Notably, mice lacking caspase-1 were resistant to SBI induced lethality (Figure 1A), suggesting that inflammasome pathways play a critical role in controlling intestinal radio-sensitivity. This commonly used caspase-1 knockout strain (7) was recently also found to be deficient in caspase-11 (herein referred to as Casp1(11)−/−). Caspase-11 mediates non-canonical inflammasome activation in response to various gram-negative bacterial infections, whereas caspase-1 is critical for the canonical inflammasome pathway downstream of several intracellular PRRs including NLRP3 and AIM2(8). We therefore repeated the experiment and found that mice lacking only caspase-1 or the adaptor protein ASC were also protected from SBI-induced lethality, indicating that a caspase-1 dependent, ASC-dependent canonical inflammasome pathway regulates intestinal radiosensitivity (Fig. 1, B and C).
Figure 1. AIM2 inflammasome deficiency protects mice from SBI induced small intestine syndrome.
(A–D) WT mice were cohoused with Casp1(11)−/−, Casp1−/−, Asc−/− or Aim2−/− mice for two weeks and then exposed to 14.2 Gy of subtotal-body irradiation (SBI). Kaplan-Meier survival analysis of Casp1(11)−/− mice (A), Casp1−/− mice (B), Asc−/− mice (C), Aim2−/− (D) mice and their cohabitated WT mice was performed. Each figure represents the pooled data from two to three independent experiments. The total number of mice in each group and the p value by log-rank comparison are indicated on the plots. (E) Representative pictures of H&E staining of the jejunum from Aim2−/− mice and their cohabitated WT mice at day 0 and day 3.5 after 14.2 Gy of SBI. Scale bar=100 μm.
AIM2 is an innate immune sensor that mediates assembly and activation of inflammasome in response to double-stranded DNA(9, 10). We found that AIM2-deficient mice were protected from SBI induced lethality and intestinal damage (Fig. 1D). In accordance with previous observations (11–13), WT mice exhibited severe loss of crypts 3.5 days after SBI, whereas crypts of Aim2-deficient mice largely maintained their integrity (Fig. 1E). In contrast, no difference in survival from the GI syndrome was observed for mice lacking other inflammasome sensors including NLRP3 or NLRC4, indicating the specific role of the AIM2 inflammasome in controlling SBI-induced intestinal damage (Fig. S1, A and B).
Upon binding to dsDNA via its HIN200 domain, AIM2 recruits the adaptor protein ASC through its pyrin domain and assembles into an inflammasome to activate caspase-1, and thus maturation and secretion of pro-inflammatory cytokines interleukin (IL)-1β and IL-18. In addition, activation of the AIM2 inflammasome in macrophages can induce caspase-1 dependent cell death known as pyroptosis(5, 9). To elucidate the pathway downstream of the AIM2 inflammasome which might mediate intestinal radiosensitivity, we studied Il1b−/−, Il1r1−/− and Il18−/− mice and found them to be equally susceptible to SBI-induced GI syndrome as WT controls (Fig. S1, C–E). Therefore, AIM2 does not act through cytokine production to regulate intestinal damage in response to radiation. As IEC death plays a critical role in SBI induced GI syndrome(3), we next examined the contribution of caspase-1-mediated cell death in this model. We selectively deleted caspase-1 in IECs by crossing mice carrying floxed caspase-1 alleles with mice expressing Cre under the control of Villin promoter (Villin-Cre). IEC-specific caspase-1 deletion protected mice from SBI induced GI syndrome (Fig. 2A), suggesting that caspase-1-mediated pyroptosis of IECs is critical in controlling SBI sensitivity downstream of the AIM2 inflammasome.. Consistently, fewer TUNEL+ cells were observed in the crypts of the jejunum of Casp1(11)−/− and Aim2−/− mice 24 hours after SBI (Fig. 2, B and C). In addition, the levels of cleaved caspase-3 and caspase-7 were not decreased in intestines of Aim2−/− mice compared to WT (Fig. S2, A and B), indicating the reduction in TUNEL+ cells in Aim2−/− mice was caused by abrogation of caspase-1 mediated pyroptosis (Fig. S2 C), not caspase-3/7 dependent apoptosis. AIM2 was suggested to inhibit AKT activation to suppress colorectal tumorigenesis(14, 15). However, we did not observe any elevation of AKT activity in Aim2−/− mice after radiation, implying that AKT might not be involved in AIM2 inflammasome signaling in response to radiation (Fig. S2 D). Mechanistically, loss of clonogenic (stem/progenitor) cells in the crypts has been suggested to be responsible for radiation-induced intestinal damage(16). We performed the microcolony formation assay in vivo and found that AIM2-deficiency significantly enhanced crypt survival and regeneration in response to a range of radiation doses as assessed by histological analysis of haematoxylin-and-eosin (H&E) staining (Fig. S3, A and B) as well as BrdU incorporation (Fig. S3C). Furthermore, intestinal organoids derived from AIM2-deficient crypts were more resistant to radiation (Fig. S3, D and E). Taken together, our data suggest that the AIM2 inflammasome mediated pyroptosis of clonogenic cells in the intestinal crypts plays a critical role in radiation-induced GI syndrome.
