Abstract
Introduction
During pregnancy, immunoglobulin G (IgG) is transferred from the mother to the fetus, providing protection from disease in early infancy. Plasmodium falciparum infections may reduce maternofetal antibody transfer efficiency, but mechanisms remain unclear.
Methods
Mother-cord paired serum samples collected at delivery from Papua New Guinea (PNG) and the Thailand-Myanmar Border Area (TMBA) were tested for IgG1 and IgG3 to four P. falciparum antigens and measles antigen, as well as total serum IgG. Multivariable linear regression was conducted to assess the association of peripheral P. falciparum infection during pregnancy or placental P. falciparum infection assessed at delivery with maternofetal antibody transfer efficiency. Path analysis assessed the extent to which associations between P. falciparum infection and antibody transfer were mediated by gestational age at delivery or levels of maternal total serum IgG.
Results
Maternofetal antibody transfer efficiency of IgG1 and IgG3 was lower in PNG compared to TMBA (mean difference in cord antibody levels (controlling for maternal antibody levels) ranged from -0.88 to 0.09, median of -0.20 log2 units). Placental P. falciparum infections were associated with substantially lower maternofetal antibody transfer efficiency in PNG primigravid women (mean difference in cord antibody levels (controlling for maternal antibody levels) ranged from -0.62 to -0.10, median of -0.36 log2 units), but not multigravid women. The lower antibody transfer efficiency amongst primigravid women with placental infection was only partially mediated by gestational age at delivery (proportion indirect effect ranged from 0% to 18%), whereas no mediation effects of maternal total serum IgG were observed.
Discussion
Primigravid women may be at risk of impaired maternofetal antibody transport with placental P. falciparum infection. Direct effects of P. falciparum on the placenta, rather than earlier gestational age and elevated serum IgG, are likely responsible for the majority of the reduction in maternofetal antibody transfer efficiency with placental infection.
Introduction
During pregnancy, immunoglobulin G (IgG) antibodies are transferred from mother to fetus. Maternally-derived antibodies provide the neonate with a pre-existing level of passive immunity to help protect from infection and disease. Vaccination of pregnant women can help protect infants against infectious diseases including influenza, tetanus and whooping cough [1]. These strategies rely on adequate maternofetal antibody transfer, but the factors that influence maternofetal antibody transfer are not fully understood. Clinical malaria caused by Plasmodium falciparum infection is uncommon during the first six months of life and, when P. falciparum infections are detected, they tend to be asymptomatic and are of lower parasite density than infections in older infants [2,3,4,5]. Protection against malaria during early infancy has been attributed to many factors [6] including the presence of maternal antibodies in the infant [7], though the relative contribution of maternal antibodies in mediating protection is unclear [8,9].
The humoral immune response is an important component of naturally acquired immunity to malaria and antibodies targeting blood-stage antigens (expressed by merozoites and infected erythrocytes) suppress high parasite densities and progression to symptomatic disease [10]. In malaria endemic areas, individuals develop naturally acquired immunity to P. falciparum infections with age, following repeated infections [10]. Breadth, magnitude and quality of antibody responses are critical, with antibody responses to a broad repertoire of antigenic targets associated with protection against symptomatic malaria in children [11,12,13,14]. Women who would otherwise be relatively immune to clinical P. falciparum malaria are again susceptible to malaria during pregnancy due in part to changes in immune function as well as the appearance of the placenta, a site of sequestration for P. falciparum variants that express PfVAR2CSA on the erythrocyte surface [15]. The predominant antibody isotypes produced in response to malarial antigens are IgG1 and IgG3 [12,16,17,18], which are known to mediate complement deposition and opsonic phagocytosis, mechanisms that have been linked with protective immunity [19,20,21].
Several factors are known or suspected to influence the rate of maternofetal antibody transfer including total maternal IgG [22,23,24,25], gestational age at delivery [25,26,27,28,29], IgG subclass composition [28,30], HIV infection [22,29,31,32] and P. falciparum infection [22,23,25,31,33,34]. Infections with P. falciparum during pregnancy have been associated with earlier gestational age at delivery and increased maternal sera total IgG in some populations [23,34,35]; both preterm birth and increased total maternal IgG have been associated with reduced transport efficiency [22,23,24,26,27,28,30]. Placental P. falciparum infection may also impact maternofetal antibody transfer efficiency directly through the induction of pathological changes in the placenta including inflammation, deposition of pigment in fibrin or inflammatory cells, syncytial knotting and thickening of the trophoblastic basement membrane [36,37,38,39,40].
Studies investigating the relationship between placental P. falciparum infection and maternofetal antibody transfer efficiency of IgG (specific for tetanus [22,32,33], measles [22,29,34], respiratory syncytial virus [23,24] and malaria [25] antigens) have reported mixed results. Of the nine studies that have investigated associations between placental P. falciparum infection and maternofetal antibody transfer, three have found placental infection is associated with reduced antibody transfer efficiency for all antibodies investigated [32,33,34], four have noted reductions in transfer efficiency for some antibodies but not others [22,23,25,31], while two have reported no association [24,29]. Only one study has investigated the effect of P. falciparum infection on malaria-specific IgG, IgG1 and IgG3, reporting significantly lower maternofetal antibody transfer efficiency in women with any detectable P. falciparum infection at delivery of antibodies specific for some antigens, but not others [31]. Discrepancies in the above findings may be due to differences in antibodies studied, definitions of P. falciparum infection, transmission intensity, antimalarial treatment, location and statistical approaches.
To date, no study has directly reported associations between maternal P. falciparum infections detected in the periphery and antibody transfer efficiency. The relative contribution of gestational age at delivery and maternal sera IgG to associations between P. falciparum infection and reduced maternofetal transfer efficiency are yet to be elucidated. To address these gaps in the literature we sought to assess the effect of maternal P. falciparum infection during pregnancy and placental P. falciparum infection on maternofetal transfer efficiency of IgG1 and IgG3 against several malaria antigens and a non-malaria antigen (measles) in an area of high P. falciparum endemicity in Papua New Guinea (PNG), and an area of low P. falciparum endemicity at the Thailand-Myanmar Border Area (TMBA). We also investigated the extent to which any associations between P. falciparum infection and maternofetal transfer efficiency are explained through associations between P. falciparum infection and early gestational age at delivery or increased maternal sera IgG.
Materials and methods
Study population–Alexishafen, Papua New Guinea
Women attending prenatal care at Alexishafen Health Centre, Madang Province, Papua New Guinea (PNG) between August 2005 and September 2007 were recruited into a longitudinal study of malaria and pregnancy following voluntary informed consent [41]. Peripheral blood (5ml) was obtained from women at enrolment, delivery and any additional antenatal clinic visits. At delivery a placental biopsy and 10ml of cord blood were collected. Clinical and demographic data were obtained at enrolment and delivery visits. Presence of microscopic parasitaemia was determined from blood smears by two independent microscopists. A subset of 204 participants with available delivery and cord samples were included in the present study. The study was approved by The Medical Research Advisory Council of Papua New Guinea (MRAC 05/05) and the Human Research Ethics Committee of Melbourne Health, Australia (06/06).
Study population—Shoklo Malaria Research Unit, Thailand-Myanmar Border Area
Women attending antenatal clinics of the Shoklo Malaria Research Unit, Thailand-Myanmar Border Area (TMBA), between November 1998 and January 2000 were invited to participate in a chloroquine prophylaxis randomized controlled trial for prevention of P. vivax [42]. P. falciparum episodes were similar in the chloroquine prophylaxis and placebo groups [42]. A total of 118 peripheral maternal blood and paired cord blood samples were utilised from a subset of pregnant women at delivery included in a previously reported study of malaria immunity [43]. At the Shoklo Malaria Research Unit, women are invited to attend an ANC as soon as they become aware of their pregnancy and were then encouraged to attend weekly thereafter.
