Abstract
We study through a reaction-diffusion algorithm the influence of landscape diversity on the efficiency of search dynamics. Remarkably, the identical optimal search strategy arises in a wide variety of environments, provided the target density is sparse and the searcher’s information is restricted to its close vicinity. Our results strongly impact the current debate on the emergentist vs. evolutionary origins of animal foraging. The inherent character of the optimal solution (i.e., independent on the landscape for the broad scenarios assumed here) suggests an interpretation favoring the evolutionary view, as originally implied by the Lévy flight foraging hypothesis. The latter states that, under conditions of scarcity of information and sparse resources, some organisms must have evolved to exploit optimal strategies characterized by heavy-tailed truncated power-law distributions of move lengths. These results strongly suggest that Lévy strategies—and hence the selection pressure for the relevant adaptations—are robust with respect to large changes in habitat. In contrast, the usual emergentist explanation seems not able to explain how very similar Lévy walks can emerge from all the distinct non-Lévy foraging strategies that are needed for the observed large variety of specific environments. We also report that deviations from Lévy can take place in plentiful ecosystems, where locomotion truncation is very frequent due to high encounter rates. So, in this case normal diffusion strategies—performing as effectively as the optimal one—can naturally emerge from Lévy. Our results constitute the strongest theoretical evidence to date supporting the evolutionary origins of experimentally observed Lévy walks.
Author summary
How organisms improve the search for food, mates, etc., is a key factor to their survival. Mathematically, the best strategy to look for randomly distributed re-visitable resources—under scarce information and sparse conditions—results from Lévy distributions of move lengths (the probability of taking a step ℓ is proportional to 1/ℓ2). Today it is well established that many animal species in different habitats do perform Lévy foraging. This fact has raised a heated debate, viz., the emergent versus evolutionary hypotheses. For the former, a Lévy foraging is an emergent property, a consequence of searcher-environment interactions: certain landscapes induce Lévy patterns, but others not. In this view, the optimal strategy depends on the particular habitat. The evolutionary explanation, in contrast, is that Lévy foraging strategies are adaptations that evolved via natural selection. In this article, through simulations we exhaustively analyze the influence of distinct environments on the foraging efficiency. We find that the optimal procedure is the same in all situations, provided density is low and landscape information is scarce. So, the best search strategy is remarkably independent of details. These results constitute the strongest theoretical evidence to date supporting the evolutionary origins of experimentally observed Lévy walks.
Introduction
How organisms improve—or even optimize—the search efficiency of randomly distributed resource targets is a key question in the study of several biological processes (e.g., foraging and pollination). The search efficiency may even determine matters such as the individuals’ survival and the viability of future generations [1, 2]. The understanding of the rules that guide animal foraging and the unveiling of the main factors leading to successful strategies are crucial topics in behavioral ecology [1–8]. Random search models have been largely employed in the last decades to address these and related issues [1, 2]. In most cases, the quest for an efficient search strategy requires the explicit or implicit definition of a proper choice for the efficiency function η, whose maximization constitutes the focus of the optimal foraging theory [1, 2, 5–9].
Thanks to the recent advances in the acquisition of huge amounts of empirical data [10–30] and to the great improvement in the statistical methods of data analysis and statistical inference [30–45], it is now settled [10–30] that several species do perform Lévy walk foraging in many circumstances. In other words, the tail of the distribution of their move lengths—while looking for targets such as food, mates, sheltering, territory invaders, etc.—is well described by a truncated power-law function.
The earliest suggestions that Lévy processes could be related to the movement of organisms, based on empirical studies, date back to the late 1980’s [46–48]. In the 1990’s the first theoretical framework to explain this evidence appeared in the form of the so-called Lévy flight foraging hypothesis [1, 49]: Organisms must have evolved via natural selection to exploit search strategies that optimize the search efficiency provided by Lévy distributions of move lengths under scarce information and sparse targets conditions (for a detailed discussion, see, e.g., [50]).
Since in several contexts the existence of Lévy foraging has ceased to be a matter of debate, other fundamental questions have progressively come into the spotlight [50–60] (but see [61]). For example, under precisely which circumstances is Lévy flight foraging advantageous over Brownian searches (e.g. described by exponential or gamma distributed move lengths) is still an ongoing issue. Another central question concerns the underlying mechanism of Lévy foraging as having emergent rather than evolutionary origins [50, 51]. According to the former, a Lévy foraging pattern should arise as an emergent property, i.e. a side-effect of complex interactions between the searcher and the environment. The evolutionary explanation, in contrast, is that Lévy foraging strategies are adaptations that evolved via natural selection.
This matter remains controversial as recent results have claimed support for both emergentist [14, 20, 21, 32, 62–68] and evolutionary [12, 16, 33, 69–76] views. As already emphasized, optimal or even suboptimal search outcomes are essential to guarantee the endurance of an individual or a species. Therefore, the question about the emergent versus evolutionary origin of the empirically observed Lévy foraging behavior becomes crucial.
Here we address this issue by investigating—through random search ideas and a (very general) reaction-diffusion model—the influence of distinct landscapes on the optimal foraging strategy. The searcher’s degree of diffusivity is controlled by the power-law exponent μ of the distribution of step lengths, P(ℓ) ∼ ℓ−μ. Hence, ballistic, superdiffusive, or diffusive search strategies correspond to μ → 1, 1 < μ < 3, or μ ≥ 3, respectively [1]. The reactive component is represented by the detection and consumption of target sites once they enter the searcher’s perceptive range.
We perform an extensive analysis of the search efficiency in a great diversity of heterogeneous landscapes, namely: (i) nonuniform patches containing homogeneous distributions of targets; (ii) targets distributed fractality as a Lévy dust; and (iii) Lévy dusts of both patches and inner targets. A large collection of assorted environments is thus studied by varying the density of targets, number and size of patches, degree of heterogeneity and fragmentation, and fractal dimension of the Lévy dusts (including the homogeneous distribution of targets as a relevant limit case).
The emergentist view, relying mostly on the presence of complex searcher-targets interactions, should be sensitive (and respond accordingly) to the specificities of the landscape. Therefore, diverse optimal search strategies should arise in rather distinct environments: the best search ‘protocol’ would depend on the particular habitat, does not existing an universal (good in all instances) behavior. In contrast, our main result is that the optimal search strategy is essentially the same in all situations considered, provided the targets density is sparse and environment information is scarce. In other words, as long as targets are not often found within the searcher’s perceptive range and leaving the present spot to look for resources remains a major activity, the general properties of the best search strategy appear to be remarkably independent of landscape details.
This basically “insensitive” character of the optimal strategy then suggests the interpretation favoring the evolutionary view, which concurs with the Lévy flight foraging hypothesis. Indeed, as we also report, deviations from this behavior are shown to be relevant in plentiful environments, in which the importance of extensive searching is reduced. Nevertheless, an alternative (though less probable) scenario supporting the emergentist view would be that in which essentially the same optimal Lévy foraging strategy arises in rather diverse landscapes, even considering the distinct specificities of the searcher-environment interactions (or trade-offs) in each particular case.
Such questions have led to a lively debate in the literature. We cite here only 3 such examples.
In an article provocatively titled “Ultimate failure of the Lévy Foraging Hypothesis”, Benhamou and Collet have argued that composite Brownian walks consisting of two scales are better than Lévy walks [77]. However, scale-specific strategies presupposes that the foragers have some information about the target distribution. Thus, the direct comparison with clueless Lévy walks should be viewed with a certain reservation. On the other hand, they claim correctly that natural selection should favor the evolution of search strategies for which environmental feedback is possible, but conclude that “strange” scale-free kinetics are somehow ruled out without giving plausible reasons for so. We disagree that scale-free kinetics cannot have evolved and as evidence cite the recent work of Gutiérrez and Cabrera involving a conductance-based neural coding scheme that reproduces foraging trajectories [78]. Moreover, for searchers with high cognitive skills see [79, 80]. In any case, the results we report below show that environmental feedback is most likely present in a wide variety of landscapes, still, organisms get rewarded for choosing generalistic optimal or near-optimal strategies.