Figure 2. AIM2 inflammasome regulates intestinal radio-sensitivity through caspase-1 mediated epithelial cell death.
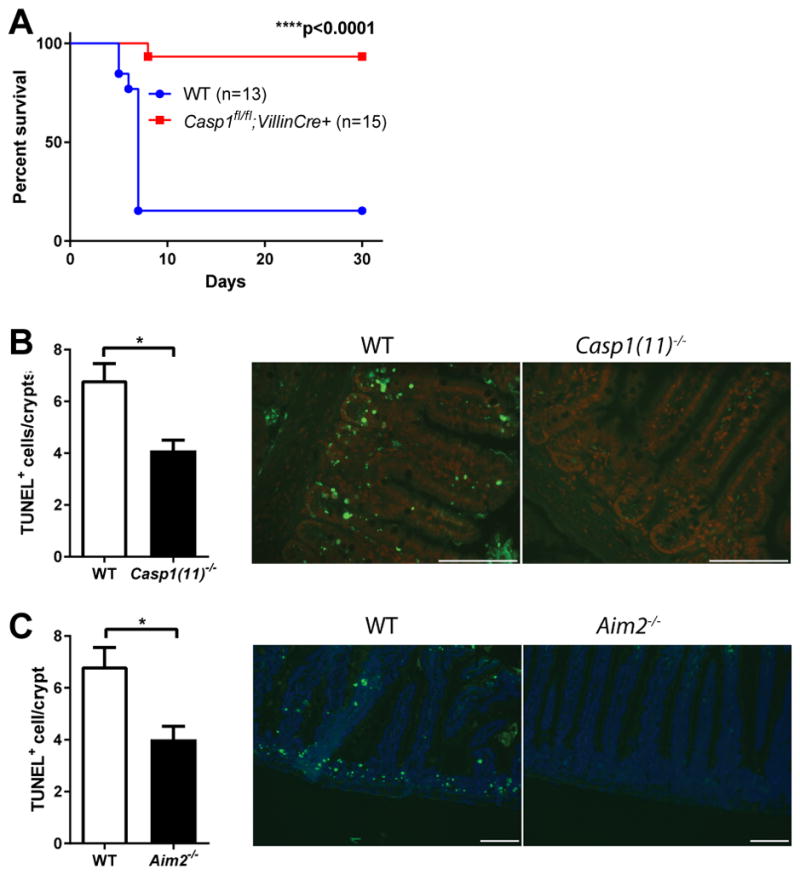
(A) WT mice were cohoused with Casp1fl/fl;VillinCre+ mice for two weeks and then exposed to 14.2 Gy of SBI. Kaplan-Meier survival analysis of Casp1fl/fl;VillinCre+ mice and their cohabitated WT mice was performed on pooled data from two independent experiments. By log-rank comparison, ****P<0.0001. (B, C) Small intestines were harvested from Casp1(11)−/−, Aim2−/− and their cohabitated WT mice 24 hours post 14.2 Gy SBI, and cell death was analyzed by TUNEL staining. Epithelial cells stained positively for TUNEL showed green fluorescence. Nuclei were stained with PI (red) or DAPI (blue). Scale bar=100 μm. Numbers of TUNEL-positive cells per crypts were quantified (n=3–5 mouse/group, at least 20 crypts of each mouse were counted) and representative pictures were shown. Results are expressed as mean ± SEM, *P<0.05 by Student’s t test.
