ABSTRACT
The exquisite regulation of cell division at the shoot apex according to the external environmental cues enables plants to adapt the ever-changing environment. We have recently shown that light direct signaling and carbohydrate (sugar) energy signaling are both essential for the activation of cell division at the shoot apex. Light is converted to auxin signal to activate small GTPase 2 (ROP2). Subsequently, the activated ROP2 directly interacts with Target of Rapamycin (TOR) protein kinase, a pivotal regulator of cell division, to promote its kinase activity. However, neither light nor auxin alone can activate TOR kinase without the presence of sugar. In this addendum, we showed that Constitutive Photomorphogenesis 1 (COP1) acts as an upstream factor of ROP2. COP1 regulates ROP2 and TOR activity in an auxin dependent manner. The development of true leaves in the cop1–6 mutant under darkness is compromised by auxin biosynthesis inhibitor yucasin and TOR chemical inhibitor torin2. Together, our results suggested that COP1 regulates auxin-ROP2-TOR signaling in response to light.
KEYWORDS: Cell division, COP1, ROP2, TOR
Cell division at the shoot apex underpins the above-ground architecture throughout the life of the plant. To adapt the ever-changing environment, plants need tightly regulated the cell division according to the environmental cues. For instance, light awaken the quiescence of cell division at shoot apexes after the seedlings break up the soil. One of the striking differences between dark- and light-grown seedlings is the emergence of true leaves in the light grown seedlings but not in dark-grown seedlings after seed germination. However, several constitutive photomorphogenesis (COP) mutants such as cop1 can even develop true leaves in darkness as grown in the light.1 COP1 functions as an E3 ubiquitin ligase, which facilitates the ubiquitylation and proteolysis of positive regulators of photomorphogenesis, such as transcription factors HY5, HYH, HFR1 and LAF1.2,3 Photoreceptors mediated light signals negatively regulate activity of COP1.4 The capacity of generating true leaves of COP1 mutants suggests that COP1 functions as a negative regulator of cell division.
In addition to its direct signals role received by photoreceptors, light also fuels the carbohydrate (sugar) production via photosynthesis, which underlines the complex roles of light in regulating cell division in shoot apexes, nevertheless, was largely debated and unclear before. Using mitotic quiescent seedlings caused by depletion of endogenous sugars, photoreceptors mutants and photosynthesis inhibitors, we recently distinguished the roles of light and sugar for the cell division at the shoot apex.5 We showed that both light and sugar signals are required to activate Target of Rapamycin (TOR), a conserved eukaryotic protein kinase that controls many processes including translation, transcription and cell division.5 Cell division at the shoot apex cannot proceed in the tor mutant or the seedlings treated with TOR chemical inhibitors rapamycin or torin2. The metabolism of sugar through glycolysis and oxidative-phosphorylation transmits the signal of sugar availability to TOR, though the detailed mechanism of glucose to activate TOR remains to be elucidated.6 Light, in the other tributary, is perceived to activate cell division through triggering accumulation of auxin in the shoot apex. Auxin then activates Rho-like small GTPase 2 (ROP2), which subsequently promotes the kinase activity of TOR. Consistently, the true leaves development of a constant active ROP2 mutant only requires glucose but no light, which suggests ROP2 specifically mediates light signal.5
However, the mechanism how light signal is transduced to auxin for ROP2 activation is still unknown. In this addendum, we investigated whether COP1 functions upstream of ROP2. The ROP2 activity in the cop1–6 is higher than in wild type grown in darkness (Fig 1A, B), indicating that COP1 suppresses ROP2 activity. COP1 may regulate ROP2 activity via affecting auxin level, as evident that the ROP2 activity in cop1–6 can be decreased to low level by auxin synthesis inhibitor, yucasin (Fig 1A, B).
Figure 1.