Pyke, in an article subtitled “It’s time to abandon the Lévy foraging hypothesis”, criticizes many aspects of the hypothesis [51]. A rebuttal of all the criticisms is beyond the scope of the present work. We merely point out here that if the evolutionary view discussed above is even partially correct, it may be premature to abandon the hypothesis. The results we report next strongly support the evolutionary view.
Finally, we mention the recent work of Reynolds, who has argued that constraining the research on Lévy walks to the confines of optimal foraging theory can be restrictive [60]. This is a valid point. We have argued in [50] that the complete answer may involve a combination of both the evolutionary and the emergentist views.
We organize this work as follows. First, we present a brief description of certain relevant aspects associated to the foraging behavior under the emergent and evolutionary points of view. Second, we build the diverse heterogeneous landscapes in which the searches take place. And third, we define the general random search model to be employed. Then, the results for the efficiency of search strategies in each of these landscapes are discussed and their connection with the issue of emergent vs. evolutionary foraging behavior is established. Finally, ending remarks and conclusions are drawn.
Factors associated to the origin of efficient foraging behavior
Emergence and evolutionary pressure
Perhaps, the most fundamental question regarding efficiency in foraging—or more generally in biological encounters (as discussed, e.g., in [81, 82])—is the real necessity for optimal strategies. Animals can display complex and rich habits, associated to the multiple tasks they must accomplish. Foraging is certainly one of them. But to develop proper characteristics (metabolical, morphological, cognitive, ecological, etc) allowing an effective random search strategy should be the case only if foraging becomes a bottleneck for survival.
Evolution through natural selection is probably the simplest yet most powerful universal mechanism driving the huge diversity and great adaptability of species on all scales, from isolated groups of species in small islands to metapopulations in biomes. Generally, selective pressure does not act (or acts only mildly) on a certain feature if it is irrelevant for the fitness of the individuals. In this context, a particular trait, say a specific foraging behavior, may be a side-effect of another evolutionary process, as proposed in [60]. Or it may just be an emergent trade-off, a direct response to the environment. But note that emergent behavior does not imperatively lead to advantageous strategies [83, 84]. This can be the case, although only fortuitously by coincidence [85, 86].
A point barely discussed in the foraging literature, however greatly emphasized in population dynamics (see, e.g. [87]), concerns the type of patterns arising in terms of the individuals responses to the environment. They are distinct if such responses are either local or global. In fact, according to the so called Moran effect [88, 89]—related to the habitat as a whole (a rather relevant aspect when the random search takes place in a reasonable fraction of it)—an important ingredient to induce emergence is the build-up of an awareness by the forager of long-range correlations existing in the landscape. Only immediate and local responses to stochastic stimuli imposes serious restrictions for the rise of a full extended environment-related behavior [90], of the kind conceived as an alternative to the evolutionary view [32, 62–68].
The above considerations bring an extra ingredient into the discussion. Either (i) encounter rate optimization is not needed or else (ii) it is needed, leading to selection pressures. We consider both cases below.
Suppose first there is no pressure for higher biological encounter rates. As already observed, then for a “brimming” emergent behavior an individual must possess certain inherent skills (like long-term memory and/or specific long-range detection power) to perceive territorial variations on a large enough scale. In some instances, the correct abilities might have evolved due to unrelated evolutionary forces, i.e. a matter of “lucky” coincidence [60]. But in others, such abilities may not be useful at all in a given landscape. Thus such abilities may not arise in the first place—hence may not bolster emergent Lévy foraging. In this case, emergence in the Lévy foraging context would not be ubiquitous.
On the contrary, if optimization is needed, the innate emergent behavior in a certain ecosystem can arise only if it corresponds to a good foraging strategy. In each distinct landscape, the necessary “proficiency” to identify the habitats’ long-range structures might be different, e.g., to spot spatial correlations of resources in the African savanna certainly requires features and traits different from those necessary to do the same in the Amazon rainforest. Pure emergence leading to optimal strategies thus demands the extra-ordinary coincidence of fortuitous matches between habitats and just the right kinds of traits. Such coincidences would be analogous to demanding eyesight to have fortuitously “emerged” independently in different mammals living in widely differing habitats as a side-effect of distinct unrelated adaptations, rather than having evolved as an adaptation via natural selection. Given the huge range of situations, although such lucky coincidences can take place, they must be extremely rare.
The evolutionary robustness of foraging strategies
Macroevolution displays a rather interesting dynamics, where bursts of high evolutionary variations and extinctions (hectic phase) are followed by long periods of metastable configurations (stasis phase). This intermittency, which has become known as punctuated equilibrium [91], is well established from the analysis of fossil records [92–94]. Regardless the basic reasons for such a pattern [95], it strongly contrasts with what should be expected from the natural selection taking place at the much shorter scales of several generations [96, 97]. Hence, pathways of normal evolution—allowing gradual adjustments to a varying habitat—might represent a somehow limited resilience mechanism if considered at long times scales [92, 93]. Indeed, depending on the magnitude and rate of the changes in the ecosystem (e.g., with the potential of strongly unbalance the trophic interactions [98]) incremental skills acquired through natural selection could not suffice to preclude intensive extinction [99].
Among distinct evolutionary behaviors, however, one can think about an hierarchical order [50], with the existence of those granting a high power of adaptability [100]. These features, which are more plastic in the sense of being more robust to distinct environmental configurations, once incorporated into the individuals habits are likely do not suffer subsequent drastic alterations [101]. For instance, specific characteristics of shorebirds are strongly related to foraging behavior as it concerns the type of food they need to detect and handle [102], e.g. the case of bill length determined by the type of hunting, either visual or tactile [103]. On the other hand, certain general morphologic aspects (as the hindlimb structure) does not seem to have affected along the time the foraging movement strategies of shorebirds [103].
A particularly intriguing evolutionary history is that of the neornithine. This whole crown group is believed to have fairly similar behaviors of the modern birds [104] since their extensive diversification in the late Cretaceous [105]. So, they have gone through the huge extinctions of the K/Pg boundary probably maintaining many of their ecological traits. It indicates strong endurance of their original behaviors [101]. In fact, Lévy foraging strategies are common among Aves [16, 25, 29, 106], a class in which flexible and generalist foraging strategies are not unusual, as exemplified by frugivorous birds [106].
It is worth mentioning that trace fossils dating fifty million years ago [22] have given support to such idea associating Lévy walks to the origin of optimal search behavior. Furthermore, present day communities still maintaining the hunter-gatherer tradition may constitute a nice window into the human past evolution. So, the fact that the Ju/’hoansi of Botswana and Namibia [107] and the Hadza of Tanzania [108] follow Lévy walk foraging patterns in their activities is another evidence for the Lévy hypothesis.
Important features of efficient foraging strategies, Lévy walks and other alternative search behaviors
There are situations where the specific foraging behavior is irrelevant [109]. In essence, either because energetic resources are plentiful or when enough information, cognitive power and strong detection skills preclude the necessity of a complete random search. In contrast, in (a) clueless scenarios and (b) scarce conditions [1, 50], the particular features of searching may constitute the difference between surviving or perishing [110]. In these cases, probably the most important characteristics that any foraging “protocol” should possess are superdiffusion and scale invariance (see, e.g., [9, 75])—incidentally, two inherent properties of unrestricted Lévy strategies [1].
