ABSTRACT
Autophagy has recently been shown to be required for postmeiotic anther development including anther dehiscence, programmed cell death-mediated degradation of the tapetum and pollen maturation in rice. Several phytohormones are known to play essential roles during male reproductive development including pollen maturation. However, the relationship between phytohormone metabolism and autophagy in plant reproductive development is unknown. We here comprehensively analyzed the effect of autophagy disruption on phytohormone contents in rice anthers at the flowering stage, and found that endogenous levels of active-forms of gibberellins (GAs) and cytokinin, trans-zeatin, were significantly lower in the autophagy-defective mutant, Osatg7–1, than in the wild type. Treatment with GA4 partially recovered maturation of the mutant pollens, but did not recover the limited anther dehiscence as well as sterility phenotype. These results suggest that autophagy affects metabolism and endogenous levels of GAs and cytokinin in rice anthers. Reduction in bioactive GAs in the autophagy-deficient mutant may partially explain the defects in pollen maturation of the autophagy-deficient mutant, but tapetal autophagy also plays other specific roles in fertilization.
KEYWORDS: Anther, Autophagy, Gibberellin, Plant hormones, Pollen development, Rice
Autophagy is an evolutionarily conserved system for the degradation of intracellular components through vacuoles/lysosomes and has been shown to play essential roles in growth, development and survival of eukaryotic cells.1 Intracellular components are enveloped by autophagosomal membranes and fuse with the vacuoles/lysosomes, where they are broken down by lytic enzymes. These changes are referred to as autophagic flux.2
In many eukaryotes, autophagy is required for normal development, e.g. for dauer development in nematodes and preimplantation in mice.3-5 In plants, autophagy has been suggested to be involved in seed development and germination, photomorphogenesis, chloroplast maturation, mineral nutrition, hormonal responses, pathogen resistance, stress protection, senescence, and fertile floret development under nutrient-limiting conditions.6-9 However, mutants of Arabidopsis and maize defective in autophagy exhibit normal life cycles, and little is known regarding the roles of autophagy during normal reproductive development in plants.10,11
Rice mutants defective in autophagy, Osatg7–1 and Osatg9, show sporophytic male sterility and limited anther dehiscence under normal growth conditions, suggesting that autophagy is crucial for sexual reproductive development of rice.12,13 Pollens of Osatg7–1 mutant are premature due to significant defects in the anther during pollen maturation. Autophagosomes emerge in the tapetum during the uninucleate stage in the wild type but not in Osatg7–1 mutant, indicating that autophagy is induced at the uninucleate stage in the postmeiotic tapetum cells and may be involved in the catabolism of intracellular components such as plastids and lipid bodies during pollen maturation.12,14
Several phytohormones are known to play essential roles during the male reproductive development in rice.15 Autophagy-deficient mutants of Arabidopsis such as atg5 have been shown to over-accumulate salicylic acid (SA) in leaves, and many of their phenotypes can be explained by the excess amount of SA.16 However, relationship between autophagy and phytohormone metabolism in plant reproductive development is unknown.
In the present study, we conducted comprehensive analyses for phytohormone levels of anthers at the flowering stage in the Osatg7–1 mutant and wild type, in which the insertion of Tos17 in OsATG7 was removed by heterozygous segregation, and investigated the effect of autophagy disruption on phytohormone contents. Endogenous levels of bioactive gibberellins (GAs; GA1, GA4 and GA7) in anthers were lower in the Osatg7–1 mutant than in the wild type and cultivar Nipponbare (NB) as controls at the flowering stage (Table 1). A cytokinin (trans-zeatin; tZ) also decreased in the mutant, while no significant difference was observed for other phytohormones including SA, jasmonic acid (JA), auxin and abscisic acid (ABA) between the wild type and the mutant (Table 1). Unlike the case in Arabidopsis leaves,16 SA content was not affected by the disruption of autophagy in anthers (Table 1).
Table 1.