Importantly, mice deficient in inflammasome components including ASC, caspase-1(11)(17), as well as AIM2(15, 18), can develop altered intestinal microbiota composition. Therefore, we cohoused WT controls used in these studies with the individual knockout strains at least two weeks before radiation in all of our experiments to equilibrate their gut microbiota (Fig. 1, Fig. 2 and Fig. S1), and thereby rule out the contribution of dysbiosis in regulating radiation-induced intestinal injury. Taken together, our data demonstrated that the AIM2 inflammasome acts intrinsically in IECs to control intestinal radiosensitivity through caspase-1 mediated pyroptosis.
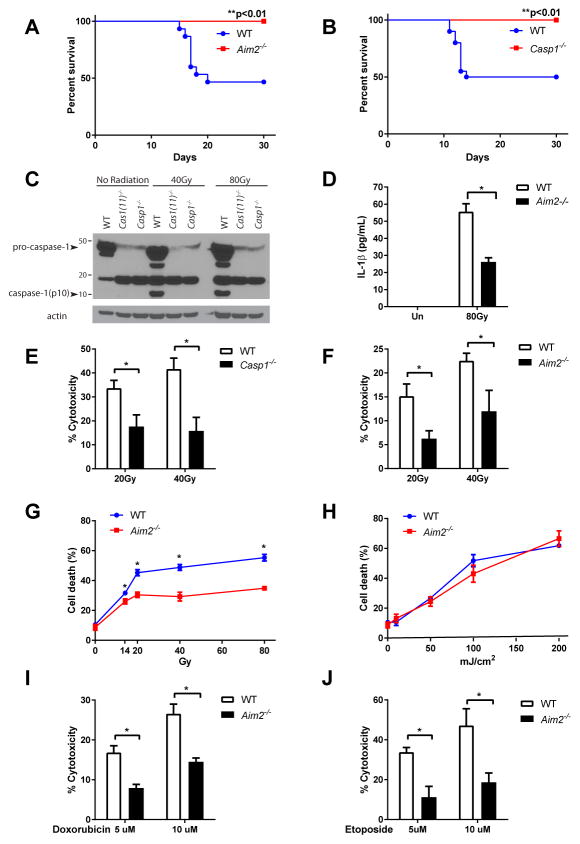
In addition to gastrointestinal toxicity (GI syndrome), acute irradiation can also induce bone marrow toxicity (hematopoietic syndrome) depending on the dose and route of radiation exposure(1, 19, 20). To further investigate whether the AIM2 inflammasome also contributes to radiosensitivity in the bone marrow compartment, we subjected mice to a lower dose (7 Gy) of total body irradiation (TBI). In this model, about 50% of WT mice died from hematopoietic syndrome starting around 2 weeks post irradiation; remarkably, Aim2−/− and Casp1−/− mice were resistant to TBI and survived beyond a month (Fig. 3, A and B; Fig. S4A).
Figure 3. AIM2 inflammasome mediates radio-sensitivity of hematopoietic cells in response to dsDNA damage.
(A, B) Kaplan-Meier survival analysis was performed on Aim2−/− mice (A), Casp1−/− mice (B) and their WT controls exposed to 7 Gy of total-body irradiation (TBI). Each figure represents the pooled data from two independent experiments. N=10–20 mice/group. (C) Caspase-1 activation in WT and Casp1−/− BMDMs 4 hours after exposure to indicated doses of radiation was assayed by immunoblotting of the cleaved form of caspase-1 (p10 subunit). (D) Supernatant was collected from un-irradiated or 80 Gy-irradiated LPS-primed WT and Aim2−/− BMDMs, and IL-1β concentration was measured by ELISA. (E, F, I, J) WT, Casp1−/− or Aim2−/− BMDMs were treated with different doses of ionizing radiation or drugs inducing dsDNA breaks, and cell death was measured by the amount of lactate dehydrogenase (LDH) released into the supernatant. (G, H) WT and Aim2−/− BMDMs were treated with different doses of ionizing radiation or UV radiation, and cell death was quantified by trypan blue staining. Determinations were performed in triplicate and expressed as the mean ± SEM. * P<0.05 by Student’s t test.