At low densities, in fact, typical Brownian-like motion (leading to normal diffusion) cannot account for an extensive territory exploration, necessary to find enough quantities of targets (e.g, resources). At the same token, for no information about eventual targets distribution patterns (defining typical spatial scales in the environment) or if the resources dynamics is sufficiently fast (not allowing specific targets spatial arrangements), then a foraging behavior too focused on specific spatial scales can be a disadvantage. Hence, a better (eventually optimal) solution would be to adopt scale invariant strategies.
These properties, especially superdiffusion, can be obtained from a variety of random walks, but there are important technical limitations. For instance, correlated random walks (CRWs)—whose step lengths have well defined mean and variance and the turning angles are (Markovian) correlated, so with a certain directional bias—can display superdiffusion, but up to a limiting time τ [111]. Actually, there are concrete conditions determining up to which τ a given random walk can maintain superdiffusion [44]. If the biological relevant time T involved in the foraging is longer than τ, then such strategy (considering (a) and (b) above) may be worthless. Remarkably, truncated Lévy walks, more commonly used as models for animal foraging [1], do present τ’s which are extremely long [112]. Thus, they are able to hold supperdifusion in many long foraging contexts [113, 114]. Furthermore, for large time scales the value of T can suffer important fluctuations. So, a robust and lasting strategy should have huge τ’s, the case of truncated Lévy walks (for usual Lévy walks τ is in fact infinite).
In a landmark work [115], it has been shown how certain kinds of Lévy flights could be written in terms of nondifferentiable infinite series, known as the Weierstrass function. This fundamental technical result (further explored in different instances, e.g, [116–118]) gives the theoretical support for a relevant proposal of composite random walks (CompRWs) in ecology (cf. [32, 119, 120]).
For a brief contextualization, let us assume the non-truncated case in 1D (a general mathematical analysis comparing CompRW with truncated and non-truncated Lévy walks will be the subject of a future contribution). In general terms, for a step length probability density function given by (for ℓ0 ≤ ℓ < ∞, with ℓ0 a lower cut-off: there is no meaning to take random steps smaller than a given distance ℓ0, related to an individual biological characteristics) [118, 121]
| (1) |
The normalization imposes that ∑n wn = 1. Here the ℓn’s are typical length scales and the wn’s the corresponding weights of the Pcomp “modes” n. Observe, therefore, that we have 2N free parameters: the N lengths ℓn, N − 1 weights wn (recall the normalization condition) and N itself. For Eq (1), the shifted first (second) moment of Pcomp, given the average step length (a quantity related to the step lengths variance) reads
| (2) |
By the central limit theorem, any distribution having finite first and second moments will converge to normal diffusion after a certain number of steps determined by τ. Thus, from the realistic assumption that all ℓn’s are finite, we can have true superdiffusion (i.e., for any time scale) only for an infinite number of modes. In this way, rigorously CompRWs can describe Lévy walks only in such N → ∞ limit. Nonetheless, it has been shown [121] that N does not need to be large for Pcomp to resemble power-law distributions (so Lévy-like), describing superdiffusive foraging for relatively long time scales.
Many foraging models, and Lévy walks are not an exception, assume that the searching is the only activity being performed by the individual. But surely ecological processes are not exclusive: while looking for targets, an animal may be forced to change the behavior for a while, say, when facing some unexpected event. But this does not mean that optimal foraging could not be pursued in multi-task assignments. For instance, a detailed study has shown that collective searching [122]—in which constraints, such as avoidance of group dispersal, are imposed—still can be profit from Lévy strategies, but then certain spatial correlations must be imposed. In such context, CompRW should be a rather interesting alternative [19, 121].
An instructive example is that of mussels movement, associated to involving individuals interactions (including both cooperation and competition). During intertidal flats, they arrange themselves in regularly spaced clumps [14]. In such complex environment with strong feedback (thus, far more sophisticated than the usually considered foraging scenario [1, 50]), it has been reported [14] that the locomotion shaping the patterned beds are well described by Lévy walks. Nevertheless, in a reexamination of the original experimental data, CompRWs seems to yield better fittings [43]. This should not be a great surprise; in fact, it illustrates that pure Lévy ‘motifs’ can be partially altered due to the group interplay [122]. Important here is that the difference between the truncated Lévy walk and CompRW observed in the fittings (Fig 1 (a) in [43]) would not lead to a significant difference between the resulting search efficiencies (see, e.g., a quantitative analysis of local correlation effects in Lévy searching outcomes in [123]). For the results of Fig 1 (b) in [43], the discrepancy is much more pronounced. However, in this case a larger data set is used. It includes many step lengths that are rather small (e.g., typically the mussels are 7 mm long and ℓ0 is set around 0.02 mm), specially considering an aquatic environment, a media that can induce involuntary movement. Hence, such a data may be considered with a certain care for the study of foraging efficiency.
The previous discussions bring about distinctions between Lévy walks and CompRWs in terms of random search optimization (but relevant to mention that selection pressures could render CompRWs to display certain characteristics of Lévy walks [124]). The fact is that to a great extent a foraging behavior taking fully advantage of the explicit form of Pcomp would be much more complex than Lévy strategies. Indeed, for an appropriate number of modes N, eventually an individual would be able to have a strategy incorporating the most important length scales in its habitat as well as the relative frequency of these scales (given by the wn’s). But then, built-in biological mechanisms should be available so that all the environment specificities could be identified. These mechanisms should be particularly refined in the case of changing environments, when the sets of wn’s and ℓn’s and even N are dynamical parameters—exactly the type of situation posing strong evolutionary challenges. Moreover, certain skills (like non-directional sensory cues [125]) are necessary to allow proper switchings between the modes of movement behavior. In this way, evolutionary CompRWs might represent a later stage to Lévy walks, with many hierarchical ecological processes successively forging the species full behavior through intermediate states.
Surely, it is plausible that some evolutionary paths would bring directly to composite random walks. However, the generality, independence on landscape details, extremely economical in terms of free parameters and ecological plasticity [126] makes Lévy walks the natural candidate in testing evolutionary versus emergent origin of optimal foraging.
The framework adopted here and some previous discussions in the literature
It becomes clear from the above the difficulty in establishing the genesis of efficient random search strategies. In fact, we share similar views of many others: that it is unlikely that an unique explanation can account for every imaginable scenario. However, solid and general trends in movement ecology may point to a common (although not universal) mechanism determining the origin of optimal foraging behavior in a large number of cases.
In this context, our starting point, or framework, is the Lévy flight foraging hypothesis (LFFH). We will demonstrate in a systematic way in this contribution that Lévy walks—in the form of an asymptotic power-law distribution of step lengths, with a single relevant parameter μ (see next section)—does lead to optimal searching efficiency in a remarkably broad range of landscapes. This fact shows clearly that Lévy walks are robust and adaptively plastic. This is distinct from emergence, where conceivably specific-environment driven forces would yield different kinds of foraging behavior.
Admittedly, dedicated strategies (DS) with fine tuned parameters (like CompRWs) may be able to provide good foraging efficiencies, but at a higher cost in an evolutionary sense. In fact, the proper traits required by DS may need more time and higher selection pressure to evolve. On the other hand, the biologically much simpler Lévy strategies (LS) (not demanding switching or long range sensorial clues) could go unchanged during habitat transformations. Thus, since LS are cheaper, throughout the evolutionary history one could expect, when the case, LS evolving to DS (LS → DS), but DS → LS would be less probable. Also, once the LS becomes an adaptation, the resulting efficiency might relieve the evolutionary pressure towards even better strategies. So, the commonly observed LS could be a relic or descendant of very ancient adaptations [22, 107, 108], whereas DS could have evolved from more recent processes. These views are in accordance with the ideas in [60] and also with a hierarchical LFFH proposal in [50].