Phytohormones in Nipponbare (NB), wild type and Osatg7–1 anthers at the flowering stage.
| hormones (pmol/gFW) | NB | wild-type | Osatg7–1 |
|---|---|---|---|
| tZ | 5.26 ± 0.91 | 4.74 ± 0.40 | 2.29 ± 0.16* |
| IAA | 32200 ± 10000 | 30500 ± 18300 | 57600 ± 20300 |
| GA1 | 48.09 ± 5.51 | 38.00 ± 16.87 | 4.78 ± 1.16* |
| GA4 | 4740 ± 119.4 | 4860 ± 545.3 | 3170 ± 684.3** |
| GA7 | 1110 ± 79.4 | 1030 ± 119.5 | 672 ± 128.4** |
| GA12 | 63699 ± 4816 | 85200 ± 4770 | 10400 ± 2310** |
| ABA | 17.76 ± 3.34 | 11.51 ± 2.42 | 16.49 ± 3.46 |
| SA | 7520 ± 3100 | 7010 ± 2880 | 7410 ± 1740 |
| JA | 430 ± 63.35 | 532 ± 59.14 | 735 ± 216.21 |
Data represent the mean ± SD of 3≧ plants.
P < 0.05,
P < 0.01; significantly different from both NB and the wild type.
Mild defects in GA biosynthesis have been shown to impair pollen function in rice.17 To investigate the effect of bioactive GA during anther development in autophagy deficient mutant, rice plants at the pollen mother cell (PMC) stage were sprayed with 10−5 M GA4, and then both the pollen maturation and germination activity were assessed. Treatment with GA4 recovered the maturation of mutant pollens at the flowering stage (Fig. 1a). In contrast, the germination activity of mutant pollens was partially recovered by the treatment of bioactive GA (Fig. 1b). Moreover, the limited anther dehiscence as well as sterility phenotype of the mutant was not recovered by the addition of bioactive gibberellin, GA4 (Fig. 2). These results suggest that autophagy affects phytohormone contents in rice anthers at the flowering stage, and the reduction in bioactive GAs may explain in part the defects in the pollen function in the Osatg7–1 mutant. GAs play important roles in seed germination and anther development in several plant species. GA deficiency or insensitivity causes abnormal development of anthers and leads to male sterility in tomato, petunia, Arabidopsis, and rice.18-22 All these studies suggest that GA is essential for normal development of tapetal cells and pollen.
Figure 1.
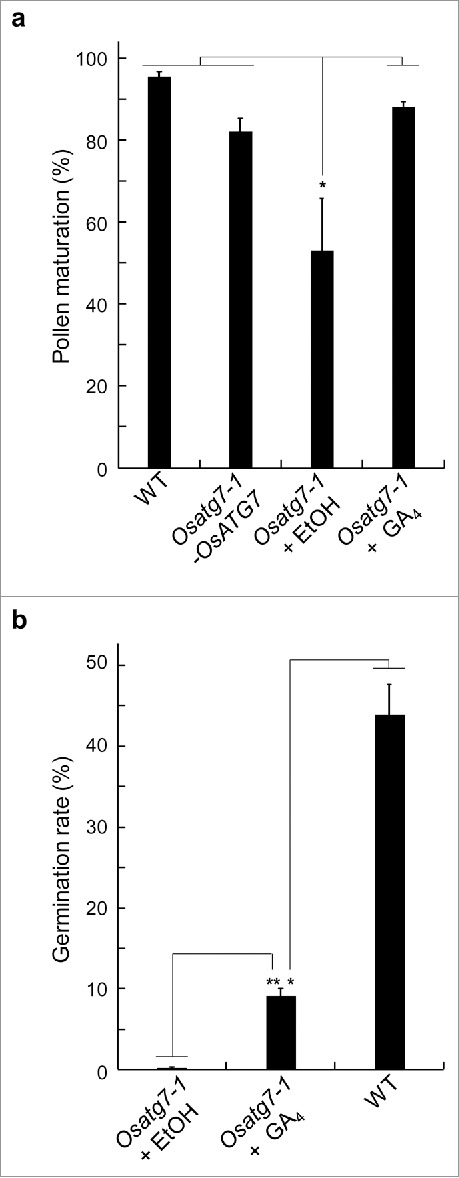
Effects of active GA on pollen maturation and germination in rice autophagy-deficient mutant. (a) Pollen grains from the wild type (WT), complementation plant (Ostg7–1-OsATG7) and Ostg7–1 mutant were stained with I2-KI solution and quantified. (b) Pollen grains from the Osatg7–1 anthers at the flowering stage were germinated for 1 h in pollen germination medium, and then observed and quantified by bright-field microscopy. Data indicates means ± SD of 10 independent samples. The average was determined from 200 pollen grains per each experiment. * P < 0.05, ** P < 0.01; significantly different from the control using an unpaired Student's t-test.
