Abstract
Darier disease is a rare and severe autosomal dominant skin disease characterised by malodorous keratotic papules in seborrheic areas of the skin. Darier disease affects up to 1 in 30 000 people and is caused by mutations in the ATP2A2 gene, which encodes to the sarco/endoplasmic reticulum calcium-ATPase isoform 2 that pumps calcium into the endoplasmic reticulum. Although many ATP2A2 variants have been described, it is not known if genotype correlates with phenotype, which could be important for prognosis and treatment. This is the first study to use whole exome sequencing to screen the ATP2A2 gene in a cohort of 28 clinically diagnosed Darier disease patients. Twenty-one different disease causing variants were identified and 15 of these were novel. Sixteen of the 21 variants were predicted to be pathogenic using in silico prediction programs. There were seven missense, four intronic/splice-sites, three frameshifts, two in-frame deletions, four nonsense and one synonymous mutations. This study also found ten patients who harbour more than one ATP2A2 variant. The phenotype of the patient cohort was assessed by photography and by patient questionnaires. The genotype-phenotype association was examined for all variants in relation to the patient’s disease severity score, and no correlation could be established.
Introduction
Darier disease (DD), also known as keratosis follicularis, is a rare and severe autosomal dominant skin disease, estimated to affect 1 in 30 000 to 100 000 people [1, 2]. The disease is characterized by keratotic papules and malodorous plaques in seborrheic areas of the skin, which can lead to large, crusted plaques. The disease often emerges during childhood and continues throughout adolescence, and can negatively influence the quality of life [2]. Disease phenotype is highly variable; even when two family members are affected by the same variant, the clinical symptoms may be different [3], which makes genotype-phenotype correlations difficult to determine. UVB irradiation, heat, friction and infections in affected areas can further exacerbate symptoms [4]. Other non-dermatological symptoms of DD include psychiatric conditions, such as intellectual disability and bipolar disease [2, 5].
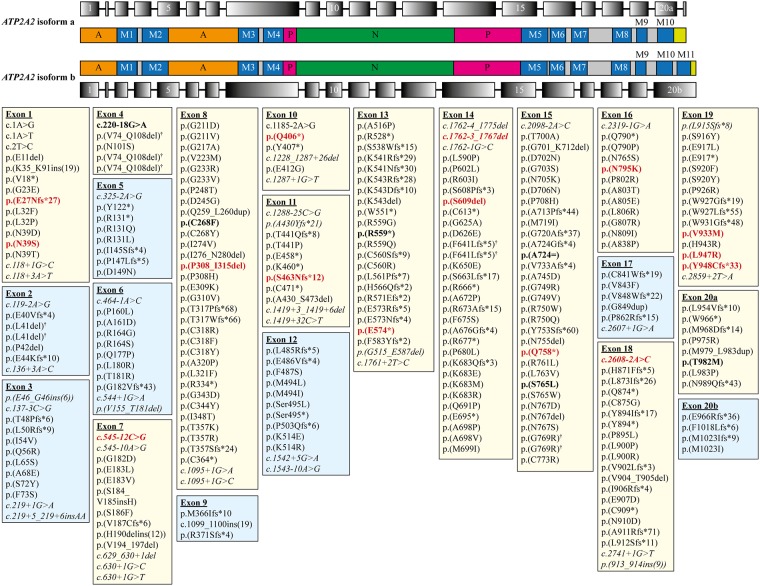
DD is caused by mutations in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium (Ca2+) -ATPase isoform 2 (SERCA2), a calcium pump in the endoplasmic reticulum (ER) [6]. The ATP2A2 gene transcript has two isoforms (isoform a and b), and consists of 21 or 20 exons, respectively (Fig 1). Both SERCA2 isoforms have a phosphorylation (P), nucleotide-binding domain (N) and the actuator domain (A; Fig 1).
Fig 1. The location of all published/reported ATP2A2 variants (in both isoforms) and variants discovered in the current study associated with Darier disease.
ATP2A2 isoform a consists of 21 exons (top row), which encodes for 10 transmembrane domains (M1 –M10, second row), and it is expressed in cardiomyocytes and slow-tiwtch skeletal muscles. The ATP2A2 isoform b consists of 20 exons (fourth row), which encodes for 11 transmembrane domains (M1 –M11, third row) and it is expressed in most tissue types [3]. Both isoforms have an actuator domain (A, orange boxes), phosphorylation domain (P, pink boxes), nucleotide binding domain (N, green boxes) and C-terminus (lime green boxes). All genetic variants are listed in either pale yellow (exon with variants discovered in this study) or blue boxes (exon with no variants from this study). Novel genetic variants found in this study are in bold and coloured red. Variants that were discovered in this study but were not novel are in bold only. Intronic variants are in italics. † indicates ATP2A2 variants with different genetic changes, but resulted in the same amino acid change.
Currently, there are over 270 unique ATP2A2 mutations associated with DD [7, 8], and there are no mutational hotspots. The genetic mutations are predominantly missense/nonsense mutations, followed by deletions, splice-site and insertion mutations. Studies have shown that mutations in the ATP2A2 gene disrupt normal SERCA2 protein function and leads to impaired/loss of Ca2+ transport in the ER, which leads to decreased ER Ca2+ stores [9–14]. Ca2+ imbalance affects cell-to-cell adhesion in skin and this could explain the DD skin phenotype [15].
To date, there has been no successful genotype-phenotype correlation established in DD. However, this could be important for the diagnosis of disease severity prediction and prognosis of patients. While an epidemiological study of intellectual disability and cognitive ability in Swedish DD patients have been conducted [16], the genetic aspect and the genotype-phenotype association have not been investigated in Sweden. In the current study, the coding sequence of ATP2A2 of a cohort of 28 clinically diagnosed Swedish DD patients was screened using whole exome sequencing (WES). Patients were asked to score their disease severity and treatment efficacy. The genotype-phenotype association was also examined for all variants in relation to the patient’s disease severity score.