Method of gestational age assessment
Gestational age was estimated from Ballard scores [44] in PNG, and in TMBA either the Dubowitz method [45] or a calculation based on fundal height [46], which performed well compared to ultrasound in term newborns [47]. Studies conducted in resource-limited settings suggest that Ballard and Dubowitz methods give comparable estimates of gestational age [48,49], however there was no attempt to quality control gestational age assessment between sites. Ballard scores and the Dubowitz method both fall short of estimation of gestational age by early ultrasound assessment [50].
P. falciparum exposures
In PNG, maternal P. falciparum peripheral infection during pregnancy was defined as any light microscopy detected infection in peripheral blood at any point during pregnancy. Women with P. falciparum infections detected were treated with curative doses of chloroquine and sulphadoxine pyrimethamine and all women were given unsupervised prophylaxis [41].
In TMBA, maternal P. falciparum peripheral infection during pregnancy was defined as any light microscopy detected infection in peripheral blood at any point during pregnancy. All women who attend TMBA ANCs were screened weekly for the presence of P. falciparum by light microscopy. When infections were detected they were treated immediately. For P. falciparum or mixed infections, a first infection was treated with quinine or artesunate and all women were given weekly chloroquine prophylaxis [42].
Placental histology
In PNG only, placental histology was performed as described by Rogerson et al [37]. We compared the presence of any parasitaemia in the placenta (pathology class 1–3) to no parasites (pathology class 4–5). As a secondary analysis we compared the presence of parasites and monocytes with malaria pigment in the placenta (pathology class 2) to parasites without malaria pigment-containing monocytes (pathology class 1 and 3) and to no parasites (pathology class 4–5).
Antibody determination at delivery
We measured total serum IgG; IgG1 and IgG3 antibodies to measles antigen (MEV-007—PROSPECbio, Rehovot, Israel) and four P. falciparum antigens (PfEBA175RII (3D7, amino acid position 146–713), PfMSP2 (3D7, amino acid position 19–249), PfAMA-1 (3D7, amino acid position 25–545) and PfDBL5 (7G8, amino acid position 2003–2270)). We did not assess IgG2 or IgG4 levels, as these isotypes have previously been found to be present at very low levels against the P. falciparum antigens assessed [12,17,18], precluding a reliable assessment of maternofetal antibody transfer. Antibodies to these P. falciparum merozoite antigens are thought to be protective [51,52]. One of these P. falciparum antigens is known to induce IgG1 dominant responses (PfAMA-1) and another is known to induce IgG3 dominant responses (PfMSP2) [18,53]. Antibodies were measured using an enzyme-linked immunosorbent assay (ELISA) as described previously [43]. P. falciparum antigens were coated at 0.5 μg/ml, measles antigen at 1 μg/ml and goat anti-human-kappa at 1/1000. Horeseradish peroxidase (HRP)-conjugated goat anti-human IgG (Sigma), mouse anti-human IgG1 (Invitrogen) and mouse anti-human IgG3 (Invitrogen) were used as the secondary antibody (at 1/1000) in the total serum IgG, specific IgG1 and specific IgG3 assays respectively. The tertiary antibody (at 1/1000) for specific IgG1 and IgG3 assays was HRP-conjugated goat anti-mouse IgG (Merck Millipore). Assay output was related to a standard curve of pooled sera using a four-parameter logistic nonlinear regression model. TMBA samples had substantially lower levels of malaria-specific antibodies than PNG samples, so immunoassays against P. falciparum antigens were run at multiple concentrations of sera (sera dilutions are provided in S1 Table, range 1/100-1/20,000 for P. falciparum antigens). When assay reactivity from a sample was too high to reliably interpolate from the standard curve, then it was rerun at a lower sera concentration. The highest reactivity in an assay was set to 100 arbitrary units (AU). All wash steps were performed using PBS with 0.05% Tween and completed using an automated plate washer within a robotic platform (Perkin Elmer, Waltham, USA). Serum addition was performed using a robotic platform (Perkin Elmer, Waltham, USA). A sample was considered to be seropositive (or in the case of total IgG: elevated) if the AU for that sample was above the mean + 3 standard deviations of those for 15 non-exposed Melbourne blood donors.
Statistical analysis
All statistical analyses were performed using Stata Version 13.1 (StataCorp, College Station, TX, USA). Correlations between cord and maternal antibodies were assessed using Spearman’s rank correlation coefficients. Wilcoxon rank-sum tests were used to assess differences in antibody levels between populations. Women who were seronegative for an antibody were not assessed for their ability to transfer that antibody. To assess the impact of study site on antibody transfer, multivariable linear regression of log2 cord antibody levels was performed with log2 maternal levels at delivery and study site as covariates, and further adjustment for the potential confounders, gravidity (primigravid/multigravid) and estimated gestational age at delivery (weeks). P. vivax infection was not included in the model.
To assess the impact of P. falciparum infection during pregnancy on cord antibody levels, all analyses were performed separately for each study site, as inherent differences in study design meant that the screening, treatment and recording of infections could not be considered equivalent for the two study sites. Multivariable linear regression was performed assessing the association of infection with log2 cord antibody levels after adjustment for log2 maternal antibody levels and gravidity (primigravid/multigravid). Effect modification by gravidity of the association between infection and cord antibody levels was assessed though the fitting of interaction terms between gravidity (primigravid/multigravid) and infection. Three different infection variables were used in the models: peripheral P. falciparum infection at any time during pregnancy (yes/no); placental histology (parasites/no parasites); placental histology (parasites and monocytes containing malaria pigment/parasites without monocytes containing malaria pigment/no parasites). Placental histology data were only recorded in the PNG study.
Where associations were observed between infection and maternofetal antibody transfer efficiency, path analysis was then conducted to investigate potential mediation of the relationship between infection and cord antibody levels through gestational age at delivery and maternal serum IgG. The proportion of the total effect due to an indirect association via gestation age at delivery or maternal serum IgG was then estimated.
Results
Study populations
IgG1 and IgG3 levels were determined for 204 maternal/cord pairs from Alexishafen, Papua New Guinea (PNG) and 118 pairs from the Thailand-Myanmar Border Area (TMBA). Gravidity and gestational age at delivery were higher in TMBA women relative to PNG women (Table 1). P. falciparum antibody seroprevalence, the proportion with detectable P. falciparum infections and the proportion with elevated serum IgG were lower in TMBA women relative to PNG women (Table 1).