Figure 2.
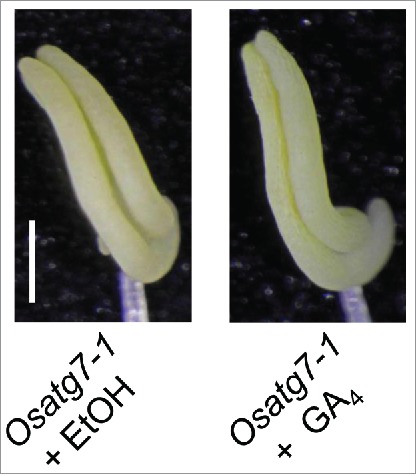
The effect of active GA on anther dehiscence in rice autophagy-deficient mutant. Anthers from the Osatg7–1 at the flowering stage were observed by bright-field microscopy. Scale bar: 0.5 mm.
As pollens develop, the tapetum is broken down to provide nutrients, metabolites, and sporopollenin precursors to the developing microspores. Defects in tapetal degradation have been suggested to result in the development of abnormal pollen coats and grains, leading to severe male sterility.23-26 Additionally, the appropriate temporal regulation of tapetal programmed cell death (PCD) is vital for normal pollen development. The signal initiating tapetal PCD has been suggested to be first produced during the tetrad stage.27 GA was previously shown to control tapetum degradation.18,19 A delay in tapetal breakdown and a switch from PCD to necrosis in the tapeta were observed in an ms1 mutant in Arabidopsis.28 Pollen wall deposition and subsequent microspore degeneration failed in a rice mutant in which tapetal degeneration and PCD was retarded.25,26
As shown in Table 1, disruption of autophagy significantly reduced endogenous levels of the precursor GA12 as well as bioactive GAs in rice anthers at the flowering stage (Table 1). Autophagy may affect the ent-kaurene pathway that supply the precursor of bioactive GAs in anthers. The ent-kaurene products are synthesized in plastids by ent-copalyl diphosphate synthase (CPS) and kaurene synthase (KS).29,30 Autophagy plays important roles in the quality control of plastids including elimination and turnover of photodamaged chloroplasts.31 Accumulation of abnormal plastids in anther cells of autophagy-deficient mutant may explain the lower activity of the ent-kaurene pathway in anther cells, suggesting the significance of autophagy in the quality control of plastids in anthers. Our present research indicates a critical role of autophagy in developmental process of crops, and shed light on the novel autophagy-mediated regulation of GA metabolism in plastids and tapetal PCD in rice reproductive development.
Jasmonic acid (JA) has been thought to play an important role in regulating anther dehiscence. Mutations in genes that participate in JA biosynthesis cause a failure or delay in anther dehiscence and can result in male sterility.32,33 Rice mutants defective in autophagy (Osatg7–1 and Osatg9) show limited anther dehiscence under normal growth conditions.12 However, Endogenous JA level was not affected by the disruption of autophagy during anther development (Table 1). In fact, the limited anther dehiscence of the Osatg7–1 mutant was not recovered by the addition of MetJA at the flowering stage (Fig. S1), indicating that the defect in anther dehiscence and the male sterile phenotype in the Osatg7–1 mutant may not explain by the reduction of JA content.
Comprehensive analyses of phytohormones in rice anther showed that endogenous levels of bioactive cytokinin tZ (trans-zeatin) was also lower in the Osatg7–1 mutant than in the wild type at the flowering stage (Table 1). However, the physiologic function of cytokinin during anther development remains largely unclear. Overexpression of cytokinin oxidase/dehydrogenase (CKX) under the control of anther specific promoter have been suggested to result in male sterility in maize.34 Interestingly, ectopic-expression of Arabidopsis ATG8 protein reduces shoot anthocyanin accumulation in response to cytokinin feeding to the roots,35 implying the participation of ATG8 protein in cytokinin-regulated root-shoot communication in Arabidopsis. Moreover, external application of cytokinin leads to the autophagosome formation in Arabidopsis root cells.35 Autophagy may participate in the control CK content during anther development, and the potential role of CK in the regulation of anther development is an important topic for future research.