Methods
Patient recruitment
Twenty-eight patients with clinically diagnosed DD were recruited from the Dermato-Venereology clinic of Karolinska University Hospital and other Dermato-Venereology clinics in Sweden, including Uppsala, Linköping and Uddevalla. All patients were Caucasian.
Ethical disclosure
All patients (and/or patients’ guardians) gave written informed consent for genetic testing.
This study was approved by the Stockholm regional ethical board (2017/1098-32).
Patient questionnaire and determination of total body surface area
A questionnaire based on the study conducted by Burge and Wilkinson, (1992) [2] was issued to the patient cohort. The questions included the age of disease onset, the type of medication currently taken, the medication effect (ranked 1–5), disease severity (mild/moderate/severe and ranked from 1–5), general skin symptoms, quality of life, and factors that worsen disease (heat/friction/menstruation/pregnancy/stress/sun). The scores from 1–5 correspond to the following: 1, bad; 2, acceptable; 3, good; 4, very good; and 5, excellent [17].
The total body surface area (TBSA) affected by disease was assessed for each patient using photographs taken at the time when blood was drawn for WES. The TBSA was based on the rule of nines, which is a method used to quantify the area of affected skin in burns victims [18]. The assessment was blinded in that the genotype was unknown to the examiner.
Whole exome sequencing and bioinformatics analysis
The Centre of Genomics and Transcriptomics (CeGAT, Tubingen, Germany) performed the WES. The exome was enriched by the Agilent SureSelectXT Human All Exon V6 (Agilent, CA, USA). The enriched libraries were sequenced using the Illumina HiSeq (Illumina, CA, USA) high-throughput sequencing platform with a read-length of 2 x 100 bp. Raw sequencing reads were processed according to the CeGAT bioinformatics streamlined process to generate a list of ATP2A2 gene variants.
Bidirectional Sanger-based sequencing confirmation
All reported ATP2A2 variants were confirmed by bi-directional Sanger-based sequencing. All ATP2A2 PCR primers were previously published by Ringpfeil et al., (2001) [19], with the addition of M13 forward or reverse sequences on the 5’ end. PCR was performed as previously described [20]. PCR products were electrophoresed in 1% UltraPure™ Agarose (Thermo Fisher Scientific, MA, USA) with GelRed™ (Biotium, CA, USA) incorporated into the gel.
Subsequent to gel electrophoresis confirmation, the PCR products were submitted to KI Gene (Centre for Molecular Medicine, Karolinska University Hospital Solna, Sweden) for bi-directional Sanger-based sequencing. The sequence analysis was performed using Geneious software (version 7.1.9) [21].
ATP2A2 variant analysis
The pathogenicity of missense and intronic/splice-site variants were assessed using in silico prediction programs. Missense variants were assessed with PolyPhen-2 [22], SIFT [23] and SNPs&GO [24], and the splice-site variants were assessed with ASSP [25] and HSF [26]. A mutation is categorised as benign or pathogenic when the majority of scores from the prediction programs are concordant [27]. For nonsense and frameshift mutations, it was assumed that the variants would be deleterious to correct protein function. The variants were not categorised solely based on the predicted outcomes; dbSNP database (build 149) [28], Exome Aggregation Consortium (ExAC) database [29], and Exome Sequencing Project (ESP) [30] database were also used to determine if variants were rare polymorphisms.
Results
Twenty-eight patients (19 females and nine males) with clinically diagnosed DD were screened for ATP2A2 variants. Nine were family members from four different families (Table 1). Fourteen of the 28 patients have a family history of DD, nine could be sporadic cases and two patients were adopted (Table 1). Twenty-one different variants were found, and 15 were novel variants that have not been previously reported in the literature or any databases (i.e. dbSNP [28] or LOVD database [7, 8]; Table 1 and Fig 1).
Table 1. List of ATP2A2 mutations found in DD patients described in this study.
| ID | Age of testing | Age of onset | Sex | Family history of disease | Nucleotide change | Amino acid change | Zygosity | Exon | Domain | Mutation type | dbSNP | ExAC/ESP | Predicted outcome | Reported |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | F | ||||||||||||
| 2 | 27 | 11 | M | Yes | c.2840T>G | p.L947R | Het | 19 | M 9 | Missense | Pathogenic | |||
| 3 | 50 | 19 | F | No | ||||||||||
| 4 | 51 | 14 | M | No | c.1762-3_1767del | Het | 14 | N | Essential splice-site | Pathogenic | ||||
| 5* | 47 | 19 | M | No | c.2172G>A | p.A724= | Het | 15 | P | Synonymous | rs56243033 | 0.055/0.05 | Benign | [31] |
| 6 | 56 | 3.5 | M | Yes | c.2840T>G | p.L947R | Het | 19 | M 9 | Missense | Pathogenic | |||