Table 1. Distribution of maternal characteristics by study site.
| Alexishafen, Papua New Guinea (n = 204) |
TMBA, Thailand (n = 118) |
|
|---|---|---|
| Maternal characteristics | ||
| Age (years) | 24 (21,28), [16–49] | 25 (21–32), [15–42] |
| Gravidity | 2 (1–4), [1–10] | 3 (2–5), [1–13] |
| Gestational age at delivery (weeks)a | 38 (37–40), [28–42] | 40 (40,41), [31–42] |
| Plasmodium spp. infection | ||
| P. falciparum infectionb | 81 (40) | 32 (27) |
| P. vivax infectionb | 12 (6) | 32 (27) |
| P. falciparum placental histology | ||
| No parasites detected | 92 (45) | Not determined |
| Parasites detected | 112 (55) | Not determined |
| Antibody measures | ||
| Maternal seropositive | ||
| PfEBA175RII IgG1 | 178 (87) | 43 (36) |
| PfEBA175RII IgG3 | 199 (98) | 114 (97) |
| PfAMA1 IgG1 | 197 (97) | 106 (90) |
| PfAMA1 IgG3 | 189 (93) | 91 (77) |
| PfMSP2 IgG1 | 199 (98) | 95 (81) |
| PfMSP2 IgG3 | 204 (100) | 106 (90) |
| PfDBL5 IgG1 | 119 (58) | 34 (29) |
| PfDBL5 IgG3 | 123 (60) | 74 (62) |
| Measles IgG1 | 195 (96) | 113 (96) |
| Measles IgG3 | 204 (100) | 117 (99) |
| Elevated serum IgGc | 50 (25) | 16 (14) |
NB—Data presented as median (inter-quartile range), [minimum-maximum] or n (%). Abbreviations: TMBA = Thailand-Myanmar Border Area.
a Estimated by Ballard scores in PNG, Dubowitz method in TMBA.
b Infection detected by light microscopy (peripheral) at any point during pregnancy. 7 and 11 women experienced a P. falciparum infection and a P. vivax infection during pregnancy in Papua New Guinea and Thailand respectively.
c Defined as a level of total IgG greater than the mean + three standard deviations of 15 Melbourne blood donors.
Higher levels of malaria-specific IgG1 and IgG3 in maternal and cord sera from PNG than from TMBA
Maternal IgG1 and IgG3 levels were strongly correlated with cord IgG1 and IgG3 levels (Spearman’s ρ ranges from 0.78–0.96, S1 Fig). Maternal and cord IgG1 and IgG3 levels against P. falciparum antigens were higher in PNG samples, where malaria transmission is higher, than in TMBA samples (Figs 1 and 2, p<0.0001 for all comparisons except PfDBL5 IgG3 (p = 0.16 and p = 0.58 for maternal and cord levels respectively)). IgG1 and IgG3 levels were lower in the cord blood than in the maternal blood for the majority of antigens (Figs 1C and 2C). The cord:maternal ratio of antibodies against all antigens (P. falciparum and measles) was lower in PNG samples than TMBA samples (Figs 1C and 2C). The cord:maternal IgG1 ratio was higher than the IgG3 ratio for each antigen investigated within each study site (Figs 1C and 2C).
Fig 1. Maternal and cord IgG1 levels by study site.
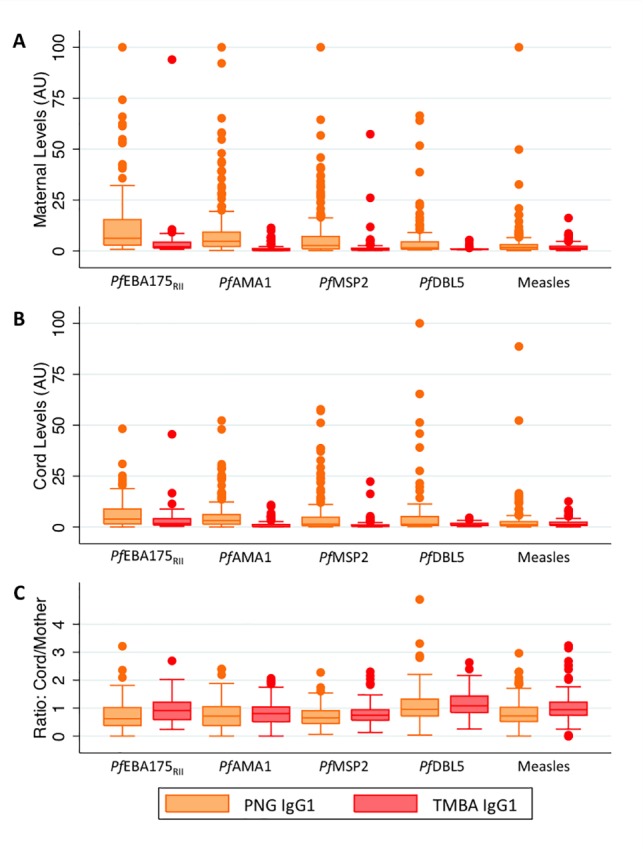
(A) Maternal IgG1 levels (arbitrary units) by study site. (B) Cord IgG1 levels (arbitrary units) by study site. (C) Cord:maternal IgG1 ratio by study site.
Fig 2. Maternal and cord IgG3 levels by study site.
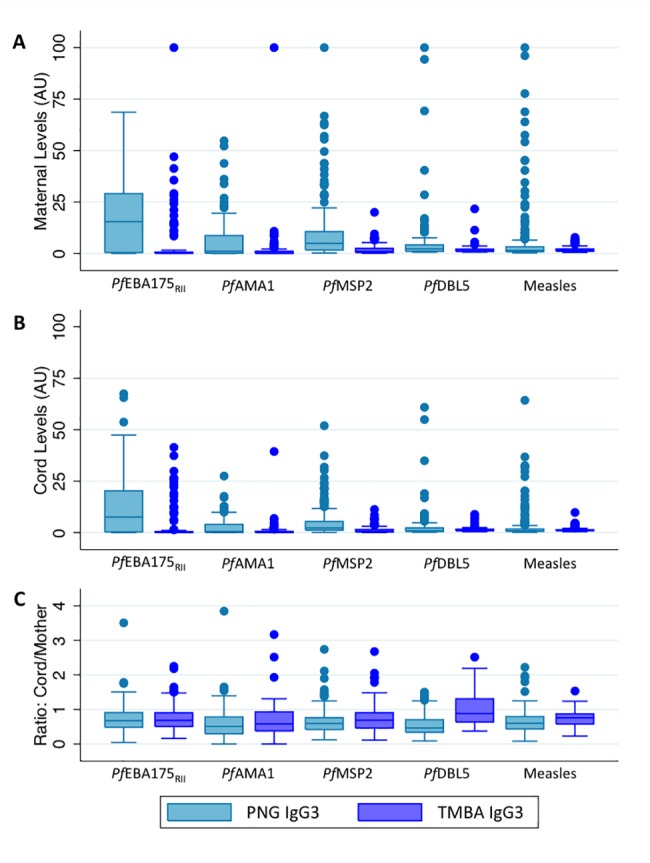
(A) Maternal IgG3 levels (arbitrary units) by study site. (B) Cord IgG3 levels (arbitrary units) by study site. (C) Cord:maternal IgG3 ratio by study site.
Maternofetal transfer efficiency of IgG1 and IgG3 antibodies specific for malaria and measles was lower in PNG women relative to TMBA women
To investigate maternofetal antibody transfer efficiency, linear regression was performed, modelling cord antibody levels (log2(AU)) with maternal levels (log2(AU)) included as a covariate. Maternofetal transfer efficiency of IgG1 and IgG3 antibodies specific for P. falciparum antigens was reduced in PNG compared to TMBA (Table 2) (estimate of adjusted mean difference of P. falciparum antibody levels in cord blood (log2 units) for PNG versus TMBA ranged from -0.88 to 0.09 with a median value of -0.15). The largest magnitude of effect was observed in DBL5 IgG3. Maternofetal transfer efficiency of antibodies specific for measles was significantly lower in PNG relative to TMBA (-0.41 log2 units (95% confidence interval (CI): -0.61, -0.21) and -0.26 (95% CI: -0.38, -0.14) for IgG1 and IgG3 respectively). Adjusting for gestational age at delivery and gravidity did not substantially alter the magnitude of associations observed (Table 2).