To investigate the cellular mechanism of AIM2-mediated radio-sensitivity, we used primary bone marrow derived macrophages (BMDMs). In WT cells, ionizing radiation activated the AIM2 inflammasome, as directly evidenced by the cleavage of caspase-1 to yield the p10 subunit and secretion of mature IL-1β, which were absent from Casp1−/− and Aim2−/− BMDMs (Fig. 3, C and D; Fig. S4B). Consistent with our in vivo findings, we observed a dose-dependent increase of cell death in response to ionizing radiation in WT BMDMs, which was abrogated in AIM2-deficient and caspase-1 deficient BMDMs (Fig. 3, E–G). As radiation-induced uric acid release from dead/damaged cells was previously suggested to activate caspase-1 in spleen cells(21), we harvested conditioned medium from irradiated bone marrow cells and found it did not affect survival of either un-irradiated WT or Aim2−/− bone marrow cells (Fig. S4C). Our data suggest that the AIM2 inflammasome acts in a cell-autonomous manner in response to radiation, independently of soluble factors released from dead/injured cells. Interestingly, WT and Aim2−/− BMDMs were equally sensitive to UV radiation which causes single-strand DNA breaks (Fig. 3H); however, Aim2−/− BMDMs showed significantly higher resistance to the commonly used chemotherapeutic agents doxorubicin and etoposide, which kill malignant cells by introducing DNA double-strand breaks (DSBs) (Fig. 3, I and J). In line with the in vitro data, Aim2−/− mice were also less sensitive to intestinal damage and lethality of high-dose doxorubicin treatment (Fig. S4, D and E). Altogether, these findings suggested that the AIM2 inflammasome is specifically involved in mediating cell death in response to DSBs such as those caused by ionizing radiation and chemotherapeutic agents.
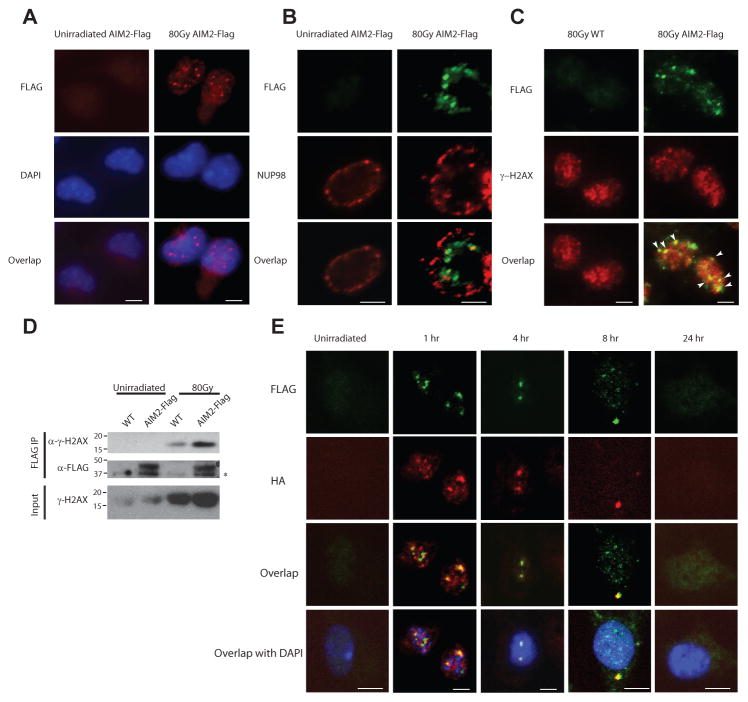
To further explore the molecular mechanism by which the AIM2 inflammasome responds to ionizing radiation, we reconstituted the AIM2 inflammasome in human embryonic kidney (HEK) 293T cells by overexpressing Flag-tagged AIM2, caspase-1 and ASC. Radiation induced the formation of AIM2-positive specks, a classical marker for inflammasome assembly, and resulted in cell death (Fig. S5, A and B). In addition, we generated a Flag-tagged AIM2 mouse in which a Flag tag was knocked into the C-terminus of AIM2 protein using the CRISPR/cas9 based genome-editing system (herein referred to as AIM2-Flag mice, Fig. S5C), because highly specific antibodies against endogenous murine AIM2 are not available(14). As we found an important role of AIM2 in regulating cell death and tissue injury in immune cells and the small intestine, we first verified the steady-state expression of endogenous AIM2 protein in the spleen and small intestine using the AIM2-Flag mice (Fig. S5D). Next, we analyzed irradiated primary macrophages from AIM2-Flag mice by immunofluorescence (IF) microscopy. Endogenous AIM2 showed very diffuse and weak staining before radiation exposure, but formed puncta in the nucleus upon radiation exposure (Fig. 4, A and B; Fig. S5E). Moreover, considerable colocalization of AIM2-containing specks and gamma-H2AX-positive foci was observed in the nucleus, suggesting that AIM2 is recruited to sites of dsDNA breaks (Fig. 4C; Fig. S5F). In support of our IF experimental observations, a strong interaction between Flag-tagged AIM2 and gamma-H2AX was detected by co-immunoprecipitation in irradiated cells (Fig. 4D). To further investigate the molecular mechanism of AIM2 inflammasome assembly, we also generated an ASC-HA knockin mouse to tag endogenous ASC protein using a similar strategy (Fig. S5G and H), and we crossed it to the AIM2-Flag mice to study the interaction between