| 7† | 68 | 20 | F | Yes | c.1675C>T | p.R559* | Het | 13 | N | Nonsense | Pathogenic | |||
| 8 | 67 | 64 | F | Adopted | c.220-18G>A | Het | i3 | M 1 | Intronic | rs35235621 | 0.015/0.018 | Benign | ||
| c.2843_2844delAT | p.Y948Cfs*33 | Het | 19 | M 9 | Frameshift | Pathogenic | ||||||||
| 9† | 30 | M | c.1675C>T | p.R559* | Het | 13 | N | Nonsense | Pathogenic | |||||
| c.2172G>A | p.A724= | Het | 15 | P | Synonymous | rs56243033 | 0.055/0.05 | Benign | [31] | |||||
| 10 | 44 | 14 | F | Adopted | c.1216C>T | p.Q406* | Het | 10 | N | Nonsense | Pathogenic | |||
| c.2172G>A | p.A724= | Het | 15 | P | Synonymous | rs56243033 | 0.055/0.05 | Benign | [31] | |||||
| 11 | 60 | 12 | F | Yes | c.2608-2A>C | Het | i17 | Essential splice-site | Pathogenic | |||||
| 12 | 64 | F | Yes | c.2608-2A>C | Het | i17 | Essential splice-site | Pathogenic | ||||||
| 13 | 78 | 15 | F | Yes | c.803G>T | p.C268F | Het | 8 | M 3 | Missense | rs121912733 | Pathogenic | [3] | |
| c.1386_1390delTTCTA | p.S463Nfs*12 | Het | 11 | N | Frameshift | Pathogenic | ||||||||
| 14 | 67 | 16 | M | Yes | c.922_945del | p.P308_I315del | Het | 8 | M 4 | Deletion (in-frame) | Likely pathogenic | |||
| 15** | 65 | 22.5 | F | Yes | c.79delG | p.E27Nfs*27 | Het | 1 | A | Frameshift | Pathogenic | |||
| 16** | 44 | 14 | F | Yes | c.79delG | p.E27Nfs*27 | Het | 1 | A | Frameshift | Pathogenic | |||
| 17 | 60 | 20 | F | No | c.2945C>T | p.T982M | Het | 20 | M 10 | Missense | rs149024535 | 0.0017/0.0003 | Benign | [32] |
| 18 | 81 | Unsure | F | No | c.2294C>T | p.S765L | Het | 15 | M 5 | Missense | Pathogenic | |||
| 19 | 61 | 16 | F | No | c.220-18G>A | Het | i3 | M 1 | Intronic | rs35235621 | 0.015/0.018 | Benign | ||
| c.1825_1827delTCC | p.S609del | Het | 14 | P | Deletion (in-frame) | rs767885880 | 0.000008 | Likely pathogenic | ||||||
| 20 | 49 | 13 | F | No | c.545-12C>G | Het | i6 | A | Intronic | Benign | ||||
| c.2797G>A | p.V933M | Het | 19 | M 9 | Missense | rs372102705 | 0.0001/0.00008 | Benign | ||||||
| 21 | 55 | 14 | F | Yes | c.2385T>G | p.N795K | Het | 16 | M 6 | Missense | Pathogenic | |||
| 22* | 48 | 20 | F | Yes | c.1720G>T | p.E574* | Het | 13 | N | Nonsense | Pathogenic | |||
| c.2172G>A | p.A724= | Het | 15 | P | Synonymous | rs56243033 | 0.055/0.05 | Benign | [31] | |||||
| 23* | 79 | 23 | F | Yes | c.1720G>T | p.E574* | Het | 13 | N | Nonsense | Pathogenic | |||
| c.2172G>A | p.A724= | Hom | 15 | P | Synonymous | rs56243033 | 0.055/0.05 | Benign | [31] | |||||
| 24 | 33 | 22 | F | Yes | c.116A>G | p.N39S | Het | 1 | A | Missense | Pathogenic | |||
| c.220-18G>A | Het | i3 | M 1 | Intronic | rs35235621 | 0.015/0.018 | Benign | |||||||
| 25 | 56 | 15 | M | No | ||||||||||
| 26†† | 66 | 16 | F | Yes | c.2272C>T | p.Q758* | Het | 15 | M 5 | Nonsense | Pathogenic | |||
| 27†† | 32 | M | c.2272C>T | p.Q758* | Het | 15 | M 5 | Nonsense | Pathogenic | |||||
| c.2172G>A | p.A724= | Het | 15 | P | Synonymous | rs56243033 | 0.055 | Benign | [31] | |||||
| 28 | 61 | 20 | M | No |
*, †, **, †† represent patients that are related. Rows that are blank are patients who did not harbour an ATP2A2 variant. F, female; M, male; Het, heterozygous; Hom, homozygous; ix, intron x; Mx, transmembrane domain x; A, actuator domain; P, phosphorylation domain; N, nucleotide binding domain.
Four patients (patients 1, 3, 25 and 28) did not have any variants in the ATP2A2 gene despite clinically diagnosed with DD, and two patients had benign ATP2A2 variants (patient 5 and 17, Table 1). Ten patients had two different ATP2A2 variants, and for most of these patients, one variant was benign and the other predicted to be pathogenic (Table 1). One patient had two benign variants (Patient 20), and one patient had two pathogenic variants (Patient 13; Table 1). The read-depth of each of the ATP2A2 exons for each patient sample is shown in S1 Fig.
Spectrum of variants
Sixteen of 21 variants were predicted to be pathogenic (Table 1 and S1 and S2 Tables). There are five missense mutations; two in-frame deletions; two essential splice-site mutations; three frameshift mutations and four nonsense mutations (Table 1). The benign variants consisted of two intronic mutations, two missense mutations, and one synonymous mutation (Table 1). All variants are scattered throughout ATP2A2 and these are shown in Fig 1 in relation to all previously reported ATP2A2 variants [7, 8]. Eleven variants are located in the transmembrane domains (M1, M3, M4, M5–7, M9 and M10), three in the actuator (A) domain, five in the nucleotide binding (N) domain, and two in the phosphorylation (P) domain (Table 1).
Phenotypic analysis
To investigate whether there is a genotype-phenotype correlation, patients were asked a series of questions regarding their disease state. Twenty-five of the 28 patients (89%) responded, 18 women and seven men. Four of these patients did not harbour an ATP2A2 genetic variant.