Table 2. Association of study site with maternofetal antibody transfer efficiency; estimated adjusted mean difference (95% confidence intervals) and p-values are presented.
| PfEBA175RII−IgG1 | PfEBA175RII−IgG3 | PfAMA-1 –IgG1 | PfAMA-1 –IgG3 | PfMSP2 –IgG1 | PfMSP2 –IgG3 | PfDBL5 –IgG1 | PfDBL5 –IgG3 | Measles–IgG1 | Measles–IgG3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted1 | ||||||||||
| TMBA | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| PNG | -0.24 (-0.57,0.09); 0.16 |
-0.07 (-0.26,0.14); 0.53 |
-0.06 (-0.40,0.29); 0.75 |
-0.16 (-0.47,0.14); 0.29 |
-0.13 (-0.34,0.08); 0.22 |
0.09 (-0.12,0.29); 0.40 |
-0.28 (-0.64,0.07); 0.12 |
-0.88 (-1.11,-0.65); <0.001 |
-0.41 (-0.61,-0.21); <0.001 |
-0.26 (-0.39,-0.14); <0.001 |
| Adjusted2 | ||||||||||
| TMBA | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| PNG | -0.11 (-0.45,0.23); 0.52 |
0.07 (-0.15,0.28); 0.53 |
0.10 (-0.25,0.45); 0.56 |
-0.03 (-0.35,0.29); 0.85 |
-0.04 (-0.26,0.19); 0.75 |
0.17 (-0.05,0.39); 0.14 |
-0.14 (-0.52,0.25); 0.48 |
-0.79 (-1.05,-0.53); <0.001 |
-0.34 (-0.56,-0.12); 0.002 |
-0.24 (-0.37,-0.11); 0.001 |
NB–Coefficients represent the differences in cord antibody levels by study site after adjustment for maternal antibody levels. Abbreviations: TMBA = Thailand-Myanmar Border Area; PNG = Papua New Guinea. 1 –Adjusted for log2 maternal levels. 2—Adjusted for log2 maternal levels, gravidity (primigravid/multigravid) and gestational age at delivery (weeks).
Maternal P. falciparum infection, detected peripherally, was not associated with antibody transfer efficiency in either study site
At PNG and TMBA there was no consistent association between peripheral P. falciparum infection and maternofetal IgG1 or IgG3 transfer efficiency (Fig 3, estimate of adjusted mean difference of antibody levels in cord blood (log2AU) for mothers with a peripheral P. falciparum infection versus those without a P. falciparum infection ranged from -0.15 to 0.21 with a median value of -0.05).
Fig 3. Association of maternal P. falciparum infection with maternofetal antibody transfer.
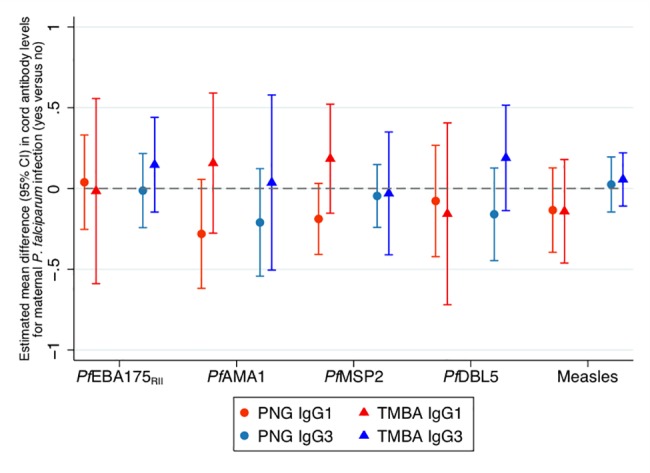
Estimates and 95% confidence intervals are presented of the mean difference in log2 cord antibody levels after adjustment for log2 maternal antibody levels and gravidity, for mothers with a P. falciparum infection during pregnancy compared to uninfected mothers. Dashed line at 0 indicates no difference in mean log2 cord antibody levels. See S2 Table for a table version of this figure.
Placental P. falciparum infection was associated with lower antibody transfer efficiency in primigravid women, but not multigravid women
No data were available on the placental histology of TMBA women, but a study conducted in the Shoklo and Maela camps during the time of this study indicated a low estimated prevalence of P. falciparum placental histopathological changes when early detection and treatment of infection, routine in this population, was performed [40]. Placental histology data were available from the PNG study allowing us to investigate the impact of placental infection on maternofetal antibody transfer efficiency. The presence of placental P. falciparum was associated with reduced maternofetal IgG1 and IgG3 transfer efficiency in primigravid women (Fig 4A, S3 Table) (estimate of mean difference of antibody levels in cord blood (log2 units) for mother-cord pairs with P. falciparum present in the placenta versus those without placental P. falciparum parasites present ranged from -0.62 to -0.10 with a median value of -0.36). There were no consistent associations between placental infection and maternofetal transfer efficiency in multigravid women (Fig 4B, S3 Table). In primigravid PNG women associations of placental infections with monocyte infiltrate were greater in magnitude than placental infections without monocyte infiltrate (S2 Fig).
Fig 4. Association of placental infection with maternofetal antibody transfer efficiency in primigravid and multigravid Papua New Guinea women.
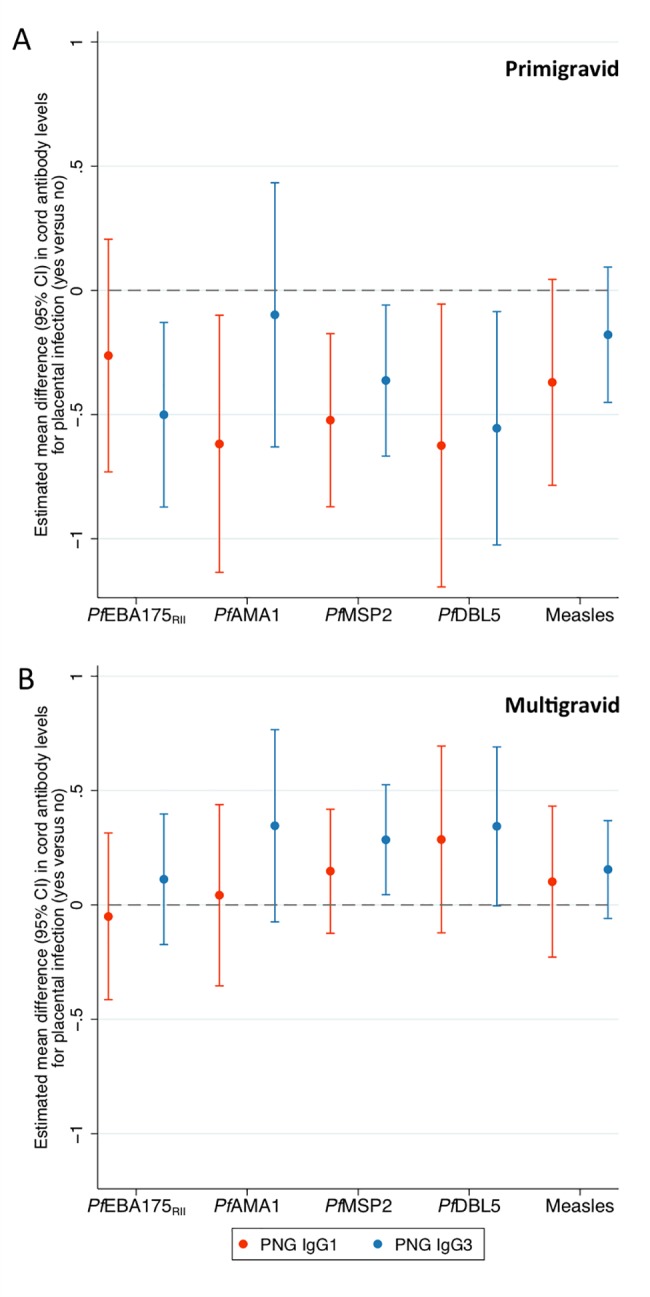
Estimates and 95% confidence intervals are presented for the mean difference in log2 cord antibody levels after adjustment for log2 maternal antibody levels for (A) primigravid or (B) multigravid mothers with a P. falciparum placental infection compared to mothers with no placental infection. Dashed line at 0 indicates no difference in mean log2 cord antibody levels. See S3 Table for a table version of this figure, including estimates for all women.