AIM2 and ASC. Importantly, using AIM2-Flag/ASC-HA double knockin mice, we found that radiation induced AIM2 and ASC co-localization and speck formation in the nuclei following radiation exposure, and the speckles containing both AIM2 and ASC later accumulated in the peri-nuclear region (Fig. 4E). Together with the data showing robust caspase-1 processing in irradiated macrophages (Fig. 3C), these results provide strong evidence for the assembly and activation of an inflammasome in response to radiation. AIM2 was previously known as a cytoplasmic DNA sensor(9, 10). Although its nuclear localization has been implicated in certain cell lines, the biological importance of nuclear AIM2 was not understood, and the sub-cellular distribution of endogenous AIM2 is unclear (22–24). Our results suggest that the recruitment of AIM2 to chromatin sites of radiation-induced DNA damage may be involved in mediating inflammasome activation and cell death.
Figure 4. Ionizing radiation induces the formation AIM2 specks in the nucleus.
Primary macrophages from AIM2-Flag mice (A, B, C, D) or AIM2-Flag/ASC-HA double knock-in mice (E) were left un-irradiated or exposed to 80 Gy ionizing radiation. For immunofluorescence microscopy, cells were fixed at 4 hours (A, B, C) or at indicated time points (E) after radiation. AIM2 was stained with anti-Flag antibody (red in A; green in B, C, E), and co-stained with nuclear envelope protein NUP98 (red, B) or gamma-H2AX (red, C) or ASC (using anti-HA antibody, red, E). Cell nuclei were visualized by DAPI (blue) in A and E. Co-localization of AIM2-Flag specks and gamma-H2AX foci was indicated by white arrowheads in C. Scale bar=5 μm. Figures represent results from three independent experiments and at least 100 cells were analyzed for each condition. (D) Co-immunoprecipitation (co-IP) of gamma-H2AX with AIM2-Flag in irradiated macrophages using anti-Flag M2 agarose beads. The immunoprecipitates (Flag IP) or the total lysates were analyzed by immunoblotting with antibodies against gamma-H2AX(Ser139) or the Flag tag. Samples from untagged WT mice were used as controls to determine the specificity of immunoblots. IB, immunoblotting. Non-specific band is indicated with an asterisk. Data represent two independent experiments.
Our present study demonstrates an unexpected role for AIM2 in sensing ionizing radiation-induced DNA damage in the nucleus. AIM2 acts thereby through the inflammasome pathway to trigger caspase-1 mediated cell death in intestinal epithelial cells and bone marrow cells. We show here that deficiency in the AIM2 inflammasome protects mice from radiation-induced small intestine syndrome as well as hematopoietic failure. While the relative contribution of pyroptosis and other forms of cell death such as apoptosis to radiation-induced tissue damage merits further investigation, our findings may have important implications for development of therapy against radiation induced GI/hematopoietic toxicity. Drugs that block the activity of the AIM2 inflammasome may be effective to treat patients exposed to ionizing radiation such as in radiation exposure via nuclear reactors or cancer patients suffering from hematopoietic or GI toxicity as a consequence of radiotherapy or chemotherapy.
Supplementary Material
Acknowledgments
We thank V.M. Dixit, N. Kayagaki and E.S. Alnemri for sharing materials and reagents, and V.A.Rathinam for technical advice. We thank P. Bongiorni for assistance with radiation experiments. The data presented in this manuscript are tabulated in the main paper and in the supplementary materials. H.B.L. is supported by NIH T32 2T32DK007356. This work was supported by the Howard Hughes Medical Institute (R.A.F.).