Finally, we stress that the origins of optimality in Lévy searches is an old question [49] (e.g., surveyed in [1]). For more recent discussions, we observe the following. In [75] simulations in different types of environments have addressed how the intrinsic exploitation/exploration trade-offs of Lévy walks can lead, under certain conditions, to a higher efficiency than other models. In our work the focus is different. We show that an intrinsic Lévy walk behavior can provide overall better performance in diverse landscape structural geometries and argue that such plastic behavior, favoring adaptation, should have evolutionary origins. In the search for possible mechanisms yielding the high efficiencies of Lévy walks, it has been proposed that an effective intermittency—arising from singular temporal distribution of reorientation events—may be key for the optimal strategy [127]. This is in sound agreement with our analysis in the super-dense landscape (see the section: The super-dense limit): even when there is no need for a fine-tuned strategy, optimal Lévy walks with μ ∼ 2 result in slightly higher efficiency. Further, the possible mechanisms underlying Lévy walks have received great attention. For instance, swarm dynamics [128], biological simulated annealing [129], and even chaotic neuronal dynamics [28], have been pointed as potential candidates generating Lévy dynamics. Our present findings seem to indicate that all such processes could be incorporated as adaptations through natural selection.
Materials and methods
Search landscapes
Patchy landscapes [130–138], including some hierarchical structures consistent with scale-free fractal patterns [10, 139–144], are plentiful in nature. To model such environments, we consider a number of heterogeneous distributions of point-like targets placed in a two-dimensional squared space of side length M = 104 and periodic boundary conditions. The use of periodic boundary conditions leads to the same qualitative results as those of random searches in large spaces with limiting borders, but with the technical advantage of improving the statistical analysis. Here we are mostly interested in the study of the impact of the environments diversity on search dynamics under sparse conditions. We describe below the landscapes analyzed in this work. But also some discussion about the targets features is in order.
Heterogeneous patches with Euclidean distribution of targets
We start by defining landscapes characterized by the presence of Np circular patches indexed by p = 1, 2, …, Np, with radius R(p) and average distance between homogeneously (Euclidean) distributed inner targets. The total number of targets is Nt = 104 and the targets density in the pth patch is inversely proportional to . We mention that the statistical analysis of searches in these heterogeneous landscapes, as well as in the uniform case of patches with same R(p) and , has been partially performed in [81, 145]. Three types of configurations are considered:
Random sizes: patches with fixed and radii uniformly distributed in the interval 0.03M ≤ R(p) ≤ 0.3M, resulting in a distribution of numbers of targets per patch [see Fig 1(A) with Np = 10 patches];
Random targets densities: patches with fixed R(p) = 0.1M and targets densities uniformly distributed in the interval , also presenting a distribution of numbers of targets per patch [Fig 1(B)]; and
Random sizes and targets densities: targets equally distributed in the patches with different radii, 0.03M ≤ R(p) ≤ 0.3M, thus generating lower (higher) inner densities in larger (smaller) patches, with [Fig 1(C)].
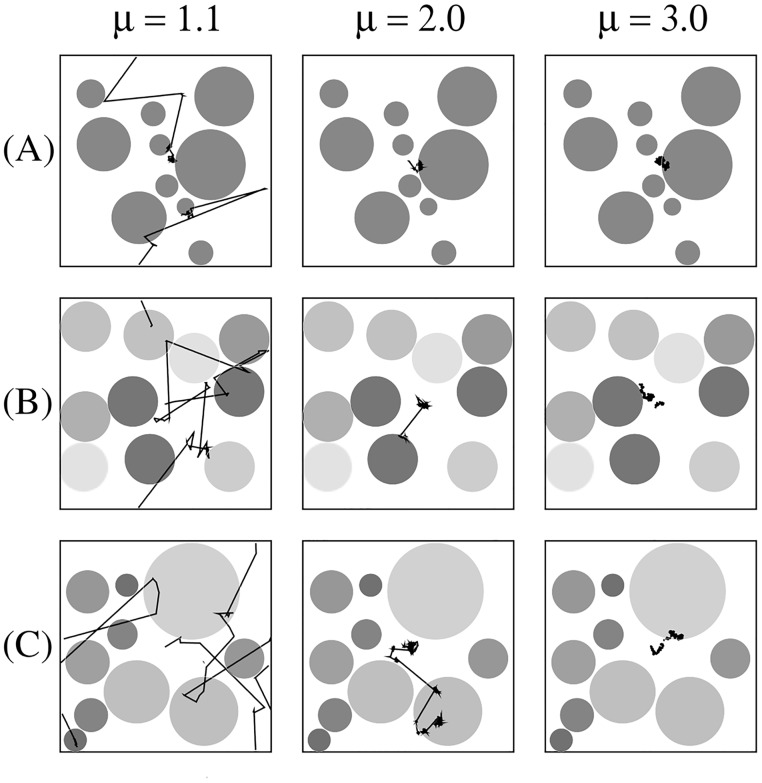
Fig 1. Heterogeneous search landscapes with representative trajectories of different strategies.
Fragmented search landscapes containing Nt = 104 targets placed in Np = 10 heterogeneous patches (gray regions) with: (A) same average distance between inner targets, , and radii uniformly distributed in the range 0.03M ≤ R(p) ≤ 0.3M, M = 104; (B) same radius, R(p) = 0.1M, and uniformly distributed in the range ; and (C) distinct sizes uniformly distributed in the range 0.03M ≤ R(p) ≤ 0.3M, but fixed number (103) of inner targets per patch, so that . The darker the patch, the higher its homogeneous density of inner targets. We also show typical paths of a searcher with power-law (Lévy-like) distributions of step lengths displaying different degrees of diffusivity: nearly ballistic (μ = 1.1), superdiffusive (μ = 2.0), and Brownian (μ = 3.0). In this illustrative example the search ends upon the finding of only 10 targets.
As we set the searcher’s perceptive range to unity, we consider that values and ∼ 102 can be respectively assigned to locally dense and sparse targets distributions. In each case above, we have also analyzed less fragmented landscapes with a smaller number Np = 5 of denser patches (see Results below).
Lévy dust distribution of targets
We also considered a distribution of Nt = 104 targets whose positions form a so-called Lévy dust. In this case, the targets locations correspond [46, 146, 147] to the points visited by a Lévy walker (not to be confounded with the searcher itself) following the probability density function (pdf) of move sizes d given by
| (3) |
and homogeneous distribution of turning angles in the interval [0, 2π). Above, the parameter d0 = 1 (dmax = M) represents the smallest (largest) move allowed, and At is the normalization constant. In the large-Nt limit, the set of points of the Lévy dust has scale-free properties within the interval (d0, dmax), and fractal dimension df = β − 1 [46] (we observe that for actual environments with fractal distributed resources the reader can see, e.g., [148, 149]). The β = 3 Brownian limit corresponds to the Euclidean aggregation of targets in two-dimensional space. Some examples are illustrated in Fig 2. Notice that larger values of β imply denser clusterings of targets occupying a relatively small fraction of the search space [see, e.g., Fig 2(D)]. On the other hand, a nearly homogeneous landscape results from the case β = 1.1, as seen in Fig 2(A). This feature allows an interesting comparison with the random searches performed in homogeneous environments, the earliest scenarios studied in the context of Lévy foraging [49].