Placental P. falciparum infection was associated with lower antibody transfer efficiency in primigravid mothers primarily through mechanisms other than earlier gestational age at delivery or increased maternal IgG
Path analysis was performed to assess the proportion of the total effect of placental infection on maternofetal IgG1 and IgG3 transfer efficiency in PNG primigravid women mediated by gestational age and changes in total levels of maternal IgG. The negative effect of placental infection in primigravid women was not substantially mediated through induction of shorter gestational age at delivery (proportion of total effect attributable to gestational age mediation ranged from 0% to 18% with a median value of 5%, p ranged from 0.21 to 0.93 for indirect effects) (Fig 5, S4 Table). None of the effect appeared to be mediated through changes in total maternal serum IgG (p ranged from 0.63 to 0.87 for indirect effects).
Fig 5. Path analysis model for cord PfAMA1 IgG1 levels and placental P. falciparum infection in primigravid women.
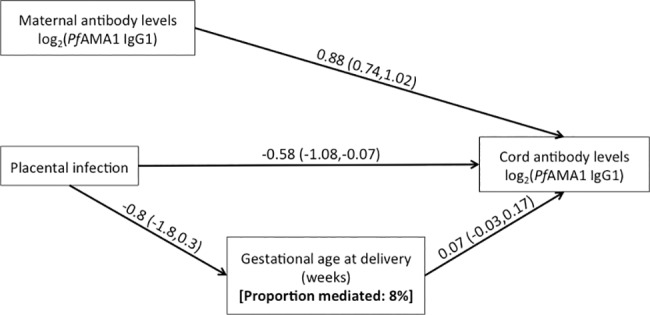
Estimated mean difference (95% CI) are presented. For other antibodies see S4 Table.
Discussion
To date, there has been considerable uncertainty regarding the effects of P. falciparum infection during pregnancy on maternofetal antibody transfer efficiency, as well as the mechanisms by which infection may mediate impaired maternofetal antibody transfer. A great strength of this study was the inclusion of women from two separate study sites with differing P. falciparum endemicity and access to antenatal clinics. Importantly, antibody data were standardised to a reference sera pool curve, which enabled direct comparisons of antibody levels across the study populations. The assessment of measles antibodies, which were present at similar levels in both study sites and should not be boosted during a placental P. falciparum infection, strengthened the generalisibility of the findings. Although some studies have included the potential mediating effects of elevated serum IgG and shorter gestation as confounders [22,23,31,33], this is the first study to investigate whether placental infection is mediated via these effects with path analysis. Malaria exposure during pregnancy was captured differently at each study site and therefore separate analyses had to be performed. The prevalence of HIV is very low (<1.5%) in these populations [54,55]) so HIV is unlikely to have a substantial effect on maternofetal transfer at the population level. The lack of placental histology data from the TMBA prevented an assessment of placental infection in that study site.
Maternofetal transfer efficiency of some antibodies was reduced in PNG compared to TMBA. Besides the direct effects of malaria, there are several factors that could account for this difference, including maternal genetic differences, gestational age at delivery and altered levels of total maternal IgG [22,23,32]. The median gestational age at delivery of PNG women was 2 weeks shorter than that observed at TMBA. However, differences in gestation cannot fully explain the difference we observed, given the persistence of an association between study site and maternofetal transfer efficiency of some antibodies even after controlling for gestational age at delivery. Very high levels of total maternal IgG are thought to saturate available receptors at the placenta, thereby reducing efficiency of transfer [56], however we did not observe an association between increased maternal IgG and decreased maternofetal transfer efficiency in either population in this study.
Greater maternofetal antibody transfer efficiency at TMBA relative to PNG may be due in part, to the prompt detection and treatment of P. falciparum infections at the TMBA site, preventing the persistence of long-term infections and thereby reducing the risk and consequences of placental infection. Women at TMBA were encouraged to attend antenatal clinics weekly, and were rapidly treated upon diagnosis, so the duration of any Plasmodium spp. infections during gestation is minimal and placental infections are rare [40]. In PNG, policy dictated weekly chloroquine prophylaxis, this was not monitored and so infections with P. falciparum may have persisted undetected. Malaria transmission was also substantially higher at the PNG site. At delivery, the majority of women presented with P. falciparum infected placentae in PNG, whereas at TMBA, placental infections are a rare outcome [40].
In primigravid PNG women we observed an association between the presence of P. falciparum parasites in the placenta and reduced maternofetal IgG1 and IgG3 transfer efficiency relative to uninfected women; no association was observed in multigravid PNG women. Primigravid women tend to have placental infections of greater parasite density and more commonly have associated placental inflammation than do multigravid women [57]. Multigravid women are not only to be less likely to present with placental infection, but also less likely to have impaired maternofetal antibody transfer when infected.
There are numerous mechanisms by which placental P. falciparum infection may reduce maternofetal antibody transfer efficiency. Placental P. falciparum infection is associated with a shorter gestational age [35] and shorter gestational age is associated with reduced maternofetal antibody transfer [26,27,28]. In our model, we found that gestational age explained a small proportion of the reduced maternofetal antibody transfer efficiency associated with placental infection. Placental infection has been associated with increased total maternal IgG in some other populations [23,34]; transfer efficiency can be reduced when maternal IgG levels are elevated [56]. However, amongst PNG women we did not observe a reduction in antibody transfer efficiency among those women with increased total serum IgG, as defined by levels greater than the mean plus three standard deviations of Melbourne control levels. The majority of the association between placental infection and reduced maternal transfer efficiency in primigravid PNG women was explained through mechanisms other than gestational age and maternal IgG in the present study. Our results indicate that a large proportion of the observed effect may be due to direct effects of placental infection. Given that placental infections with malaria-pigment containing monocytes tended to have even lower transfer efficiency than placental infections without monocytes, inflammation at the placenta is likely to be involved. Disruptions to the placental architecture that occur during placental infection [36,58,59,60] may have a negative impact on maternofetal transfer and receptor expression. Placental infection with inflammation seems to impair transplacental transport of glucose and amino acids via a reduction in expression of transport receptors [61,62]; if expression of placental immunoglobulin transport receptors were also reduced then this would explain some of the reduction in antibody transfer efficiency. As IgG1 outcompetes IgG3 for placental receptor binding [63], a reduction in transport receptors would likely have a greater impact on IgG1 transfer relative to IgG3 transfer. Further research is needed to elucidate the precise mechanisms by which placental infection with P. falciparum mediates reduced efficiency of maternofetal transfer, but our findings indicate direct effects of P. falciparum placental infection play a substantial role, emphasising the need for highly efficacious prevention in pregnancy.
Our study was subject to limitations. Our knowledge of infection status over the entire course of pregnancy was incomplete as the infection status of a woman was necessarily restricted to instances when they presented to antenatal care. Infection was detected via microscopy, so sub-microscopic infections were not captured. The infection status of cord blood was not determined in both study settings. Notably in the TMBA study we lacked placental histology data, limiting our analysis of placental histology exposures to pregnant women from PNG. We did not have data available on the outcomes of the children in their first year of life, precluding an assessment of the relationship between maternofetal antibody transfer and infant outcomes.