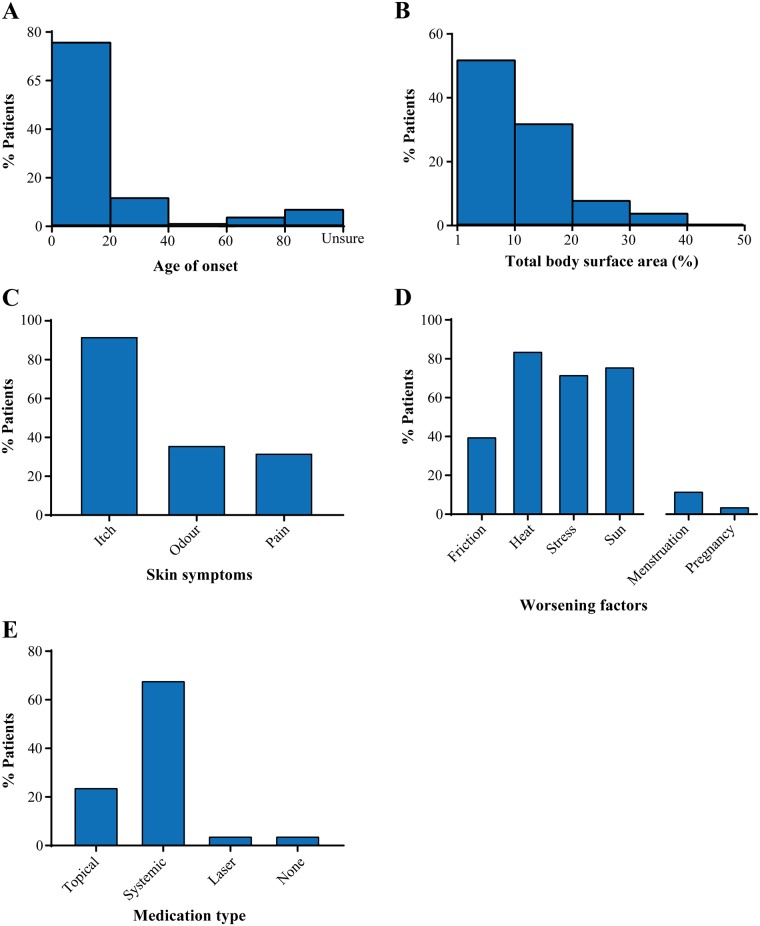
The age of onset for the majority of patients was below 20 years of age (76%; Fig 2A). The total body surface area (TBSA) affected by disease was also variable, with the majority of patients having disease on 1–20% of their bodies (84%; Fig 2B). The most commonly affected skin areas were the skin folds (56%) and trunk of the body (52%; Table 2). Itchy skin (92%), heat, sun and stress (72–84%) were the most common exacerbating factor for many patients (Fig 2C and 2D). More than half of the patients who responded to the questionnaire (60%) were negatively affected by DD and found that it affected their life quality (Table 2). Most patients were treated systemically with either acitretin or isotretionin (68%), and some were treated topically (24%; Fig 2E).
Fig 2. Clinical characteristics of the cohort.
Age of onset (A), total body surface area affected (B), skin symptoms (C), worsening factors (D) and medication type (E) in the patient cohort.
Table 2. Patient clinical characteristics.
| ID | Sex | Nucleotide change | Type of medication | TBSA (%) | Affected skin areas | Reduction in quality of life? | General skin symptoms | Factors that worsens disease |
|---|---|---|---|---|---|---|---|---|
| 1 | F | |||||||
| 2 | M | c.2840T>G | Moisturizer | 18 | Face, scalp, trunk, only mildly in skin folds, mild elsewhere | Yes | Itch | Friction, heat, friction, stress, sun |
| 3 | F | Topical | Back, shoulders, trunk | No | Itch | Heat, sun | ||
| 4 | M | c.1762-3_1767del | Acitretin | 4 | Face, scalp, trunk, only mildly in skin folds | No | Itch | Heat, stress, sun |
| 5* | M | c.2172G>A | Topical | 1 | Trunk, shoulders, back | No | Itch | Heat, stress, sun |
| 6 | M | c.2840T>G | Acitretin | 10 | Back, shoulders, trunk | No | Itch | Stress |
| 7† | F | c.1675C>T | Acitretin | 1 | Small defined area only on one side | No | Itch | Heat, sun |
| 8 | F | c.220-18G>A | Acitretin | 5 | Trunk | No | NA | Sun |
| c.2843_2844delAT | ||||||||
| 9† | M | c.1675C>T | ||||||
| c.2172G>A | ||||||||
| 10 | F | c.1216C>T | Isotretinoin | 6 | Trunk | Yes | Itch | Heat, sun |
| c.2172G>A | ||||||||
| 11 | F | c.2608-2A>C | Acitretin | 17 | Hands, feet | Yes | Itch, pain | Friction, heat, stress, sun |
| 12 | F | c.2608-2A>C | Acitretin | 12 | Face, folds, trunk | Yes | Itch | Friction, heat, stress, sun |
| 13 | F | c.803G>T | Acitretin | 18 | Face, hands, scalp | Yes | Odour, pain | Friction, heat, menstruation, pregnancy, stress, sun |
| c.1386_1390delTTCTA | ||||||||
| 14 | M | c.922_945del | Acitretin | 14 | Folds, only mild elsewhere | No | Itch | Heat, stress, sun |
| 15** | F | c.79delG | None | 2 | Folds, only mild elsewhere | Yes | Itch, odour, pain | Friction, heat, stress, sun |
| 16** | F | c.79delG | Acitretin | 12 | Entire body | Yes | Itch, odour, pain | Friction, heat, stress |
| 17 | F | c.2945C>T | Laser | 1 | Face, scalp, trunk, only mildly in skin folds | Yes | Itch, odour | Heat |
| 18 | F | c.2294C>T | Acitretin | 12 | Lower back, knee folds, waist | No | Itch | Heat |
| 19 | F | c.220-18G>A | Acitretin | 6 | Back, hands, feet, thighs, trunk | Yes | Itch, odour, pain | Heat, stress, sun |
| c.1825_1827delTCC | ||||||||
| 20 | F | c.545-12C>G | Acitretin | 10 | Face, scalp, trunk, only mildly in skin folds | Yes | Itch, pain | Friction, heat, menstruation, stress |
| c.2797G>A | ||||||||
| 21 | F | c.2385T>G | Acitretin | 22 | Entire body, also pharynx | Yes | Itch, odour, pain | Friction, heat, stress, sun |
| 22* | F | c.1720G>T | Isotretinoin | 20 | Folds, mild elsewhere | No | Itch | Heat, stress, sun |
| c.2172G>A | ||||||||
| 23* | F | c.1720G>T | Isotretinoin | 25 | Trunk | No | Itch | Heat, stress, sun |
| c.2172G>A | ||||||||
| 24 | F | c.116A>G | Topical | 1 | Face, genital area, hands | Yes | Itch, odour, pain | Menstruation, stress |
| c.220-18G>A | ||||||||
| 25 | M | Acitretin | 38 | Face, scalp, trunk, only mildly in skin folds | Yes | Itch | Friction, stress, sun | |
| 26†† | F | c.2272C>T | Topical | 2 | Trunk, only mildly in skin folds, mild elsewhere | Yes | Itch, odour | Heat, sun |
| 27†† | M | c.2272C>T | ||||||
| c.2172G>A | ||||||||
| 28 | M | Topical | 10 | Scalp, trunk, only mildly in skin folds | Yes | Itch, pain | Friction, heat, friction, stress |
*, †, **, †† represent patients that are related. Rows that are blank are patients who did not harbour an ATP2A2 variant or they did not answer the questionnaire. TBSA, total body surface area; F, female; M, male; Het, heterozygous; Hom, homozygous; ix, intron x; Mx, transmembrane domain x; A, actuator domain; P, phosphorylation domain; N, nucleotide binding domain.
