ABSTRACT
Thioredoxins (Trxs) play a relevant role in thiol-dependent redox regulation, which allows the rapid adaptation of chloroplast metabolism to unpredictable environmental conditions. In chloroplasts, Trxs use reducing equivalents provided by photoreduced ferredoxin (Fdx) via the action of a ferredoxin-thioredoxin reductase (FTR), thus linking redox regulation to light. In addition, these organelles contain an NADPH-thioredoxin reductase, NTRC, with a Trx domain at the C-terminus. NTRC efficiently reduces 2-Cys peroxiredoxins (Prxs), hence having antioxidant function. However, NTRC also participates in the redox regulation of processes, such as starch and chlorophyll biosynthesis, which are known to be regulated by Trxs. Thus, the question arising is whether there is a cross-talk between the 2 redox systems. Arabidopsis mutants simultaneously devoid of NTRC and Trx x or Trxs f show a dramatic growth inhibition phenotype, indicating that NTRC is required for the function of these unrelated Trxs. Remarkably, both the ntrc-trxx double mutant and, to a higher extent, the ntrc-trxf1f2 triple mutant show high mortality at the seedling stage, which is rescued by sucrose. These findings show the relevant role of redox regulation for chloroplast performance and uncover the key function of cotyledons chloroplasts at the transition to autotrophic metabolism during seedling establishment.
KEYWORDS: Chloroplast, NTRC, redox regulation, seedling, thioredoxin
Chloroplasts, the organelles in which light energy is converted in organic material, are the source of metabolic precursors for plant growth. Therefore, the deep influence of light on chloroplast performance and, hence, on plant development is not surprising. Central to the metabolic plasticity of chloroplasts is thiol-dependent redox regulation of enzyme activity, a regulatory mechanism in which the protein disulphide reductase activity of thioredoxins (Trxs) plays an important role.1 While in heterotrophic organisms Trxs are reduced by NADPH with the participation of an NADPH-thioredoxin reductase (NTR), the complex set of chloroplast Trxs are reduced by photosynthetically reduced ferredoxin (Fdx) with the participation of ferredoxin-thioredoxin reductase (FTR).2 Therefore, by using photoreduced Fdx as source of reducing power the FTR-Trxs system links chloroplast redox regulation to light. Virtually any process taking place in this organelle is redox sensitive, the different types of Trxs showing specific functions. While Trxs f are considered to participate in redox regulation of metabolic pathways, such as the Calvin-Benson cycle enzymes, Trx x has been proposed to have antioxidant function.3
This classical view of chloroplast redox regulation was modified by the discovery of an NTR with a joint Trx domain at the C-terminus, termed NTRC,4 which is exclusively found in organisms that perform oxygenic photosynthesis.5 Based on the finding that NTRC is a very efficient reductant of the thiol-dependent peroxidase 2-Cys peroxiredoxin (2-Cys Prxs), it was proposed the participation of NTRC in the antioxidant defense mechanism of the chloroplast.6 However, Arabidopsis mutants devoid of NTRC show growth retard phenotype6,7 and low efficiency of light energy utilization,8,9 suggesting additional functions for this enzyme. In line with this notion, it was shown that NTRC participates in redox regulation of previously identified Trx-regulated pathways such as starch10,11 and chlorophyll biosynthesis,12,13 hence raising the question of the relationship of NTRC and the Fdx/FTR/Trxs redox system in chloroplast redox regulation.