References
- 1.Mettler FA, Jr, Voelz GL. Major radiation exposure--what to expect and how to respond. N Engl J Med. 2002;346:1554–1561. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 2.Qiu W, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirsch DG, et al. p53 controls radiation-induced gastrointestinal syndrome in mice independent of apoptosis. Science. 2010;327:593–596. doi: 10.1126/science.1166202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paris F, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 5.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 7.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 8.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komarova EA, Christov K, Faerman AI, Gudkov AV. Different impact of p53 and p21 on the radiation response of mouse tissues. Oncogene. 2000;19:3791–3798. doi: 10.1038/sj.onc.1203717. [DOI] [PubMed] [Google Scholar]
- 12.Merritt AJ, Allen TD, Potten CS, Hickman JA. Apoptosis in small intestinal epithelial from p53-null mice: evidence for a delayed, p53-independent G2/M-associated cell death after gamma-irradiation. Oncogene. 1997;14:2759–2766. doi: 10.1038/sj.onc.1201126. [DOI] [PubMed] [Google Scholar]
- 13.Merritt AJ, et al. The role of p53 in spontaneous and radiation-induced apoptosis in the gastrointestinal tract of normal and p53-deficient mice. Cancer Res. 1994;54:614–617. [PubMed] [Google Scholar]
- 14.Wilson JE, et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med. 2015;21:906–913. doi: 10.1038/nm.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man SM, et al. Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell. 2015;162:45–58. doi: 10.1016/j.cell.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Booth C, Tudor G, Tudor J, Katz BP, MacVittie TJ. Acute gastrointestinal syndrome in high-dose irradiated mice. Health Phys. 2012;103:383–399. doi: 10.1097/hp.0b013e318266ee13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elinav E, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu S, et al. The DNA Sensor AIM2 Maintains Intestinal Homeostasis via Regulation of Epithelial Antimicrobial Host Defense. Cell Rep. 2015;13:1922–1936. doi: 10.1016/j.celrep.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger ME, Christensen DM, Lowry PC, Jones OW, Wiley AL. Medical management of radiation injuries: current approaches. Occup Med (Lond) 2006;56:162–172. doi: 10.1093/occmed/kql011. [DOI] [PubMed] [Google Scholar]
- 20.Waselenko JK, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–1051. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 21.Stoecklein VM, et al. Radiation exposure induces inflammasome pathway activation in immune cells. J Immunol. 2015;194:1178–1189. doi: 10.4049/jimmunol.1303051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cresswell KS, et al. Biochemical and growth regulatory activities of the HIN-200 family member and putative tumor suppressor protein, AIM2. Biochem Biophys Res Commun. 2005;326:417–424. doi: 10.1016/j.bbrc.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 23.Diner BA, et al. The functional interactome of PYHIN immune regulators reveals IFIX is a sensor of viral DNA. Mol Syst Biol. 2015;11:787. doi: 10.15252/msb.20145808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerur N, et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe. 2011;9:363–375. doi: 10.1016/j.chom.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Case CL, et al. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A. 2013;110:1851–1856. doi: 10.1073/pnas.1211521110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lara-Tejero M, et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med. 2006;203:1407–1412. doi: 10.1084/jem.20060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeda K, et al. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- 29.Glaccum MB, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 30.Zheng H, et al. Resistance to fever induction and impaired acute-phase response in interleukin-1 beta-deficient mice. Immunity. 1995;3:9–19. doi: 10.1016/1074-7613(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 31.Rathinam VA, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henao-Mejia J, et al. Generation of Genetically Modified Mice Using the CRISPR-Cas9 Genome-Editing System. Cold Spring Harb Protoc. 2016;2016 doi: 10.1101/pdb.prot090704. pdb prot090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammed H, et al. Rapid immunoprecipitation mass spectrometry of endogenous proteins (RIME) for analysis of chromatin complexes. Nat Protoc. 2016;11:316–326. doi: 10.1038/nprot.2016.020. [DOI] [PubMed] [Google Scholar]
- 34.Tustison KS, Yu J, Cohn SM. Assessment of intestinal stem cell survival using the microcolony formation assay. Methods Mol Med. 2001;50:267–273. doi: 10.1385/1-59259-084-5:267. [DOI] [PubMed] [Google Scholar]
- 35.Beyaz S, et al. High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature. 2016;531:53–58. doi: 10.1038/nature17173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.