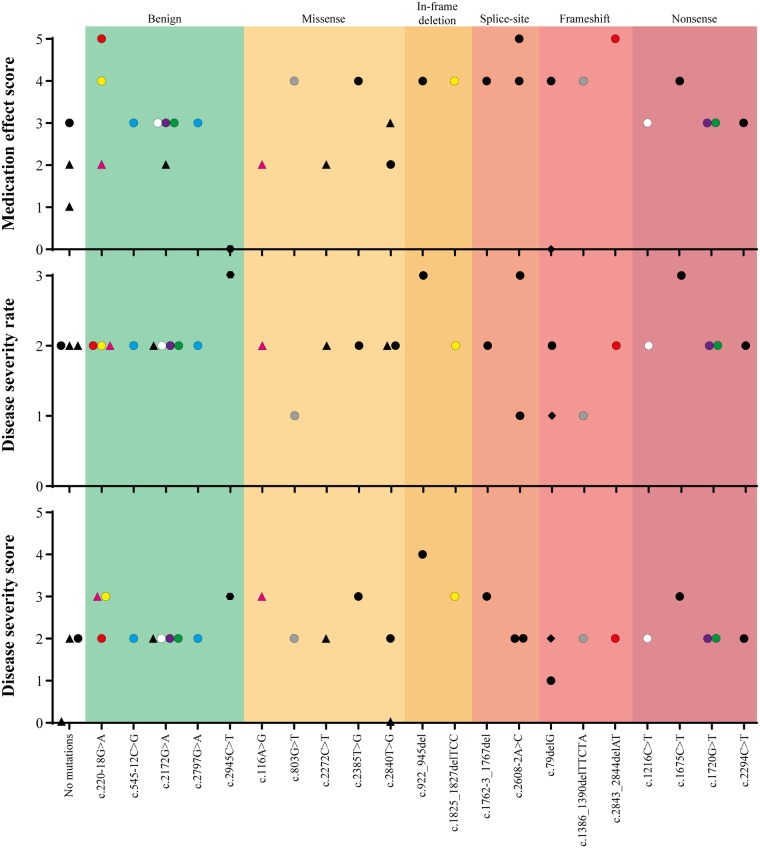
Patients were asked to score the medication effect and disease severity (Fig 3 and S2 Fig). Disease severity was scored in two different ways: whether disease was mild, moderate or severe; and ranked from 1–5 (1 corresponding to bad and 5 to excellent). Both medication effect and disease severity were analysed based on the ATP2A2 variant and the protein domain location (S2 Fig), and the type of mutation (i.e. missense, frameshift, etc.; Fig 3).
Fig 3. Medication effect (1–5), disease severity (mild–severe) and disease severity (1–5) scores sorted by mutation type.
Medication effect and disease severity score (Top and bottom), a score of 1 = bad, 2 = acceptable, 3 = good, 4 = very good or 5 = excellent. Disease severity rate (Middle), a score of 1 = severe, 2 = moderate and 3 = mild. A score of 0 corresponds to patient’s lack of answer to a particular category. The type of ATP2A2 mutation is shown on the x-axis, and they are sorted into seven categories: No mutations, benign, missense, in-frame deletion, splice-site, frameshift and nonsense mutations. ● represent patients receiving systemic treatment; ▲represents patients receiving topical treatment; ◆ represent patients receiving no treatment and  represent patients who have received laser treatment. The different coloured points represent the patients with two ATP2A2 variants. Points with the same colours are variants in the same patient.
represent patients who have received laser treatment. The different coloured points represent the patients with two ATP2A2 variants. Points with the same colours are variants in the same patient.
Patients treated systemically were more satisfied with medication effect (good to excellent scores) than topically treated patients, who scored their medication effect as bad to good (Fig 3 and S2 Fig–top graphs). Genotype-negative patients scored their medication effects as bad to good, while benign variants scored medication effect as generally good (Fig 3, top graph). Medication effect for patients harbouring missense mutations were acceptable to very good regardless of treatment type (Fig 3, top graph). All patients with in-frame deletion, splice site, frameshift and nonsense mutations were systemically treated and ranked the medication effect at good to excellent (Fig 3, top graph).
The disease severity of genotype-negative patients or those with benign ATP2A2 variants were rated as moderate (Fig 3, middle graph). Many patients scored disease severity at 2 (using the 1–5 scoring system), which equated to acceptable (Fig 3, bottom graph). Missense and in-frame deletion mutations caused mild to moderate disease, which is similar to the scores of 2 to 3 (acceptable to good) in disease severity (Fig 3, middle and bottom graphs). Splice-site and frameshift mutations scores were contradictory between the two different ranking systems. The scores varied widely for patients with either type of mutations (mild to severe); however, the corresponding disease severity scores ranged from 1 to 3 (bad to good).