We have observed that placental P. falciparum infection was associated with reduced maternofetal antibody transfer efficiency in primigravid women and that only a small proportion of this association is mediated by gestational age. Adequate maternofetal transfer of antibodies is essential for maternal vaccination strategies to effectively protect infants; efforts to prevent and treat malaria in pregnancy should continue to be encouraged.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Scatter plots of cord and maternal IgG1 and IgG3 levels against PfEBA175RII, PfAMA1, PfMSP2, PfDBL5 and Measles. Samples from Alexishafen, Papua New Guinea are denoted as green closed circles and from Shoklo Malaria Research Unit, Thailand-Myanmar Border Area as closed red circles. Spearman ρ values (IgG1 and IgG3): PfEBA175RII (0.91,0.96); PfAMA1 (0.89,0.95); PfMSP2 (0.94,0.94); PfDBL5 (0.79,0.78); Measles (0.87,0.86).
(TIF)
Estimates and 95% confidence intervals are presented for the mean difference in log2 cord antibody levels after adjustment for log2 maternal antibody levels for (A) primigravid or (B) multigravid mothers with a P. falciparum placental infection without monocyte infiltrate (orange triangles, n = 22 and n = 59 in primigravid and multigravida respectively) or a P. falciparum placental infection with monocyte infiltrate (blue triangles, n = 23 and n = 8 in primigravid and multigravida respectively) compared to mothers with placentas with no P. falciparum parasites present. Dashed line at y = 0 indicates no difference in mean log2 cord antibody levels.
(TIF)
Acknowledgments
We thank the staff of the Alexishafen Health Centre, PNG Institute of Medical Research and Shoklo Malaria Research Unit for their assistance with the study. We thank Joe Smith, Robin Anders, Damien Drew and Annie Mo for provision of antigens. We thank Sarah Charnaud and Kerryn Moore for assistance with analysis. We thank all the study participants for their participation.
Data Availability
All relevant data is within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Health and Medical Research Council of Australia (project grant and training award to Freya J. I. Fowkes; Infrastructure for Research Institutes Support Scheme grant, Senior Research Fellowship and Program Grant to JGB and IM, NHMRC Independent Research Institutes Infrastructure Support to the Burnet Institute and WEHI), Australian Research Council (Future Fellowship to Freya J. I. Fowkes), and Victorian State Government Operational Infrastructure Support grant to the Burnet Institute. Alistair R. D. McLean is supported by an Australian Postgraduate Award. Shoklo Malaria Research Unit is part of the Mahidol Oxford University Tropical Medicine Research Unit supported by the Wellcome Trust of Great Britain. The Christophe and Rodolphe Mérieux Foundation supported the study through a prize (2008) to François Nosten. No individuals employed or contracted by the funders (other than the named authors) played any role in: study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chu HY, Englund JA. Maternal immunization. Clin Infect Dis. 2014;59(4):560–8. Epub 2014/05/07. doi: 10.1093/cid/ciu327 ; PubMed Central PMCID: PMC4168293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce-Chwatt LJ. Malaria in African infants and children in Southern Nigeria. Ann Trop Med Parasitol. 1952;46(2):173–200. Epub 1952/09/01. . [DOI] [PubMed] [Google Scholar]
- 3.Garnham PC. Malarial immunity in Africans; effects in infancy and early childhood. Ann Trop Med Parasitol. 1949;43(1):47–61. Epub 1949/04/01. . [DOI] [PubMed] [Google Scholar]
- 4.Sehgal VM, Siddjiqui WA, Alpers MP. A seroepidemiological study to evaluate the role of passive maternal immunity to malaria in infants. Trans R Soc Trop Med Hyg. 1989;83 Suppl:105–6. Epub 1989/01/01. . [DOI] [PubMed] [Google Scholar]
- 5.McGuinness D, Koram K, Bennett S, Wagner G, Nkrumah F, Riley E. Clinical case definitions for malaria: clinical malaria associated with very low parasite densities in African infants. Trans R Soc Trop Med Hyg. 1998;92(5):527–31. Epub 1998/12/23. . [DOI] [PubMed] [Google Scholar]
- 6.Kangoye DT, Nebie I, Yaro JB, Debe S, Traore S, Ouedraogo O, et al. Plasmodium falciparum malaria in children aged 0–2 years: the role of foetal haemoglobin and maternal antibodies to two asexual malaria vaccine candidates (MSP3 and GLURP). PLoS ONE. 2014;9(9):e107965 Epub 2014/09/23. doi: 10.1371/journal.pone.0107965 ; PubMed Central PMCID: PMC4169582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobbs KR, Dent AE. Plasmodium malaria and antimalarial antibodies in the first year of life. Parasitology. 2016;143(2):129–38. Epub 2016/01/09. doi: 10.1017/S0031182015001626 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley EM, Wagner GE, Akanmori BD, Koram KA. Do maternally acquired antibodies protect infants from malaria infection? Parasite Immunol. 2001;23(2):51–9. Epub 2001/03/10. . [DOI] [PubMed] [Google Scholar]
- 9.Riley EM, Wagner GE, Ofori MF, Wheeler JG, Akanmori BD, Tetteh K, et al. Lack of association between maternal antibody and protection of African infants from malaria infection. Infect Immun. 2000;68(10):5856–63. Epub 2000/09/19. ; PubMed Central PMCID: PMC101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22(1):13–36, Table of Contents. Epub 2009/01/13. doi: 10.1128/CMR.00025-08 ; PubMed Central PMCID: PMC2620631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osier FHA, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KKA, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76(5):2240–8. doi: 10.1128/IAI.01585-07 . Language: English. Language Code: eng. Date Revised: 20091118. Date Created: 20080421. Date Completed: 20080505. Update Code: 20111122. Publication Type: Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards JS, Stanisic DI, Fowkes FJ, Tavul L, Dabod E, Thompson JK, et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis. 2010;51(8):e50–60. Epub 2010/09/17. doi: 10.1086/656413 . [DOI] [PubMed] [Google Scholar]