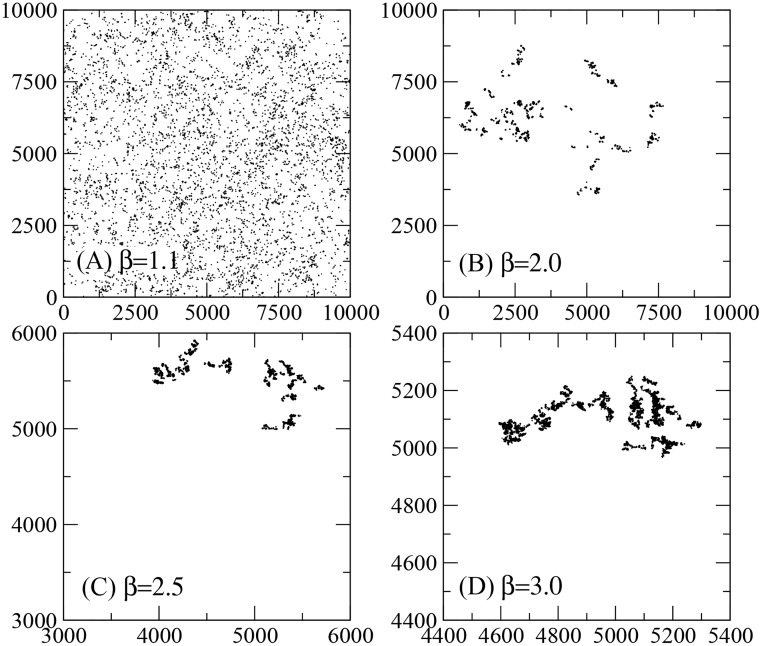
Fig 2. Lévy dust distribution of targets.
Search landscapes containing Lévy dust distributions of Nt = 104 targets (see main text), drawn from Eq (1) with d0 = 1, dmax = M = 104, and (A) β = 1.1, (B) β = 2.0, (C) β = 2.5, and (D) β = 3.0. Larger values of β increase the degree of clustering of targets. The bouncing of coordinates technique applied to the β = 1.1 case results in a nearly homogeneous targets distribution.
We observe that, due to the finite landscape, border effects should be carefully considered along the construction of the Lévy dust. Indeed, the frequency of ultra-long steps increases as β approaches unity. The undesirable situation in which the end coordinates of a given move eventually reach a region beyond the borders of the environment must be fixed. In order to do it, we have applied the bouncing of coordinates technique [147].
Lévy dust distributions of patches and inner targets
The previous types of landscapes can be combined to create a great diversity of environments with heterogeneous (Lévy dust) distributions of both patches and targets.
We now place Nt targets equitably among Np patches. Inside each patch, the targets are distributed as a Lévy dust of exponent β, as in Eq (3). Moreover, the patches “seeds” (or centers) also form a Lévy dust characterized by another exponent γ, i.e. the distances r between the patches seeds are drawn from the pdf
| (4) |
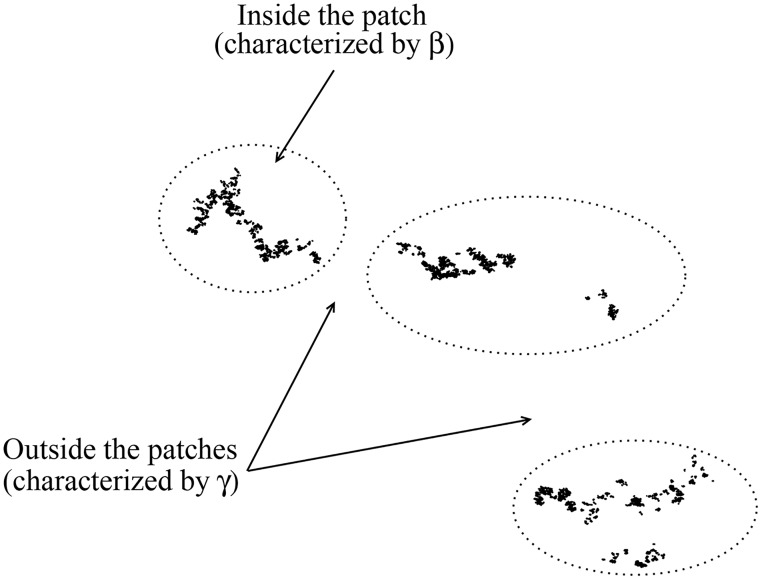
where Ap denotes the normalization constant. A careful choice of parameters is necessary in order to avoid overlap of patches: the inferior limit r0 should be larger than the typical size of the patch, whereas the superior limit rmax should be naturally smaller than the environment length. An example is shown in Fig 3 for Np = 3 and Nt = 15000 (5000 targets per patch), with distributions of patches and targets, respectively, set by γ = 2.0, r0 = 500 and rmax = M, and β = 2.5, d0 = 2 and dmax = M/10. In this case, it no longer makes sense to define the radius R(p) of a patch, since the parameter β governs the degree of clustering of the targets distribution within each patch (the dotted lines in Fig 3 are only a visual guide).
Fig 3. Construction of a fractal patch environment.
Illustration of a search landscape with Np = 3 patches and Nt = 15000 targets (5000 targets per patch), forming Lévy dust distributions (see main text). Here, β = 2.5, d0 = 2, dmax = M/10 in Eq (1), and γ = 2.0, r0 = 500, rmax = M = 104 in Eq (2). The parameters are chosen so that the patches do not overlap. Dotted lines are only a guide to visually delimit the patches regions.
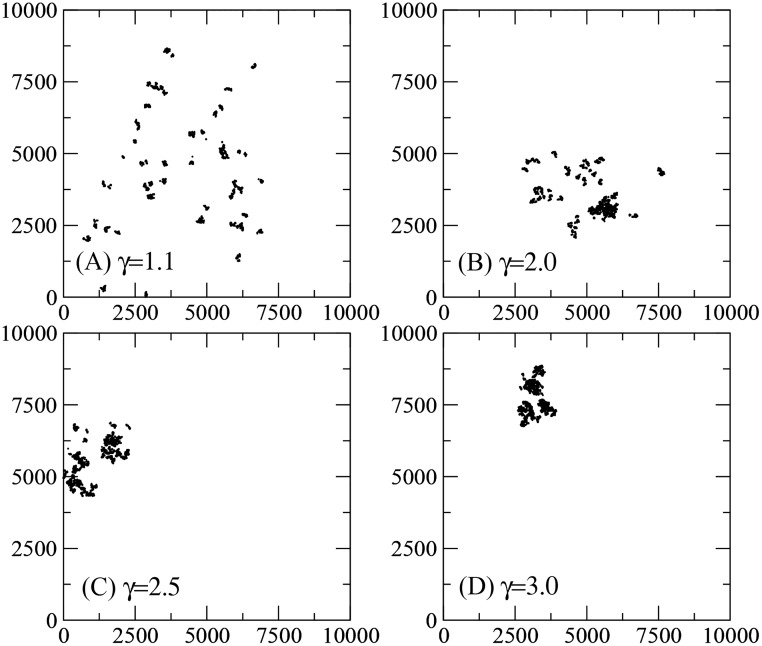
In Fig 4 Np = 50 patches are depicted (this time no dotted lines helping to delimit the patches are displayed), with a total of Nt = 50000 targets (1000 targets per patch) distributed according to β = 3.0, d0 = 2 and dmax = M/10. Four arrangements of Lévy dusts of patches are shown, with r0 = 500, dmax = M, and γ = 1.1, 2.0, 2.5, 3.0. Notice that a value of γ close to unity gives rise to a more widespread distribution of patches, occupying a larger area of the landscape. In contrast, for γ = 3 the patches are so close that one cannot distinguish them only by visual inspection. In this case, a large empty space surrounds the region rich in targets.
Fig 4. Fractal patches obtained by combining two Lévy dust distributions.
Search landscapes containing Lévy dust distributions located in Np = 50 patches. Here, Nt = 50000 (1000 targets per patch), β = 3.0, d0 = 2, dmax = M/10 in Eq (1), and r0 = 100, rmax = M = 104, (A) γ = 1.1, (B) γ = 2.0, (C) γ = 2.5, (D) γ = 3.0, in Eq (2). For large γ the patches are so close that one cannot distinguish them only by visual inspection.
Search dynamics and efficiency
Once the several search landscapes have been defined, we next consider the random search model used to evaluate the performance of distinct search strategies.