Discussion
To date, no study has successfully correlated genotype to phenotype in DD. In the current study, we screened a Swedish cohort of 28 clinically diagnosed DD patients using WES and found 15 novel variants that have not been reported previously. We estimate that this cohort comprises more than 10% of DD patients in Sweden. Genotype-phenotype correlation was investigated based on disease severity scores and failed to show mutations with specific clinical picture.
Genotypic results
Twenty-one ATP2A2 variants were identified in this study, 15 are novel variants that have not been previously reported. Sixteen of these variants are predicted to be pathogenic by in silico prediction programs. Despite the lack of functional studies investigating these variants, previous studies of different ATP2A2 mutants (missense, nonsense and deletion mutations) showed that they all affect SERCA2 function by either decreasing protein expression, Ca2+-ATPase activity, Ca2+ transport or alter protein kinetic properties [9–14]. There is also evidence that mutant SERCA2 protein is an initiator of ER stress that causes human epidermal keratinocytes to round up, detach and induce apoptosis [10]. From these previous studies, it could be concluded that the 16 pathogenic variants reported in this study are likely to affect SRECA2 protein function. The variants are also associated with clinical data.
There were ten patients who had two ATP2A2 variants (one double pathogenic and one double benign variants). To the authors’ best knowledge there has been no reported cases of patients harbouring two ATP2A2 variants. Therefore, it is unknown whether the patient with two pathogenic variants have an additive effect on disease severity.
The four patients (14%) who are genotype-negative, but show skin symptoms could be wrongly diagnosed for DD. The number of genotype-negative patients in this study is similar to other reports in literature, which have a range of 7–33% of patients who are genotype-negative [8, 33–35]. Three of the four patients have rated their disease as moderately severe. The fact that no ATP2A2 variants were detected by WES does not necessarily mean that there is no mutation in the gene. There could be large deletions/insertions or cryptic splice-sites present that could not be detected using WES and require other methods (i.e. comparative genomic hybridisation microarray–CGH arrays, or multiplex ligation-dependent probe amplification–MLPA) to detect the copy number changes. There is also the possibility that some variants are in the unscreened regions of the ATP2A2 gene, such as the promoter regions, intronic regions or the 3’ untranslated region, which could affect expression/or function of the SERCA2 protein.
Phenotypic/clinical results
The clinical features/symptoms of the current cohort are similar to previously described DD patients [2, 36], including age of onset, affected skin areas, skin symptoms and factors that exacerbate disease. The patients in this study are treated with either oral retinoids (acitretin or isotretinoin) or topical treatments, which are also typical for other DD patients [36]. Many patients have ranked the systemic treatment as effective against DD symptoms; however, the treatment is often not well tolerated and cannot be taken long term [2]. Like other reported DD cases, disease severity is varied [3, 19, 32, 35, 37–39]. Four patients in the current cohort ranked their disease as severe (16%), 18 as moderate (72%) and three as mild (12%, based on the disease severity rating results).
Genotype-phenotype correlation
Similar to previous reports, establishing a cross-sectional genotype-phenotype correlation for DD was difficult [3, 19, 32, 35, 37–39]. Previous reports have shown that family members harbouring the same variant manifest different phenotypes [3], which was seen in the current study. Even when the ATP2A2 variants were separated into protein region and mutation type (i.e. benign, missense, etc.), it was still difficult to find a correlation between genotype and phenotype. One possible reason could be that for a majority of ATP2A2 variants, SERCA2 protein function is affected independent of the type or location of the mutation [9–14]. It could also be due to the variability of disease symptoms over time, which is influenced by environmental factors.
It is interesting to note that patients with only benign variants have moderate DD symptoms. Whether these variants have an effect on SERCA2 protein function is unknown, as none have been characterised. It is also possible that these patients could be DD phenocopies (non-genetic forms of the disease); however, Berg and Basset (1993) suggest the likelihood of this is rare [40]. This is because DD is a rare disease that is clearly inherited in an autosomal dominant manner [40]. There is also the possibility that the patients could harbour a second undetected ATP2A2 variant (i.e. large deletion/insertion).
Limitations
Currently, DD is diagnosed by its appearance and histopathology. There are some dermatological diseases that may also resemble DD. We believe that this “crude” non-molecular diagnostic method will be supported with genetic testing in the future to further confirm the clinical diagnosis. As well as analytical limitations that may not be detecting all DD variants, patients may be wrongly diagnosed with DD as their symptoms resemble DD, which confounds result interpretation. Other limitations of the study is that patients determined the medication efficacy and disease severity, and there will be variability in how these two factors were determined between patients. As the study only examined cross-sectional disease severity a longer and larger multicentre prospective study to investigate disease severity may be a better method of determining this factor.
Conclusion
Despite being unable to establish a genotype-phenotype correlation as the disease severity is variable amongst patients, we identified 15 novel variants that have not been previously reported in DD. This study is the first to use WES to screen DD patients, and it is the first to investigate the genetic aspect in Swedish DD patients.
Supporting information
An x represents each patient sample and yellow circles represent patients who are genotype-negative. The red bars show the median and 95% confidence intervals.