This issue is currently being addressed by genetic approaches. In this regard, it is worth mentioning that Arabidopsis single mutants lacking specific isoforms of chloroplast Trxs such as those of the types x,14 f,15-17 or m18,19 show almost wild type phenotype, which is in contrast with the important function assigned to these Trxs based on biochemical analyses.20 Interestingly, mutants combining the deficiencies of NTRC and chloroplast Trxs, such as the ntrc-trxf1 mutant, show a very severe phenotype,21 suggesting that both systems have overlapping functions in chloroplast redox regulation. In support of this notion, Arabidopsis mutants devoid of NTRC and FTR are not viable.22 Our finding that Arabidopsis mutants lacking NTRC and Trx x, and, to a higher extent, mutants lacking NTRC and Trxs f1 and f2 show a very severe growth inhibition phenotype,23 indicate that the deficiency of NTRC impairs the activity of chloroplast Trxs. While these findings further support the notion that the 2 chloroplast redox systems act concertedly, the molecular basis of this interaction remains unknown. One possibility is that NTRC and the different Trxs modulate the activity of common targets. If this is the case, the deficiency of one of the systems could be counteracted by the other while the simultaneous deficiency of both systems would cause the severe impairment of redox regulation of these targets. However, the light-dependent reduction of fructose 1,6-bisphosphatase (FBPase), a well-established redox regulated enzyme of the Calvin-Benson cycle, was more affected in the ntrc than in the trxx and trxf1f2 mutants despite the fact that the enzyme was reduced by Trxs f, and less efficiently by Trx x, but not by NTRC in vitro.23 These results suggest that NTRC may exert an indirect effect on the redox regulation of FBPase. Since NTRC is the most efficient reductant of 2-Cys Prxs in vivo,14 an additional possibility to be taken into account is that the lack of NTRC affects hydrogen peroxide homeostasis provoking oxidative stress, which would affect Trx activity. However, the biochemical basis of the functional relationship of NTRC, 2-Cys Prxs and the Trxs remains to be elucidated.
The ntrc-trxx and the ntrc-trxf1f2 mutants show a severe growth inhibition phenotype; moreover, a remarkable feature of the phenotype of these mutants was the low number of individuals that reached the adult stage when grown on soil, the mortality of the ntrc-trxx and ntrc-trxf1f2 seedlings being of approx. 50% and 95%, respectively.23 These results not only show the profound relationship of NTRC and functionally unrelated Trxs x and f, they also highlight the relevance of redox regulation on chloroplast performance at early stages of development. Seedling establishment, defined as the formation of the first true leaves, was delayed in ntrc-trxx and ntrc-trxf1f2 surviving seedlings. In line with these results, seedlings of these mutant lines germinated in synthetic media without exogenous carbon source displayed impaired root growth. This phenotypic effect was partially rescued by sucrose, indicating that the photosynthetic activity of cotyledon chloroplasts is critical to reach autotrophic growth and for the development of new tissues such as roots and true leaves.
Therefore, the impairment of the chloroplast redox network in the ntrc-trxx and, more severely, in ntrc-trxf1f2 mutants causes growth retard but not lethality at the adult phase of development. In contrast, this impairment of the redox network is critical for post-germinative seedling establishment. The analysis of soil-germinated seedlings of the ntrc-trxf1f2 triple mutant (Fig. 1A) shows short hypocotyls and expanded cotyledons, indicating that the developmental program of photomorphogenesis is not affected by the deficient chloroplast redox regulation in these mutants. However, these seedlings are unable to generate the new organs and undergo progressive cotyledon bleaching (Fig. 1A), suggesting that the photosynthetic performance of the cotyledon chloroplasts is not sufficient to provide the sucrose required for further development of the new tissues.24 In contrast with the wild type (Fig. 1B), chloroplasts of the ntrc-trxf1f2 seedlings show symptoms of thylakoid dismantling and the presence of clear regions in the stroma indicating active degradation of chloroplast structures (Fig. 1C-E). Moreover, chloroplasts in advanced degree of degeneration with the appearance of gerontoplasts25 were also detected at this stage of development (Fig. 1E). Chloroplast degeneration is characterized by the increase in the number and size of plastoglobules and an increase of plastoglobule attachment (Fig. 1C-E), which is indicative of oxidative stress.26 This feature suggests that once autotrophic growth is arrested by the deficiency of the redox system of cotyledon chloroplasts, these cells suffer increasing oxidative stress and normal development of the new organs, leaves and roots, is inhibited. The dramatic effect of the lack of the redox systems here analyzed at the seedling stage uncovers the relevance of redox regulation for chloroplast performance, which is critical for the transition to autotrophic growth at the seedling stage.
Figure 1.