- 13.Rono J, Osier FH, Olsson D, Montgomery S, Mhoja L, Rooth I, et al. Breadth of anti-merozoite antibody responses is associated with the genetic diversity of asymptomatic Plasmodium falciparum infections and protection against clinical malaria. Clin Infect Dis. 2013;57(10):1409–16. Epub 2013/08/29. doi: 10.1093/cid/cit556 ; PubMed Central PMCID: PMC3805176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJ, et al. Identification and Prioritization of Merozoite Antigens as Targets of Protective Human Immunity to Plasmodium falciparum Malaria for Vaccine and Biomarker Development. J Immunol. 2013;191(2):795–809. Epub 2013/06/19. doi: 10.4049/jimmunol.1300778 ; PubMed Central PMCID: PMC3702023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khunrae P, Dahlbäck M, Nielsen MA, Andersen G, Ditlev SB, Resende M, et al. Full-Length Recombinant Plasmodium falciparum VAR2CSA Binds Specifically to CSPG and Induces Potent Parasite Adhesion-Blocking Antibodies. J Mol Biol. 2010;397:826–34. doi: 10.1016/j.jmb.2010.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elliott SR, Brennan AK, Beeson JG, Tadesse E, Molyneux ME, Brown GV, et al. Placental malaria induces variant-specific antibodies of the cytophilic subtypes immunoglobulin G1 (IgG1) and IgG3 that correlate with adhesion inhibitory activity. Infect Immun. 2005;73(9):5903–7. doi: 10.1128/IAI.73.9.5903-5907.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Megnekou R, Staalsoe T, Taylor DW, Leke R, Hviid L. Effects of pregnancy and intensity of Plasmodium falciparum transmission on immunoglobulin G subclass responses to variant surface antigens. Infect Immun. 2005;73(7):4112–8. doi: 10.1128/IAI.73.7.4112-4118.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77(3):1165–74. Epub 2009/01/14. doi: 10.1128/IAI.01129-08 ; PubMed Central PMCID: PMC2643653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osier FH, Feng G, Boyle MJ, Langer C, Zhou J, Richards JS, et al. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med. 2014;12:108 Epub 2014/07/02. doi: 10.1186/1741-7015-12-108 ; PubMed Central PMCID: PMC4098671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, et al. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity. 2015;42(3):580–90. Epub 2015/03/19. doi: 10.1016/j.immuni.2015.02.012 ; PubMed Central PMCID: PMC4372259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joos C, Marrama L, Polson HE, Corre S, Diatta AM, Diouf B, et al. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS ONE. 2010;5(3):e9871 Epub 2010/04/03. doi: 10.1371/journal.pone.0009871 ; PubMed Central PMCID: PMC2845614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, Milligan PJ, Wesumperuma L, Broadhead RL, et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79(3):F202–5. Epub 1999/04/09. ; PubMed Central PMCID: PMC1720856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okoko BJ, Wesuperuma LH, Ota MO, Banya WA, Pinder M, Gomez FS, et al. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-foetal transfer of measles and tetanus antibodies in a rural west African population. J Health Popul Nutr. 2001;19(2):59–65. Epub 2001/08/16. . [PubMed] [Google Scholar]
- 24.Atwell JE, Thumar B, Robinson LJ, Tobby R, Yambo P, Ome-Kaius M, et al. Impact of Placental Malaria and Hypergammaglobulinemia on Transplacental Transfer of Respiratory Syncytial Virus Antibody in Papua New Guinea. J Infect Dis. 2015. Epub 2015/08/05. doi: 10.1093/infdis/jiv401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dechavanne C, Cottrell G, Garcia A, Migot-Nabias F. Placental Malaria: Decreased Transfer of Maternal Antibodies Directed to Plasmodium falciparum and Impact on the Incidence of Febrile Infections in Infants. PLoS ONE. 2015;10(12):e0145464 Epub 2015/12/25. doi: 10.1371/journal.pone.0145464 ; PubMed Central PMCID: PMC4689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okoko BJ, Wesumperuma HL, Fern J, Yamuah LK, Hart CA. The transplacental transfer of IgG subclasses: influence of prematurity and low birthweight in the Gambian population. Ann Trop Paediatr. 2002;22(4):325–32. Epub 2003/01/18. doi: 10.1179/027249302125001985 . [DOI] [PubMed] [Google Scholar]
- 27.Costa-Carvalho BT, Vieria HM, Dimantas RB, Arslanian C, Naspitz CK, Sole D, et al. Transfer of IgG subclasses across placenta in term and preterm newborns. Braz J Med Biol Res. 1996;29(2):201–4. Epub 1996/02/01. . [PubMed] [Google Scholar]
- 28.Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36(5):248–55. Epub 1996/11/01. . [DOI] [PubMed] [Google Scholar]
- 29.Scott S, Cumberland P, Shulman CE, Cousens S, Cohen BJ, Brown DW, et al. Neonatal measles immunity in rural Kenya: the influence of HIV and placental malaria infections on placental transfer of antibodies and levels of antibody in maternal and cord serum samples. J Infect Dis. 2005;191(11):1854–60. Epub 2005/05/05. doi: 10.1086/429963 . [DOI] [PubMed] [Google Scholar]
- 30.Einarsdottir HK, Stapleton NM, Scherjon S, Andersen JT, Rispens T, van der Schoot CE, et al. On the perplexingly low rate of transport of IgG2 across the human placenta. PLoS ONE. 2014;9(9):e108319 Epub 2014/09/25. doi: 10.1371/journal.pone.0108319 ; PubMed Central PMCID: PMC4177109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moro L, Bardaji A, Nhampossa T, Mandomando I, Serra-Casas E, Sigauque B, et al. Malaria and HIV infection in Mozambican pregnant women are associated with reduced transfer of antimalarial antibodies to their newborns. J Infect Dis. 2015;211(6):1004–14. Epub 2014/10/02. doi: 10.1093/infdis/jiu547 . [DOI] [PubMed] [Google Scholar]
- 32.Cumberland P, Shulman CE, Maple PA, Bulmer JN, Dorman EK, Kawuondo K, et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis. 2007;196(4):550–7. Epub 2007/07/13. doi: 10.1086/519845 . [DOI] [PubMed] [Google Scholar]
- 33.Brair ME, Brabin BJ, Milligan P, Maxwell S, Hart CA. Reduced transfer of tetanus antibodies with placental malaria. Lancet. 1994;343(8891):208–9. Epub 1994/01/22. . [DOI] [PubMed] [Google Scholar]
- 34.Owens S, Harper G, Amuasi J, Offei-Larbi G, Ordi J, Brabin BJ. Placental malaria and immunity to infant measles. Arch Dis Child. 2006;91(6):507–8. Epub 2006/05/23. doi: 10.1136/adc.2005.085274 ; PubMed Central PMCID: PMC2082802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1–2 Suppl):28–35. . [DOI] [PubMed] [Google Scholar]
- 36.Bulmer JN, Rasheed FN, Francis N, Morrison L, Greenwood BM. Placental malaria. I. Pathological classification. Histopathology. 1993;22(3):211–8. Epub 1993/03/01. . [DOI] [PubMed] [Google Scholar]
- 37.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–9. [PubMed] [Google Scholar]
- 38.Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, et al. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31(1):85–93. Epub 2000/02/09. . [DOI] [PubMed] [Google Scholar]
- 39.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol. 1982;109(3):330–42. Epub 1982/12/01. ; PubMed Central PMCID: PMC1916118. [PMC free article] [PubMed] [Google Scholar]
- 40.McGready R, Davison BB, Stepniewska K, Cho T, Shee H, Brockman A, et al. The effects of Plasmodium falciparum and P. vivax infections on placental histopathology in an area of low malaria transmission. Am J Trop Med Hyg. 2004;70(4):398–407. Epub 2004/04/22. . [PubMed] [Google Scholar]
- 41.Stanisic DI, Moore KA, Baiwog F, Ura A, Clapham C, King CL, et al. Risk factors for malaria and adverse birth outcomes in a prospective cohort of pregnant women resident in a high malaria transmission area of Papua New Guinea. Trans R Soc Trop Med Hyg. 2015;109(5):313–24. Epub 2015/03/12. doi: 10.1093/trstmh/trv019 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villegas L, McGready R, Htway M, Paw MK, Pimanpanarak M, Arunjerdja R, et al. Chloroquine prophylaxis against vivax malaria in pregnancy: a randomized, double-blind, placebo-controlled trial. Trop Med Int Health. 2007;12(2):209–18. Epub 2007/02/16. doi: 10.1111/j.1365-3156.2006.01778.x . [DOI] [PubMed] [Google Scholar]