(TIF)
Medication effect and disease severity score (Top and bottom), a score of 1 = bad, 2 = acceptable, 3 = good, 4 = very good or 5 = excellent. Disease severity rate (Middle), a score of 1 = severe, 2 = moderate and 3 = mild. A score of 0 corresponds to patient’s lack of answer to a particular category. The type of ATP2A2 mutation is shown on the x-axis, and they are sorted into protein domains. ● represent patients receiving systemic treatment; ▲ represents patients receiving topical treatment; ◆ represent patients receiving no treatment and  represent patients who have received laser treatment.
represent patients who have received laser treatment.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank the families for kindly consenting to be part of this study and permitting this report. Further, we thank clinical research nurses Helena Griehsel, Papely Kassiri and Kristina Skoglund for excellent assistance. Finally, we express our gratitude to Prof. Mona Ståhle for senior mentorship.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financially supported by the Swedish Research Council, Swedish Society for Medical Research (SSMF), Olle Engkvist stiftelse, Hudfonden, Tore Nilssons stiftelse, Jeanssons stiftelse, Lars Hiertas stiftelse and OE Ostermanns stiftelse to Jakob D Wikstrom. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Tavadia S, Mortimer E, Munro CS. Genetic epidemiology of Darier’s disease: A population study in the west of Scotland. British Journal of Dermatology. 2002;146:107–109. [DOI] [PubMed] [Google Scholar]
- 2.Burge SM, Wilkinson JD. Darier-White disease: A review of the clinical features in 163 patients. Journal of the American Academy of Dermatology. 1992;27:40–50. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Perez VL, Carter SA, Healy E, Todd C, Rees JL, Steijlen PM, et al. ATP2A2 mutations in Darier’s and disease: Variant and cutaneous phenotypes are associated with missense and mutations and but neuropsychiatric features are and independent of mutation class. Human Molecular Genetics. 1999;8:1621–1630. [DOI] [PubMed] [Google Scholar]
- 4.Foggia L, Hovnanian A. Calcium pump disorders of the skin. American Journal of Medical Genetics Part C, Seminars in Medical Genetics. 2004;131C:20–31. doi: 10.1002/ajmg.c.30031 [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen NJ, Lyons I, Hoogendoorn B, Burge S, Kwok PY, O’Donovan MC, et al. ATP2A2 mutations in Darier’s disease and their relationship to neuropsychiatric phenotypes. Human Molecular Genetics. 1999;8:1631–1636. [DOI] [PubMed] [Google Scholar]
- 6.Sakuntabhai A, Ruiz-Perez V, Carter S, Jacobsen N, Burge S, Monk S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nature Genetics. 1999;21:271–277. doi: 10.1038/6784 [DOI] [PubMed] [Google Scholar]
- 7.Fokkema IF, Taschner PE, Schaafsma GC, Celli J, Laros JF, den Dunnen JT. LOVD v.2.0: The next generation in gene variant databases. Human Mutation. 2011;32:557–563. doi: 10.1002/humu.21438 [DOI] [PubMed] [Google Scholar]
- 8.Nellen RG, Steijlen PM, van Steensel MA, Vreeburg M, Frank J, van Geel M. Mendelian disorders of cornification caused by defects in intracellular calcium pumps: Mutation update and database for variants in ATP2A2 and ATP2C1 associated with Darier disease and Hailey-Hailey disease. Human Mutation. 2017;38:343–356. doi: 10.1002/humu.23164 [DOI] [PubMed] [Google Scholar]
- 9.Ahn W, Lee MG, Kim KH, Muallem S. Multiple effects of SERCA2b mutations associated with Darier’s disease. Journal of Biological Chemistry. 2003;278:20795–20801. doi: 10.1074/jbc.M301638200 [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Bruce AT, Tu C, Ma K, Zeng L, Zheng P, et al. Protein aggregation of SERCA2 mutants associated with Darier disease elicits ER stress and apoptosis in keratinocytes. Journal of Cell Science. 2011;124:3568–3580. doi: 10.1242/jcs.084053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dode L, Andersen JP, Leslie N, Dhitavat J, Vilsen B, Hovnanian A. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. Journal of Biological Chemistry. 2003;278:47877–47889. doi: 10.1074/jbc.M306784200 [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Yamasaki K, Daiho T, Miyauchi Y, Takahashi H, Ishida-Yamamoto A, et al. Distinct types of abnormality in kinetic properties of three Darier disease-causing sarco(endo)plasmic reticulum Ca2+-ATPase mutants that exhibit normal expression and high Ca2+ transport activity. Journal of Biological Chemistry. 2004;279:35595–3560. doi: 10.1074/jbc.M404887200 [DOI] [PubMed] [Google Scholar]
- 13.Miyauchi Y, Daiho T, Yamasaki K, Takahashi H, Ishida-Yamamoto A, Danko S, et al. Comprehensive analysis of expression and function of 51 sarco(endo)plasmic reticulum Ca2+-ATPase mutants associated with Darier disease. Journal of Biological Chemistry. 2006;281:2282–22895. [DOI] [PubMed] [Google Scholar]
- 14.Leinonen PT, Myllyla RM, Hagg PM, Tuukkanen J, Koivunen J, Peltonen S, et al. Keratinocytes cultured from patients with Hailey-Hailey disease and Darier disease display distinct patterns of calcium regulation. British Journal of Dermatology. 2005;153:113–117. doi: 10.1111/j.1365-2133.2005.06623.x [DOI] [PubMed] [Google Scholar]
- 15.Leinonen PT, Hagg PM, Peltonen S, Jouhilahti EM, Melkko J, Korkiamaki T, et al. Reevaluation of the normal epidermal calcium gradient, and analysis of calcium levels and ATP receptors in Hailey-Hailey and Darier epidermis. Journal of Investigative Dermatology. 2009;129:1379–1387. doi: 10.1038/jid.2008.381 [DOI] [PubMed] [Google Scholar]
- 16.Cederlof M, Karlsson R, Larsson H, Almqvist C, Magnusson PK, Nordlind K, et al. Intellectual disability and cognitive ability in Darier disease: Swedish nation-wide study. British Journal of Dermatology. 2015;173:155–158. doi: 10.1111/bjd.13740 [DOI] [PubMed] [Google Scholar]