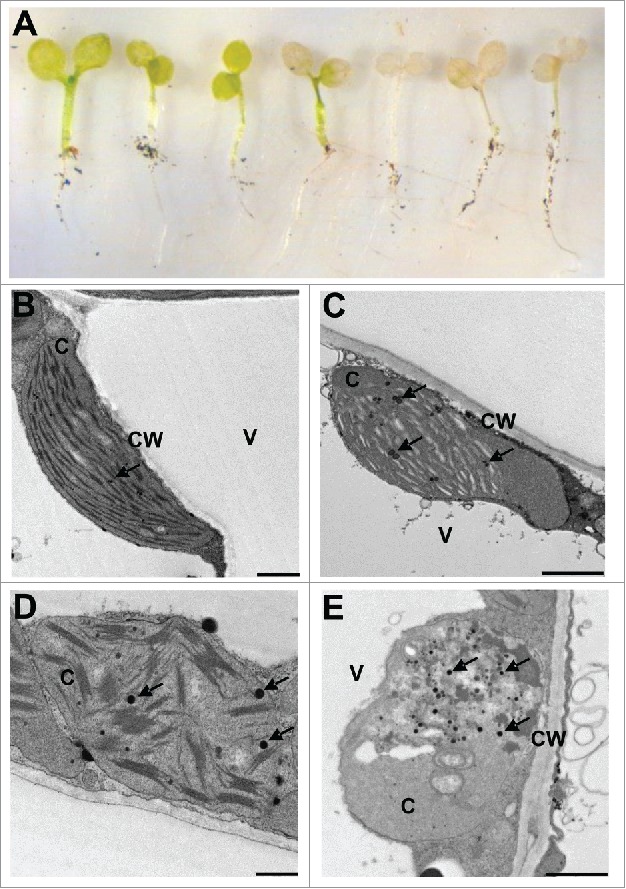
Cotyledon chloroplast structure of wild type and ntrc-trxf1f2 bleaching seedlings. (A) Seeds of ntrc-trxf1f2 triple mutant were allowed to germinate on soil and seedlings at different stages of bleaching are shown. (B-E) Electron transmission microscopy analysis of chloroplast structure from wild-type plants (B) and the mutant line seedlings (C-E). Plants were germinated on soil for 10 days, and seedlings were collected just before the appearance of the first true leaves and fixed in glutaraldehyde. Transmission electron microscopy analysis was performed as previously reported.23 Bars represent 1 µm (B, C, E) and 0.5 μm (D). c, chloroplasts; cw, cell wall; v, vacuoles. Arrows indicate plastogobules.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by European Regional Development Fund-cofinanced grant (BIO2013–43556-P) from the Spanish Ministry of Innovation and Competiveness (MINECO) and Grant CVI-5919 from Junta de Andalucía, Spain.
Abbreviations
- Fdx
ferredoxin
- FTR
ferredoxin-dependent thioredoxin reductase
- NTRC
NADPH-dependent thioredoxin reductase C
- Prx
peroxiredoxin
- Trx
thioredoxin
References
- 1.Rouhier N, Cerveau D, Couturier J, Reichheld JP, Rey P. Involvement of thiol-based mechanisms in plant development. Biochimica Biophys Acta 2015; 1850:1479-96; PMID:25676896; https://doi.org/ 10.1016/j.bbagen.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 2.Schürmann P, Buchanan BB. The ferredoxin/thioredoxin system of oxygenic photosynthesis. Antioxid Redox Signal 2008; 10:1235-74; PMID:18377232; https://doi.org/ 10.1089/ars.2007.1931 [DOI] [PubMed] [Google Scholar]
- 3.Geigenbeger P, Thormälen I, Daloso DM, Fernie AR. The unprecedented versatility of the plant thioredoxin system. Trends Plant Sci 2017; 22:249-62; PMID:28139457; https://doi.org/ 10.1016/j.tplants.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Serrato AJ, Pérez-Ruiz JM, Spinola MC, Cejudo FJ. A novel NADPH thioredoxin reductase, localized in the chloroplast, which deficiency causes hypersensitivity to abiotic stress in Arabidopsis thaliana. J Biol Chem 2004; 279:43821-27; PMID:15292215; https://doi.org/ 10.1074/jbc.M404696200 [DOI] [PubMed] [Google Scholar]
- 5.Nájera VA, González MC, Pérez-Ruiz JM, Cejudo FJ. An event of alternative splicing affects the expression of the NTRC gene, encoding NADPH-thioredoxin reductase C, in seed plants. Plant Sci 2017; 258:21-8; PMID:28330560; https://doi.org/ 10.1016/j.plantsci.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Ruiz JM, Spínola MC, Kirchsteiger K, Moreno J, Sahrawy M, Cejudo FJ. Rice NTRC is a high-efficiency redox system for chloroplast protection against oxidative damage. Plant Cell 2006; 18:2356-68; PMID:16891402; https://doi.org/ 10.1105/tpc.106.041541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepistö A, Kangasjarvi S, Luomala EM, Brader G, Sipari N, Keranen M, Keinanen M, Rintamäki E. Chloroplast NADPH-thioredoxin reductase interacts with photoperiodic development in Arabidopsis. Plant Physiol 2009; 149:1261-76; PMID:19151130; https://doi.org/ 10.1104/pp.108.133777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrillo LR, Froehlich JE, Cruz JA, Savage L, Kramer DM. Multi-level regulation of the chloroplast ATP synthase: The chloroplast NADPH thioredoxin reductase C (NTRC) is required for redox modulation specifically under low irradiance. Plant J 2016; 87:654-63; PMID:27233821; https://doi.org/ 10.1111/tpj.13226 [DOI] [PubMed] [Google Scholar]