Supplementary Material
Disclosure of potential conflicts of interests
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank Mr. Kotaro Nihira for technical assistance.
Fundings
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology for Young Scientists (B) (15K21450) to T.K., for Innovative Areas (16H01207; 15H01239) to K.K., for challenging Exploratory Research (15K14681) to K.K., and in part by the Asahi Glass Foundation to T.K.
References
- 1.F Li, Autophagy Vierstra RD. A multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci. 2012;17:526–37. doi: 10.1016/j.tplants.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meléndez A, Levine B “Autophagy in C. elegans,” in WormBook. In: Kramer JM, Moerman DC, editors; 2009. doi: 10.1895/wormbook.1.147.1. [DOI] [PubMed] [Google Scholar]
- 4.N Mizushima, Komatsu M. Autophagy: Renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 5.Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N. Autophagy is essential for preimplantation development of mouse embryos. Science. 2008;321:117–20. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]
- 6.Chung T, Suttangkakul A, Vierstra RD. The ATG autophagic conjugation system in maize: ATG transcripts and abundance of the ATG8-lipid adduct are regulated by development and nutrient availability. Plant Physiol. 2009;149:220–34. doi: 10.1104/pp.108.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghiglione HO, Gonzalez FG, Serrago R, Maldonado SB, Chilcott C, Curá JA, Miralles DJ, Zhu T, Casal JJ. Autophagy regulated by day length determines the number of fertile florets in wheat. Plant J. 2008; 55:1010–24. doi: 10.1111/j.1365-313X.2008.03570.x. [DOI] [PubMed] [Google Scholar]
- 8.Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, Ohsumi Y, Hanson MR, Mae T. Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol. 2008;148:142–55. doi: 10.1104/pp.108.122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumi M, Hidema J, Wada S, Kondo E, Kurusu T, Kuchitsu K, Makino A, Ishida H. Establishment of monitoring methods for autophagy in rice reveals autophagic recycling of chloroplasts and root plastids during energy limitation. Plant Physiol. 2015;167:1307–20. doi: 10.1104/pp.114.254078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li F, Chung T, Pennington JG, Federico ML, Kaeppler HF, Kaeppler SM, Otegui MS, Vierstra RD. Autophagic recycling plays a central role in maize nitrogen remobilization. Plant Cell. 2015;27:1389–408. doi: 10.1105/tpc.15.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshimoto K. Beginning to understand autophagy, an intracellular self-degradation system in plants. Plant Cell Physiol. 2012;53:1355–65. doi: 10.1093/pcp/pcs099. [DOI] [PubMed] [Google Scholar]
- 12.Kurusu T, Koyano T, Hanamata S, Kubo T, Noguchi Y, Yagi C, Nagata N, Yamamoto T, Ohnishi T, Okazaki Y, et al.. OsATG7 is required for autophagy-dependent lipid metabolism in rice postmeiotic anther development. Autophagy. 2014;10:878–88. doi: 10.4161/auto.28279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurusu T, Kuchitsu K. Autophagy, programmed cell death and reactive oxygen species in sexual reproduction in plants. J Plant Res. 2017;130:491–99. doi: 10.1007/s10265-017-0934-4. [DOI] [PubMed] [Google Scholar]
- 14.Hanamata S, Kurusu T, Kuchitsu K. Roles of autophagy in male reproductive development in plants. Front Plant Sci. 2014;5:e457. doi: 10.3389/fpls.2014.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano K, Aya K, Hobo T, Sakakibara H, Kojima M, Shim RA, Hasegawa Y, Ueguchi-Tanaka M, Matsuoka M.. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol. 2008;49:1429–50. doi: 10.1093/pcp/pcn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, Panstruga R, Ohsumi Y, Shirasu K. Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell. 2009;21:2914–2927. doi: 10.1105/tpc.109.068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chhun T, Aya K, Asano K, Yamamoto E, Morinaka Y, Watanabe M, Kitano H, Ashikari M, Matsuoka M, Ueguchi-Tanaka M.. Gibberellin regulates pollen viability and pollen tube growth in rice. Plant Cell. 2007;19:3876–88. doi: 10.1105/tpc.107.