The searcher starts from a random position in the depleted region outside the patches. The step lengths ℓ are taken from the truncated power-law (Lévy-like) pdf
| (5) |
with B denoting the normalization constant, and the turning angles are homogeneously distributed in the interval [0, 2π). The searcher can detect a target within a unit perceptive range rv = 1, with unlimited visits allowed to the targets (but see the next Section). This regime has been termed non-destructive in the literature [1, 49]. The step is truncated if the searcher encounters a target within its perceptive range rv before traversing the distance ℓ. We choose ℓ0 = rv, since search steps shorter than the perceptive range are meaningless, and ℓmax = M corresponding to the limit scale of the environment. In fact, it is clear that unbounded (infinite) displacements are naturally forbidden in realistic searches.
Eq (5) with ℓmax → ∞ corresponds to the asymptotic large-ℓ limit of Lévy α-stable distributions governed by the generalized central limit theorem (CLT), with Lévy index 0 < α = μ − 1 ≤ 2 [1, 2]. Extremely ballistic and superdiffusive random walk dynamics are represented by μ → 1 and 1 < μ < 3, respectively. In contrast, values μ ≥ 3 lead to the α = 2 Brownian (diffusive) dynamics due to the CLT, whereas μ < 1 does not correspond to normalizable functions. In the present case of a large but finite ℓmax, if steps that end without a target detection outnumber considerably the truncations by targets encounters—as it happens under sparse conditions—then the heavy-tailed Lévy statistical properties of the move lengths distribution actually sustain for a huge number of steps, with ultraslow convergence to the CLT [112].
An appropriate statistical definition of the search efficiency [1, 49] is the ratio between the number of targets found and the total distance traversed during the search, η = Nfound/Ltotal. Thus, η is equivalent to the inverse of the average distance traveled between successive encounters.
For the numerical simulations, each random search lasts until the searcher encounters Nfound = 104 targets. Averages are taken over 2.5 × 103 runs, with each run corresponding to a landscape realization defined by a given set of parameters (R(p), , β, γ, etc.). In the next section we study some representative examples. In fact, we have also exhaustively tested many other different parameter sets, which actually led to the same qualitative conclusions discussed below.
On the targets features and optimal strategies
In the present work we study non-destructive targets (allowing unrestricted revisits, see above). A trivial example would be a tree with plenty of fruits. An animal could get a fruit (the target) and leave the site (the tree location). Any time later, it can come back to that site and get a new fruit, which is effectively the same non-destructive target. Nonetheless, there are many other possibilities that could also be addressed, and we will mention a few of them in the following.
But first, we emphasize that a Lévy strategy means that a forager is following the specific rules described above. Thus, the distribution of step lengths is always given by Eq (5), with 1 < μ ≤ 3. Note then that the whole family of walks is characterized by a single parameter μ. An optimal Lévy search means that there exists a particular value of μ = μopt, for which the searching efficiency is very high, actually the best value among all the possible μ’s. Since by varying μ we have a huge diversity of statistical features for the resulting walk patterns [1, 2], this simple Lévy strategy is ubiquitous in terms of distinct movement behavior (considering it is define by just an unique parameter).
The targets could be destructible, i.e., once a site is visited and the target is gathered, revisits to the now empty location are useless. Hence, for randomly distributed destructible targets, the optimal Lévy search strategy results from a ballistic-like dynamics, i.e., by setting μ → 1 (see, e.g., [1, 49, 150]). A more general situation is that of regenerative targets, in which a target site can become profitable again, but only after a certain regeneration (or delay) time τd. Then, for distinct landscapes, the optimal value of μ (ranging from 1 to 2) would depend only on the specific value of τd [151, 152]. Lastly, we mention targets that are not all equal (e.g, representing different diet resources) and the forager is not looking only for quantity but also for diversity. In this case, it has been shown that the optimal μ can assume different values, depending on how the distinct types of targets are grouped together [153]. Incidentally, one possible solution [153] is μopt = 3/2 (or values around it), a Lévy foraging exponent experimentally observed in diverse contexts [24, 74, 154–158].
Therefore, the exact value of μopt might depend on the targets’ specificities. But the important fact is that the search optimization mechanisms and the step length distributions have the same structure. In other words, the basic trade-offs remain the same. In terms of evolution, this is a relevant fact because to respond to a transforming environment, an individual following a general Lévy walk behavior could simply change its μ value (e.g., to the optimal one corresponding to the targets new features). This ability represents a cheap adaptation. Thus, the discussions here for non-destructive targets would apply exactly in the same way for other target scenarios.
We observe that in some situations μopt is the same regardless of the targets’ features. For instance, for patchy landscapes the optimal foraging behavior for both destructive and non-destructive targets have been investigated in [81] and in the two cases one finds μopt ≈ 2. This result can be understood by noticing that the patchy regions are rich in resources, with a high inner density of targets. Thus, the consumption (or destruction) of a target found within a patch only slightly influences the searcher dynamics, as there is always another nearby target available for the forager.
In our simulations we consider only 2D environments (since these are computationally less time-consuming). Surely, many evidences of Lévy flight foraging behavior come from flying and swimming animals (see the many examples discussed and cited along the present work), i.e., in true 3D landscapes. However, we mention that the Lévy walk optimization process is essentially the same in 2D and 3D (e.g., refer to the numerical and analytical approach in [9, 159]). Therefore, our qualitative results here should also be fairly valid in 3D. Finally, we only mention a particular issue about 3D foraging strategies, for both short- and long-term search efficiencies. Optimal long-term searching strategies are independent of the foraging space. But this independence may not hold for the short-term component. There is the possibility that, in a 3D environment, the incorporation of long helical paths (i.e., as a short-scale searching strategy) [47, 160] improves the statistics of encounters by decreasing the probability of “missing” near targets, and/or by avoiding midterm curvilinear biases, or yet by reducing some energetic costs. This possibility remains an open question that would merit further consideration.
Results
Lévy as the optimal strategy for several distinct environments
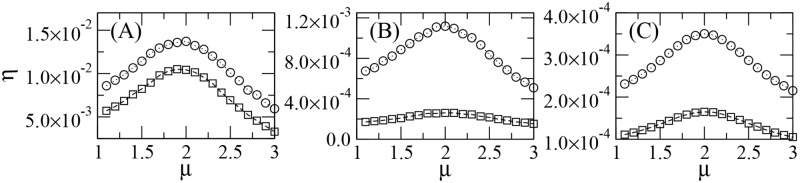
Fig 5 displays the search efficiency η as a function of the power-law exponent μ in the cases of Np = 5 and Np = 10 heterogeneous patches with Euclidean distribution of targets (see Methods section). Results shown in Fig 5(A)–5(C) correspond [145], respectively, to configurations with patches of random sizes, random targets densities, and random sizes and target densities, which are exemplified in Fig 1(A)–1(C). Some search trajectories with different degrees of diffusivity are also shown in Fig 1(A)–1(C): nearly ballistic (μ = 1.1), superdiffusive (μ = 2.0), and Brownian (μ = 3.0).
Fig 5. Search efficiency η vs. power-law exponent μ of Lévy searches for 104 targets in fragmented landscapes.
In the simulations, Np = 10 (circles) and Np = 5 (squares) heterogeneous patches contain a total of Nt = 104 inner targets (see Methods section). In (A)-(C) the parameters determining the radii and average distances between inner targets are respectively set as in Fig 1(A)–1(C). Ballistic and Brownian limits correspond to μ → 1 and μ = 3, respectively. In all cases, the efficiency η is maximized for the superdiffusive dynamics with μopt ≈ 2.
We remark in Fig 5 that distinct degrees of heterogeneity achieved by varying the density of targets and number and size of patches do not affect the classical result μopt ≈ 2 for the optimal strategy, which has been also obtained in homogeneous environments [49, 161], as well as in landscapes with uniform patches [81]. Once the searcher leaves a patch, the detection of a new fragment is a task optimized by the ballistic strategy μ → 1 [145]. Nevertheless, this trend has to be balanced by the return to the patch just visited and an intensive local scanning of inner targets, which favor oversampling and are best promoted in diffusive strategies with μ → 3. Thus, the intermediate value μopt ≈ 2 emerges as a tradeoff between these opposite tendencies.