- 9.Naranjo B, Mignee C, Krieger-Liszkay A, Hornero-Mendez D, Gallardo-Guerrero L, Cejudo FJ, Lindahl M. The chloroplast NADPH thioredoxin reductase C, NTRC, controls non-photochemical quenching of light energy and photosynthetic electron transport in Arabidopsis. Plant Cell Environ 2016; 39:804-22; PMID:26476233; https://doi.org/ 10.1111/pce.12652 [DOI] [PubMed] [Google Scholar]
- 10.Lepistö A., Pakula E, Toivola J, Krieger-Liszkay A, Vignols F, Rintamäki E.. Deletion of chloroplast NADPH-dependent thioredoxin reductase results in inability to regulate starch synthesis and causes stunted growth under short-day photoperiods. J Exp Bot 2013; 64:3843-54; PMID:23881397; https://doi.org/ 10.1093/jxb/ert216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michalska J, Zauber H, Buchanan BB, Cejudo FJ, Geigenberger P. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc Natl Acad Sci USA 2009; 106:9908-13; PMID:19470473; https://doi.org/ 10.1073/pnas.0903559106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pérez-Ruiz JM, Guinea M, Puerto-Galán L, Cejudo FJ. NADPH thioredoxin reductase C is involved in redox regulation of the Mg-chelatase I subunit in Arabidopsis thaliana chloroplasts. Mol Plant 2014; 7:1252-55; PMID:24658415; https://doi.org/ 10.1093/mp/ssu032 [DOI] [PubMed] [Google Scholar]
- 13.Richter AS, Peter E, Rothbart M, Schlicke H, Toivola J, Rintamäki E, Grimm B. Posttranslational influence of NADPH-dependent thioredoxin reductase C on enzymes in tetrapyrrole synthesis. Plant Physiol 2013; 162:63-73; PMID:23569108; https://doi.org/ 10.1104/pp.113.217141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulido P, Spínola MC, Kirchsteiger K, Guinea M, Pascual MB, Sahrawy M, Sandalio LM, Dietz KJ, González M, Cejudo FJ. Functional analysis of the pathways for 2-Cys peroxiredoxin reduction in Arabidopsis thaliana chloroplasts. J Exp Bot 2010; 61:4043-54; PMID:20616155; https://doi.org/ 10.1093/jxb/erq218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naranjo B, Díaz-Espejo A, Lindahl M, Cejudo FJ. Type-f thioredoxins have a role in the short-term activation of carbon metabolism and their loss affects growth under short-day conditions in Arabidopsis thaliana. J Exp Bot 2016; 67:1951-64; PMID:26842981; https://doi.org/ 10.1093/jxb/erw017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thormählen I, Ruber J, von Roepenack-Lahaye E, Ehrlich SM, Massot V, Hummer C, Tezycka J, Issakidis-Bourguet E, Geigenberger P. Inactivation of thioredoxin f1 leads to decreased light activation of ADP-glucose pyrophosphorylase and altered diurnal starch turnover in leaves of Arabidopsis plants. Plant Cell Environ 2013; 36:16-29; PMID:22646759; https://doi.org/ 10.1111/j.1365-3040.2012.02549.x [DOI] [PubMed] [Google Scholar]