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aya K, Ueguchi-Tanaka M, Kondo M, Hamada K, Yano K,Nishimura M, Matsuoka M. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell. 2009;21:1453–72. doi: 10.1105/tpc.108.062935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng H, Qin L, Lee S, Fu X, Richards DE, Cao D, Luo D, Harberd NP, Peng J.. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development. 2004;131:1055–64. doi: 10.1242/dev.00992. [DOI] [PubMed] [Google Scholar]
- 20.Izhaki A, Borochov A, Zamski E, Weiss D. Gibberellin regulates post-microsporogenesis processes in petunia anthers. Physiol Plant. 2002;115:442–7. doi: 10.1034/j.1399-3054.2002.1150314.x. [DOI] [PubMed] [Google Scholar]
- 21.Jacobsen SE, Olszewski NE. Characterization of the arrest in anther development associated with gibberellin deficiency of the gib-1 mutant of tomato. Plant Physiol. 1991;97:409–14. doi: 10.1104/pp.97.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nester JE, Zeevaart JAD. Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. Am J Bot. 1998;75:45–55. doi: 10.2307/2443904. [DOI] [Google Scholar]
- 23.Ariizumi T, Toriyama K. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol. 2011;62:437–60. doi: 10.1146/annurev-arplant-042809-112312. [DOI] [PubMed] [Google Scholar]
- 24.Ku S, Yoon H, Suh HS, Chung YY. Male-sterility of thermosensitive genic male-sterile rice is associated with premature programmed cell death of the tapetum. Planta. 2003;217:559–65. doi: 10.1007/s00425-003-1030-7. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Zhang DS, Liu HS, Yin CS, Li XX, Liang WQ, Yuan Z, Xu B, Chu HW, Wang J, et al.. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell. 2006;18:2999–3014. doi: 10.1105/tpc.106.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang DS, Liang WQ, Yuan Z, Li N, Shi J, Wang J, Liu YM, Yu WJ, Zhang DB.. Tapetum degeneration retardation is critical for aliphatic metabolism and gene regulation during rice pollen development. Mol Plant. 2008;1:599–610. doi: 10.1093/mp/ssn028. [DOI] [PubMed] [Google Scholar]
- 27.Kawanabe T, Ariizumi T, Kawai-Yamada M, Uchimiya H, Toriyama K. Abolition of the tapetum suicide program ruins microsporogenesis. Plant Cell Physiol. 2006;47:784–7. doi: 10.1093/pcp/pcj039. [DOI] [PubMed] [Google Scholar]
- 28.Vizcay-Barrena G, Wilson ZA. Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J Exp Bot. 2006;57:2709–17. doi: 10.1093/jxb/erl032. [DOI] [PubMed] [Google Scholar]
- 29.Hedden P, Phillips AL. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000;5:523–30. doi: 10.1016/S1360-1385(00)01790-8. [DOI] [PubMed] [Google Scholar]
- 30.Lange T. Molecular biology of gibberellin synthesis. Planta. 1998;204:409–19. doi: 10.1007/s004250050274. [DOI] [PubMed] [Google Scholar]
- 31.Izumi M, Ishida H, Nakamura S, Hidema J.. Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell. 2017;29:377–94. doi: 10.1105/tpc.16.00637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in. Arabidopsis. Plant Cell. 2001;13:2191–209. do: 10.1105/tpc.13.10.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanders PM, Lee PY, Biesgen C, Boone JD, Beals TP, Weiler EW, Goldberg RB. The arabidopsis DELAYED DEHISCENCE1 gene encodes an enzyme in the jasmonic acid synthesis pathway. Plant Cell. 2000;12:1041–61. doi: 10.1105/tpc.12.7.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang S, Cerny RE, Qi Y, Bhat D, Aydt CM, Hanson DD, Malloy KP, Ness LA. Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiol. 2003;131:1270–82. doi: 10.1104/pp.102.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slavikova S, Ufaz S, Avin-Wittenberg T, Levanony H, Galili G. An autophagy-associated Atg8 protein is involved in the responses of Arabidopsis seedlings to hormonal controls and abiotic stresses. J Exp Bot. 2008;59:4029–43. doi: 10.1093/jxb/ern244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