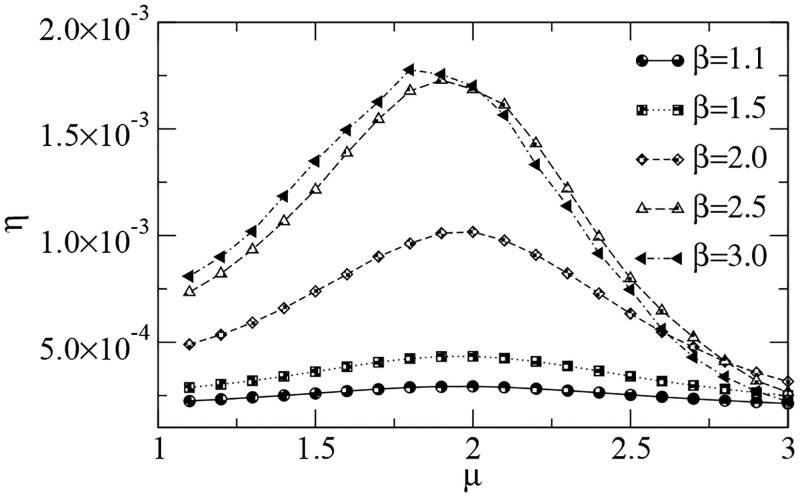
The efficiency of searches performed in Lévy dust distributions of targets is shown in Fig 6. Again, the value μopt ≈ 2 for the optimal strategy holds irrespective of the degree of clustering of targets driven by the exponent β. In fact, we notice that μopt is slightly lowered when clustering is high (e.g., μopt ≈ 1.8 for β = 3). Indeed, in landscapes similar to those depicted in Fig 4(C) and 4(D)—with a huge “open space” available —, once the searcher departs from the region plenty of targets it needs large steps (more likely for smaller μ) and enhanced superdiffusivity to come back. On the other hand, as seen in Fig 4(A), the homogeneous environment resembles the case with β = 1.1, for which μopt ≈ 2 is well known to apply [49]. The fact that a larger β implies a higher efficiency η means that landscapes containing a smaller occupied area with denser concentration of targets are generally more profitable than those with a larger occupied area of dispersed targets. Nevertheless, this might not be the case when other biological factors besides foraging are also taken into account along the searcher’s dynamics [81, 82, 145] (a nice example being that the risk of predation tends to favor smaller values of μ, see [82, 110, 162–164]).
Fig 6. Search efficiency η vs. power-law exponent μ in Lévy dust distributions.
The searcher detected 104 targets in a landscape with Lévy dust distributions of Nt = 104 targets (see Methods section). Parameters are set as in Fig 2. High clustering of targets and nearly homogeneous landscapes correspond to β = 3 and β = 1.1, respectively. In all cases, η is maximized for μopt ≈ 2, with a slight decrease in the optimal value (i.e. enhanced superdiffusion) observed as β → 3.
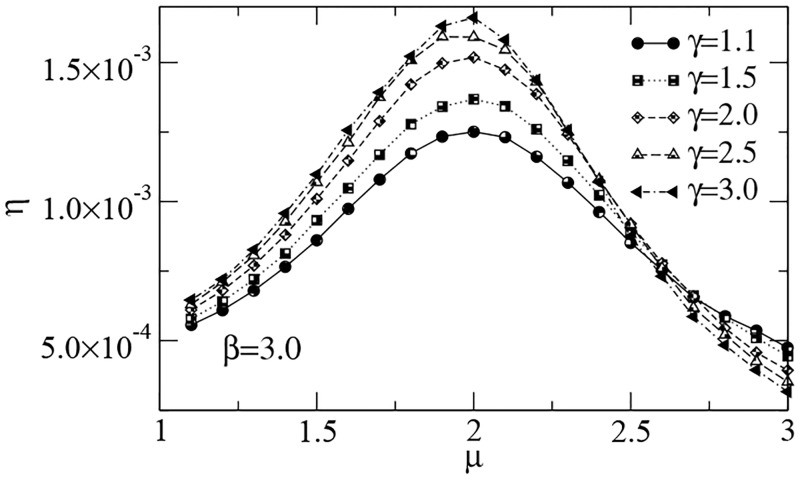
A similar scenario is found in Fig 7, in which the efficiency of searches in landscapes with Lévy dust distributions of both patches and inner targets is displayed. Here, the degree of clustering of the inner targets is fixed (β = 3), and results for several patch aggregation, driven by the exponent γ, are shown. The optimal search strategy, with μopt ≈ 2, is obtained once more. We also note that varying the value of β does not alter the qualitative behavior of the η vs. μ curves in this case.
Fig 7. Search efficiency η vs. power-law exponent μ in fractal patches.
The searcher detected 104 targets in a landscape with Lévy dust distributions of Nt = 50000 targets in Np = 50 patches (see Methods section). Parameters are set as in Fig 4. In all cases, η is maximized for μopt ≈ 2.
Conceivably, the most common pattern of spatial distribution of resources in actual ecosystems is the patchy one (see, e.g., [82] and references therein). In this sense, by assuming a wide range of distributions of targets inside the patches, from highly fractal to fairly homogeneous (as β varies), and several degrees of patches aggregation (as γ varies), one is able to cover a broad diversity of search environments. Hence, it is a remarkable fact that in all these cases, and under the reasonable assumptions made in this work, essentially the same Lévy search strategy with a unique free parameter, μ, is able to optimize the search outcome. In the next section, we radically move away from the sparse-targets limit and investigate on a regime of searches in which the relative advantages of μ ≈ 2 search strategies are no longer so evident.
The super-dense limit: Brownian searches as an emergent behavior
How significant is an optimal search strategy in a super-dense environment in which targets are so plentiful that long steps rarely occur? In this case, the key aspects determining efficient searches should be conceivably much different from those of the sparse regime. The reaction component of the search dynamics (e.g., the skills in getting the targets) becomes more relevant than the diffusion part of the process. As a consequence, the importance of extensive searching is reduced and one may anticipate that the relative advantage of strategies with μopt ≈ 2 tends to diminish. Thus, we now turn to the issue of how the preceding results obtained for generally sparse conditions are affected in super-dense homogeneous landscapes with very short average distance between targets, say lt ≤ 5.
From the numerical perspective, in the super-dense limit the landscape construction and search dynamics are time consuming, moreover requiring extra technical attention. For instance, to avoid dynamical traps in the tight arrangement of targets we have constrained the distance between any two targets to be always greater than 1.1 (recall that rv = 1). This condition prevents the searcher from artificially bouncing back and forth between two targets which are less than rv away.