- 17.Yoshida K, Hara S, Hisabori T. Thioredoxin selectivity for thiol-based redox regulation of target proteins in chloroplasts. J Biol Chem 2015; 290:14278-88; PMID:25878252; https://doi.org/ 10.1074/jbc.M115.647545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courteille A, Vesa S, Sanz-Barrio R, Cazale AC, Becuwe-Linka N, Farrán I, Havaux M, Rey P, Rumeau D. Thioredoxin m4 controls photosynthetic alternative electron pathways in Arabidopsis. Plant Physiol 2013; 161:508-20; PMID:23151348; https://doi.org/ 10.1104/pp.112.207019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thormählen I, Zupok A, Rescher J, Leger J, Weissenberger S, Groysman J, Orwat A, Chatel-Innocenti G, Issakidis-Bourguet E, Armbruster U, Geigenberger P. Thioredoxins play a crucial role in dynamic acclimation of photosynthesis in fluctuating light. Mol Plant 2016; 10:168-82; PMID:27940305; https://doi.org/ 10.1016/j.molp.2016.11.012 [DOI] [PubMed] [Google Scholar]
- 20.Michelet L, Zaffagnini M, Morisse S, Sparla F, Pérez-Pérez ME, Francia F, Danon A, Marchand CH, Fermani S, Trost P, Lemaire SD. Redox regulation of the Calvin-Benson cycle: something old, something new. Front Plant Sci 2013; 4:470; PMID:24324475; https://doi.org/ 10.3389/fpls.2013.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thormählen I, Meitzel T, Groysman J, Ochsner AB, von Roepenack-Lahaye E, Naranjo B, Cejudo FJ, Geigenberger P. Thioredoxin f1 and NADPH-dependent thioredoxin reductase C have overlapping functions in regulating photosynthetic metabolism and plant growth in response to varying light conditions. Plant Physiol 2015; 169:1766-86; PMID:26338951; https://doi.org/ 10.1104/pp.15.01122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida K, Hisabori T. Two distinct redox cascades cooperatively regulate chloroplast functions and sustain plant viability. Proc Natl Acad Sci USA 2016; 113:E3976-76; PMID:27335455; https://doi.org/ 10.1073/pnas.1604101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ojeda V, Pérez-Ruiz JM, González MC, Nájera VA, Sahrawy M, Serrato AJ, Geigenberger P, Cejudo FJ. NADPH thioredoxin reductase C and thioredoxins act concertedly in seedling development. Plant Physiol 2017; 174(3):1436-48; PMID:28500266; https://doi.org/ 10.1104/pp.17.00481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kircher S, Schopfer P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc Natl Acad Sci USA 2012; 109:11217-21; PMID:22733756; https://doi.org/ 10.1073/pnas.1203746109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Golczyk H, Greiner S, Wanner G, Weihe A, Bock R, Borner T, Herrmann RG. Chloroplast DNA in mature and senescing leaves: a reappraisal. Plant Cell 2014; 26:847-54; PMID:24668747; https://doi.org/ 10.1105/tpc.113.117465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin JR, Frost E, Vidi PA, Kessler F, Staehelin LA. Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 2006; 18:1693-03; PMID:16731586; https://doi.org/ 10.1105/tpc.105.039859 [DOI] [PMC free article] [PubMed] [Google Scholar]