- 43.Fowkes FJ, McGready R, Cross NJ, Hommel M, Simpson JA, Elliott SR, et al. New insights into acquisition, boosting and longevity of immunity to malaria in pregnant women. J Infect Dis. 2012;206(10):1612–21. Epub 2012/09/12. doi: 10.1093/infdis/jis566 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119(3):417–23. Epub 1991/09/01. . [DOI] [PubMed] [Google Scholar]
- 45.Dubowitz LM, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970;77(1):1–10. Epub 1970/07/01. . [DOI] [PubMed] [Google Scholar]
- 46.Nosten F, McGready R, Simpson JA, Thwai KL, Balkan S, Cho T, et al. Effects of Plasmodium vivax malaria in pregnancy. Lancet. 1999;354(9178):546–9. . Language Code: eng. Date Revised: 20090929. Date Created: 19990914. Date Completed: 19990914. Update Code: 20111122. Publication Type: Comparative Study. [DOI] [PubMed] [Google Scholar]
- 47.Moore KA, Simpson JA, Thomas KH, Rijken MJ, White LJ, Lu Moo Dwell S, et al. Estimating Gestational Age in Late Presenters to Antenatal Care in a Resource-Limited Setting on the Thai-Myanmar Border. PLoS ONE. 2015;10(6):e0131025 Epub 2015/06/27. doi: 10.1371/journal.pone.0131025 ; PubMed Central PMCID: PMC4482646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verhoeff FH, Milligan P, Brabin BJ, Mlanga S, Nakoma V. Gestational age assessment by nurses in a developing country using the Ballard method, external criteria only. Ann Trop Paediatr. 1997;17(4):333–42. Epub 1998/05/14. . [DOI] [PubMed] [Google Scholar]
- 49.Sunjoh F, Njamnshi AK, Tietche F, Kago I. Assessment of gestational age in the Cameroonian newborn infant: a comparison of four scoring methods. J Trop Pediatr. 2004;50(5):285–91. Epub 2004/10/30. . [DOI] [PubMed] [Google Scholar]
- 50.Wylie BJ, Kalilani-Phiri L, Madanitsa M, Membe G, Nyirenda O, Mawindo P, et al. Gestational age assessment in malaria pregnancy cohorts: a prospective ultrasound demonstration project in Malawi. Malar J. 2013;12:183 Epub 2013/06/06. doi: 10.1186/1475-2875-12-183 ; PubMed Central PMCID: PMC3679840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev. 2016;40(3):343–72. Epub 2016/02/03. doi: 10.1093/femsre/fuw001 ; PubMed Central PMCID: PMC4852283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srivastava A, Gangnard S, Round A, Dechavanne S, Juillerat A, Raynal B, et al. Full-length extracellular region of the var2CSA variant of PfEMP1 is required for specific, high-affinity binding to CSA. Proc Natl Acad Sci U S A. 2010;107(11):4884–9. Epub 2010/03/03. doi: 10.1073/pnas.1000951107 ; PubMed Central PMCID: PMC2841952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tongren JE, Drakeley CJ, McDonald SLR, Reyburn HG, Manjurano A, Nkya WMM, et al. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun. 2006;74(1):257–64. doi: 10.1128/IAI.74.1.257-264.2006 . Language Code: eng. Date Revised: 20091118. Date Created: 20051221. Date Completed: 20060207. Update Code: 20111122. Publication Type: Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Unger HW, Ome-Kaius M, Wangnapi RA, Umbers AJ, Hanieh S, Suen CS, et al. Sulphadoxine-pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: a randomised controlled trial. BMC Med. 2015;13:9 Epub 2015/01/17. doi: 10.1186/s12916-014-0258-3 ; PubMed Central PMCID: PMC4305224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plewes K, Lee T, Kajeechewa L, Thwin MM, Lee SJ, Carrara VI, et al. Low seroprevalence of HIV and syphilis in pregnant women in refugee camps on the Thai-Burma border. Int J STD AIDS. 2008;19(12):833–7. Epub 2008/12/04. doi: 10.1258/ijsa.2008.008034 . [DOI] [PubMed] [Google Scholar]
- 56.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646 Epub 2012/01/12. doi: 10.1155/2012/985646 ; PubMed Central PMCID: PMC3251916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker PG, Griffin JT, Cairns M, Rogerson SJ, van Eijk AM, Ter Kuile F, et al. A model of parity-dependent immunity to placental malaria. Nat Commun. 2013;4:1609 Epub 2013/03/21. doi: 10.1038/ncomms2605 ; PubMed Central PMCID: PMC3615483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaikitgosiyakul S, Rijken MJ, Muehlenbachs A, Lee SJ, Chaisri U, Viriyavejakul P, et al. A morphometric and histological study of placental malaria shows significant changes to villous architecture in both Plasmodium falciparum and Plasmodium vivax infection. Malar J. 2014;13:4 Epub 2014/01/07. doi: 10.1186/1475-2875-13-4 ; PubMed Central PMCID: PMC3900675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Souza RM, Ataide R, Dombrowski JG, Ippolito V, Aitken EH, Valle SN, et al. Placental histopathological changes associated with Plasmodium vivax infection during pregnancy. PLoS Negl Trop Dis. 2013;7(2):e2071 Epub 2013/03/06. doi: 10.1371/journal.pntd.0002071 ; PubMed Central PMCID: PMC3573078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bulmer JN, Rasheed FN, Morrison L, Francis N, Greenwood BM. Placental malaria. II. A semi-quantitative investigation of the pathological features. Histopathology. 1993;22(3):219–25. Epub 1993/03/01. . [DOI] [PubMed] [Google Scholar]
- 61.Chandrasiri UP, Chua CL, Umbers AJ, Chaluluka E, Glazier JD, Rogerson SJ, et al. Insight into the pathogenesis of fetal growth restriction in placental malaria: decreased placental glucose transporter isoform 1 expression. J Infect Dis. 2014;209(10):1663–7. Epub 2013/12/12. doi: 10.1093/infdis/jit803 . [DOI] [PubMed] [Google Scholar]
- 62.Boeuf P, Aitken EH, Chandrasiri U, Chua CL, McInerney B, McQuade L, et al. Plasmodium falciparum malaria elicits inflammatory responses that dysregulate placental amino acid transport. PLoS Pathog. 2013;9(2):e1003153 Epub 2013/02/15. doi: 10.1371/journal.ppat.1003153 ; PubMed Central PMCID: PMC3567154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stapleton NM, Andersen JT, Stemerding AM, Bjarnarson SP, Verheul RC, Gerritsen J, et al. Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat Commun. 2011;2:599 Epub 2011/12/22. doi: 10.1038/ncomms1608 ; PubMed Central PMCID: PMC3247843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Scatter plots of cord and maternal IgG1 and IgG3 levels against PfEBA175RII, PfAMA1, PfMSP2, PfDBL5 and Measles. Samples from Alexishafen, Papua New Guinea are denoted as green closed circles and from Shoklo Malaria Research Unit, Thailand-Myanmar Border Area as closed red circles. Spearman ρ values (IgG1 and IgG3): PfEBA175RII (0.91,0.96); PfAMA1 (0.89,0.95); PfMSP2 (0.94,0.94); PfDBL5 (0.79,0.78); Measles (0.87,0.86).
(TIF)
Estimates and 95% confidence intervals are presented for the mean difference in log2 cord antibody levels after adjustment for log2 maternal antibody levels for (A) primigravid or (B) multigravid mothers with a P. falciparum placental infection without monocyte infiltrate (orange triangles, n = 22 and n = 59 in primigravid and multigravida respectively) or a P. falciparum placental infection with monocyte infiltrate (blue triangles, n = 23 and n = 8 in primigravid and multigravida respectively) compared to mothers with placentas with no P. falciparum parasites present. Dashed line at y = 0 indicates no difference in mean log2 cord antibody levels.
(TIF)
Data Availability Statement
All relevant data is within the paper and its Supporting Information files.