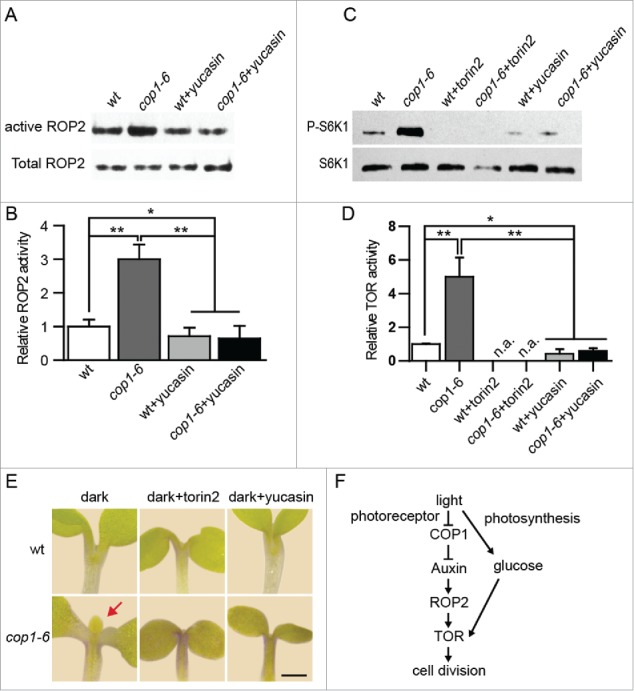
COP1 acts upstream of auxin-ROP2-TOR signaling pathway. ROP2 activity (A) and TOR activity (C) of dark grown wild type and cop1–6 seedlings treated with 25 μM torin2 or 100 μM yucasin. Quantification of ROP2 activity (B) and TOR activity (D) from 3 replicates. Error bar: SD, n.a.: not available, ** and * indicate significant difference, p<0.05 and p<0.01 respectively, ANOVA test, n = 3. (E)True leaves production in cop1–6. True leaves marked by red arrow. Typical images are presented, n>30. Bar: 1 mm. (F) The model for COP1 mediated light signaling.
Furthermore, we found increased TOR activity in the cop1–6 mutant than wild type (Fig. 1C, D). Therefore, COP1 functions as a negatively upstream regulator of the auxin-ROP2-TOR axis. The seedlings of cop1–6 developed true leaves in the darkness. However, the generation of true leaves is abolished by yucasin and torin2 (TOR inhibitor) treatment (Fig. 1E). This suggests that TOR kinase activity is required for the cell cycle activation in the dark- grown cop1-6. Taken together, our data showed that the light signaling integrator COP1 modifies auxin to regulate ROP2 and TOR in the processes of controlling true leaves development (Fig. 1F).
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This research is supported by the Horticultural Biology and Metabolomics Center, Fujian Agriculture and Forestry University (W.C.), by the Shanghai Innovation Program for Basic Research (14JC1406800, T.X.), by the Recruitment Program of Global Experts (People's Republic of China, T.X. and Y.X.), and funding from the Chinese Academy of Science (T.X. and Y.X.).
References
- 1.Hardtke CS, Deng X-W, The cell biology of the COP/DET/FUS proteins. Regulating proteolysis in photomorphogenesis and beyond? Plant Physiol. 2000;124:1548-57. doi: 10.1104/pp.124.4.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seo HS, Yang JY, Ishikawa M, Bolle C, Ballesteros ML, Chua NH. LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature. 2003;423:995-9. doi: 10.1038/nature01696. [DOI] [PubMed] [Google Scholar]
- 3.Yi C, Deng XW. COP1 – from plant photomorphogenesis to mammalian tumorigenesis. Trends Cell Biol. 2005;15:618-25. doi: 10.1016/j.tcb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Huang X, Ouyang X, Deng X-W. Beyond repression of photomorphogenesis: Role switching of COP/DET/FUS in light signaling. Curr Opin Plant Biol. 2014;21:96-103. doi: 10.1016/j.pbi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Cai W, Liu Y, Li H, Fu L, Liu Z, Xu L, Liu H, Xu T, Xiong Y, et al.. Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc Natl Acad Sci USA. 2017;114:2765-70. doi: 10.1073/pnas.1618782114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong Y, McCormack M, Li L, Hall Q, Xiang C, Sheen J. Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature. 2013;496:181-6. doi: 10.1038/nature12030. [DOI] [PMC free article] [PubMed] [Google Scholar]