- 17.Di Palo M. Rating satisfaction research: Is it poor, fair, good, very good, or excellent? Arthritis Care and Research. 1997;10:422–430. [DOI] [PubMed] [Google Scholar]
- 18.Knaysi GA, Crikelair GF, Cosman B. The role of nines: Its history and accuracy. Plastic and Reconstructive Surgery. 1968;41:560–563. [PubMed] [Google Scholar]
- 19.Ringpfeil F, Raus A, DiGiovanna J, Korge B, Harth W, Mazzanti C, et al. Darier disease–novel mutations in ATP2A2 and genotype–phenotype correlation. Experimental Dermatology. 2001;10:19–27. [DOI] [PubMed] [Google Scholar]
- 20.Leong IUS, Dryland PA, Prosser DO, Lai SW, Graham M, Stiles M, et al. Splice Site Variants in the KCNQ1 and SCN5A Genes: Transcript Analysis as a Tool in Supporting Pathogenicity. Journal of Clinical Medicine Research. 2017;9:709–718. doi: 10.14740/jocmr2894w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nature Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Research. 2012;40:W452–457. doi: 10.1093/nar/gks539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capriotti E, Calabrese R, Fariselli P, Martelli PL, Altman RB, Casadio R. WS-SNPs&GO: A web server for predicting the deleterious effect of human protein variants using functional annotation. BMC Genomics. 2013;14 Suppl 3:S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Marin A. Characterization and prediction of alternative splice sites. Gene. 2006;366:219–227. doi: 10.1016/j.gene.2005.07.015 [DOI] [PubMed] [Google Scholar]
- 26.Desmet FO, Hamroun D, Lalande M, Collod-Beroud G, Claustres M, Beroud C. Human Splicing Finder: A n online bioinformatics tool to predict splicing signals. Nucleic Acids Research. 2009;37:e67 doi: 10.1093/nar/gkp215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leong IU, Stuckey A, Lai D, Skinner JR, Love DR. Assessment of the predictive accuracy of five in silico prediction tools, alone or in combination, and two metaservers to classify long QT syndrome gene mutations. BMC Medical Genetics. 2015;16:34 doi: 10.1186/s12881-015-0176-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: The NCBI database of genetic variation. Nucleic Acids Research. 2001;29:308–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Exome Variant Server, NHLBI GO Exome Sequencing Project (ESP). Seattle, WA;.http://evs.gs.washington.edu/EVS/.
- 31.Kiec-Wilk B, Dembinska-Kiec A, Olszanecka A, Bodzioch M, Schmitz G, Kawecka-Jaszcz K. A724A polymorphism of sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2) in hypertensive patients. Clinical Chemistry and Laboratory Medicine. 2007;45:467–470. doi: 10.1515/CCLM.2007.092 [DOI] [PubMed] [Google Scholar]
- 32.Klausegger A, Nischler E, Wagner RN, Pletschacher F, Hintner H, Bauer JW. Seven novel mutations in the ATP2A2 gene of Austrian patients with Darier’s disease. Archives of Dermatological Research. 2011;303:371–374. doi: 10.1007/s00403-011-1148-6 [DOI] [PubMed] [Google Scholar]
- 33.Bchetnia M, Benmously R, Ben Brick AS, Charfeddine C, Ben Ameur Y, Fajraoui M, et al. New mutations of Darier disease in Tunisian patients. Archives of Dermatological Research. 2009;301:615–619. doi: 10.1007/s00403-009-0963-5 [DOI] [PubMed] [Google Scholar]
- 34.Dodiuk-Gad RP, Cohen-Barak E, Khayat M, Milo H, Amariglio-Diskin L, Danial-Faran N, et al. Darier disease in Israel: Combined evaluation of genetic and neuropsychiatric aspects. British Journal of Dermatology. 2015;174:562–568. doi: 10.1111/bjd.14220 [DOI] [PubMed] [Google Scholar]
- 35.Sakuntabhai A, Burge S, Monk S, Hovnanian A. Spectrum of novel ATP2A2 mutations in patients with Darier’s disease. Human Molecular Genetics. 1999;8:1611–1619. [DOI] [PubMed] [Google Scholar]
- 36.Takagi A, Kamijo M, Ikeda S. Darier disease. The Journal of Dermatology. 2016;43:275–279. doi: 10.1111/1346-8138.13230 [DOI] [PubMed] [Google Scholar]
- 37.Bchetnia M, Charfeddine C, Kassar S, Zribi H, Guettiti HT, Ellouze F, et al. Clinical and mutational heterogeneity of Darier disease in Tunisian families. Archives of Dermatology. 2009;145:654–656. doi: 10.1001/archdermatol.2009.52 [DOI] [PubMed] [Google Scholar]
- 38.Godic A, Strazisar M, Zupan A, Korosec B, Kansky A, Glavac D. Darier disease in Slovenia: Spectrum of ATP2A2 mutations and relation to patients’ phenotypes. European Journal of Dermatology. 2010;20:271–275. doi: 10.1684/ejd.2010.0913 [DOI] [PubMed] [Google Scholar]
- 39.Green E, Gordon-Smith K, Burge S, Grozeva D, Munro C, Tavadia S, et al. Novel ATP2A2 mutations in a large sample of individuals with Darier disease. The Journal of Dermatology. 2013;40:259–266. doi: 10.1111/1346-8138.12082 [DOI] [PubMed] [Google Scholar]
- 40.Berg D, Bassett AS. Darier’s disease: Current understanding of pathogenesis and future role of genetic studies. International Journal of Dermatology. 1993;32:397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An x represents each patient sample and yellow circles represent patients who are genotype-negative. The red bars show the median and 95% confidence intervals.
(TIF)
Medication effect and disease severity score (Top and bottom), a score of 1 = bad, 2 = acceptable, 3 = good, 4 = very good or 5 = excellent. Disease severity rate (Middle), a score of 1 = severe, 2 = moderate and 3 = mild. A score of 0 corresponds to patient’s lack of answer to a particular category. The type of ATP2A2 mutation is shown on the x-axis, and they are sorted into protein domains. ● represent patients receiving systemic treatment; ▲ represents patients receiving topical treatment; ◆ represent patients receiving no treatment and  represent patients who have received laser treatment.
represent patients who have received laser treatment.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.