ABSTRACT
In our recent paper, we biochemically analyzed autophagosome-related membranes at the initiation stage of macroautophagy/autophagy using atg knockout (KO) cells and demonstrated that the ULK complex is recruited to 2 distinct membranes: the ER membrane and ATG9A-positive autophagosome precursors. We have also identified phosphatidylinositol synthase (PIS)-enriched ER subdomains as the initiation site of autophagosome formation. Based on these findings, we propose that the ULK complex, the PIS-enriched ER subdomain, and ATG9A vesicles together initiate autophagosome formation.
KEYWORDS: ATG9A, autophagy, phosphatidylinositol synthase, RB1CC1, ULK complex
After the discovery of ATG genes, the whole picture of autophagy is becoming clearer. Core Atg/ATG proteins are categorized into several functional units: the Atg1/ULK complex, Atg14-containing phosphatidylinositol (PtdIns) 3-kinase complex, Atg2-Atg18 complex, Atg9/ATG9A, and Atg12 and Atg8 conjugation systems. Among them, the Atg1/ULK complex and Atg9/ATG9A function at the initiation stage of autophagosome formation. The involvement of ER-related membranes has also been suggested; however, fundamental key questions about the initiation stage remain unanswered. For example, although electron microscopy has revealed close contacts between the phagophore and ER, the physiological role of the ER remains unclear. The relationship among the Atg1/ULK complex, ER membrane and Atg9/ATG9A vesicles is unknown. Furthermore, localization of the Atg1/ULK complex at the initiation stage remains obscure.
To answer these key questions, we biochemically isolated autophagosome-related membranes from various atg knockout (KO) cells, in which the ULK complexes are enriched. We found the following (Fig. 1):
-
•
The ULK complex component RB1CC1 associates with 2 distinct membranes: the ER membrane and ATG9A-enriched membrane.
-
•
Upon autophagy induction, the ULK complex is first recruited to an ER subdomain enriched in phosphatidylinositol synthase (PIS).
-
•
PtdIns in the PIS-enriched ER membrane is required for autophagosome formation.
-
•
The ULK complex translocates to the ATG9A-positive autophagosome precursors in a phosphatidylinositol-3-phosphate (PtdIns3P)-dependent manner.
Figure 1.
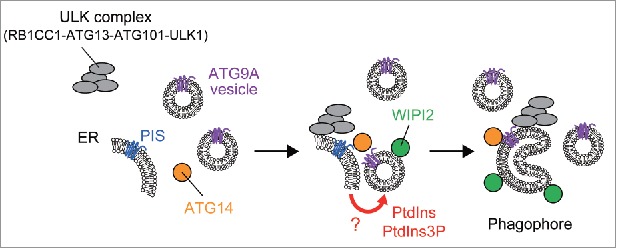
A model of autophagosome formation at the initiation stage. The ULK complex (RB1CC1-ATG13-ATG101-ULK1) translocates to PIS-enriched ER subdomains and induces recruitment of downstream factors such as ATG14. At least a part of the ATG9A population is incorporated into the phagophore membrane. The PIS-enriched ER membrane supplies PtdIns (and/or PtdIns3P) to ATG9A vesicles or a phagophore precursor, leading to elongation of the phagophore membrane, to which WIPI2 and other ATG proteins are recruited.
Our results suggest that autophagy initiation requires at least 2 distinct membranes: the ER membrane and ATG9A-enriched membrane. Considering that ATG9A is incorporated into autophagic membranes, it is possible that the ATG9A vesicle is a seed membrane to nucleate the phagophore. However, as the amount of ATG9A in the phagophore membrane is not abundant, ATG9A vesicles should not be the sole lipid/membrane source. Lipid/membrane sources from other compartments will be needed for ATG9A vesicles to transform into the phagophore. By contrast, the PIS-enriched ER subdomain seems to contribute to autophagy initiation in a more specific way. We identified the PIS-enriched ER subdomain as the initiation site of autophagy. As PtdIns is a substrate of PtdIns 3-kinase, the PIS-enriched ER subdomain will afford a desirable environment for PtdIns3P formation, a key step of autophagy induction. We speculate that the PIS-enriched ER subdomain contacts ATG9A vesicles and ensures expansion/progression of ATG9A vesicles to generate a phagophore precursor by suppling PtdIns and/or PtdIns3P (Fig. 1). It is not clear why the ULK complex needs to be recruited to both the ER membrane and the ATG9A-positive membrane. One possibility is that the ULK complex might facilitate reorganization of the ER membrane and induce the formation of ER-ATG9A membrane contacts.
In addition to PIS, our screen identified several other phospholipid biosynthetic enzymes accumulating with the ULK complex at the initiation stage. These results evoke an assumption that the ER membrane associated with ATG9A vesicles might be specialized for phospholipid biosynthesis. Accumulating evidence has shown that phospholipids are synthesized and transported at membrane contact sites between the ER and other organelles. Formation of the ER-ATG9A vesicle contact might facilitate transport of phospholipids between these 2 compartments.
The relationship between the PIS-enriched domain and the omegasome is also interesting. The omegasome has been well characterized as a PtdIns3P-positive subdomain, which serves as a cradle for the phagophore, whereas the PIS-enriched domain is supposed to be enriched with PtdIns. Thus, there should be some crosstalk between these 2 compartments.
New insights in this study have raised the following questions about the initiation stage:
-
1.
How are ER-ATG9A membrane contacts formed?
-
2.
How are lipids supplied to the ATG9A-positive autophagosome precursor from the ER membrane?
-
3.
How is the destination of the ULK complex determined?
-
4.
What is the relationship between the PIS-enriched ER subdomain and the omegasome?
Further experiments are required to uncover the exact mechanisms of autophagy initiation.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by JSPS KAKENHI Grant-in-Aid for Young Scientists (A) (Grant Number 26711011) (to T.N.); Grant-in-Aid for Scientific Research on Innovative Areas (Grant Number 25111001 and 25111005) (to N.M.); the Naito Foundation (to T.N.); the Japan Foundation for Applied Enzymology (to T.N.).