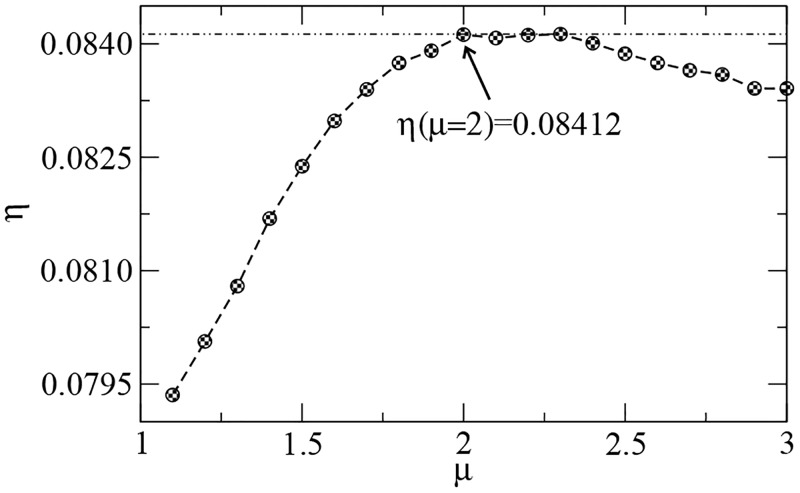
The random search model is the same as considered in the previous sections. Fig 8 displays the efficiency η vs. the power-law exponent μ for lt = 5, with averages taken over a smaller number (200) of landscapes due to the much longer simulations (in some cases, we have numerically checked that the qualitative results do not seem to alter if a larger number of averages is considered). The most evident finding is that less superdiffusive search walks (2 < μ < 3) and even diffusive ones (μ = 3) now perform nearly as efficient as the optimal strategy with μopt ≈ 2. Indeed, long steps ℓ ≫ lt, more likely to occur for small values of μ, are notably unnecessary in the super-dense scenario (see, e.g., [109]).
Fig 8. Search efficiency η vs. power-law exponent μ in a super-dense landscape.
The simulations lasted for 104 detected targets in a landscape with lt = 5 and homogeneous distribution of Nt = 50000 targets. Note that μopt ≈ 2 is still the optimal value. However, in contrast with the sparse regime (see previous η vs. μ plots), less superdiffusive search walks (2 < μ < 3) and even diffusive ones (μ = 3) perform nearly as efficient as the optimal superdiffusive strategy.
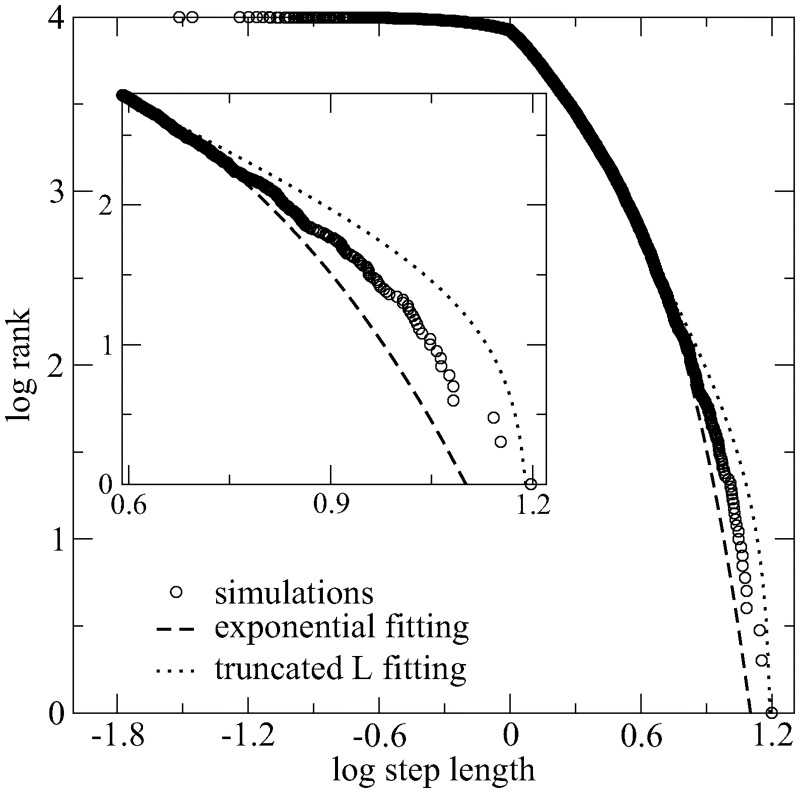
The effect of the high truncation rate by the encounter of targets in the super-dense landscape can be realized in Fig 9. We considered a μ = 2 Lévy search walk in a homogeneous environment with lt = 2.5. In Fig 9 we show the output distribution of step lengths, which is the frequency of actual step sizes performed by the searcher during the full search. It computes non-truncated steps that end up without detecting a target, as well as the relatively large number of truncated moves due to targets encounters. Actually, the ratio of the number of truncated steps to the non-truncated ones decreases for larger lt as in one-dimensional space [165], and decays even faster in two dimensions [166]. The output distribution of step lengths is thus expected to be strongly driven by the landscape properties in the case of super-dense regimes.
Fig 9. Output distribution of step lengths for a μ = 2.0 Lévy searcher in a super-dense landscape.
In the simulations, lt = 2.5 with Nt = 50000 targets homogeneously placed. The distribution takes into account the first 104 search steps, including non-truncated moves that end up without detecting a target and also a relatively large number of truncated steps due to targets encounters. Numerical simulation data are represented by circles. Dashed and dotted lines are, respectively, best fits to Brownian-like exponential and truncated power-law pdfs. The inset details the large-steps regime. Statistical data inference (MLE and AIC methods) indicates that the output distribution of step lengths in the super-dense regime is not properly described by a superdiffusive power-law (Lévy-like) pdf. Instead, it shows the signature of a Brownian motion.
By focusing on the tail of the output distribution of step lengths (see inset of Fig 9), we can infer the quality of fits by a truncated power-law and a Brownian-like exponential function. Through the maximum likelihood estimation (MLE) method, we found that the log-likelihood of the truncated power-law (−2947) is larger (in absolute value) than that of the exponential distribution (−2938). Moreover, the Akaike information criterion (AIC) provides Akaike weights which equal 0 for the truncated power-law and 1 for the exponential. Therefore, the statistical analysis supports the conclusion that the output distribution of step lengths in a super-dense environment has the signature of a Brownian motion. Indeed, even the best-fit exponent for the truncated power-law output distribution (μ = 3.3) reflects the Brownian character of the optimal search walk.
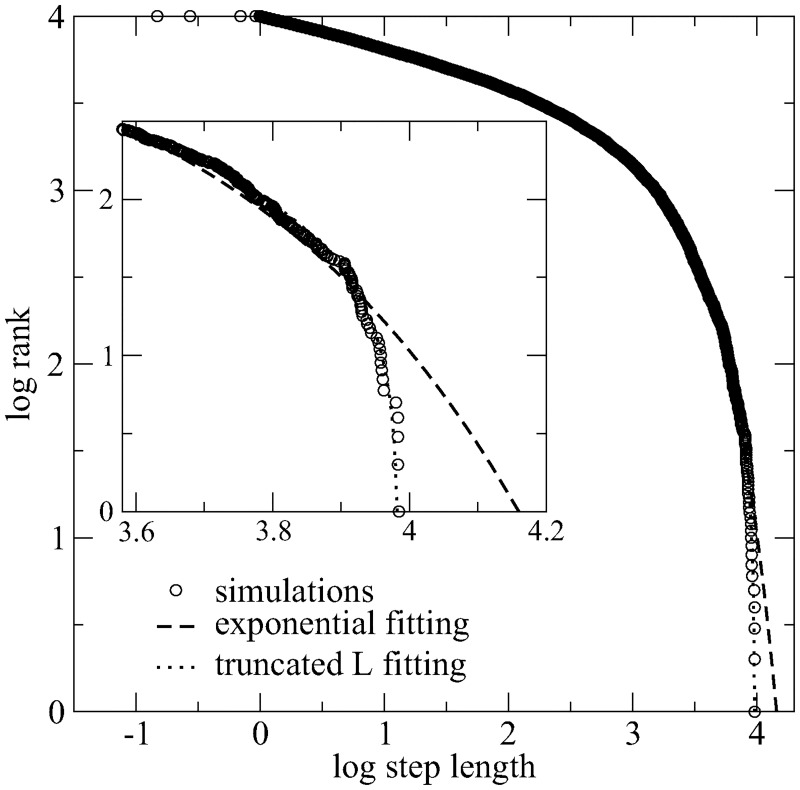
For comparison, a similar study was performed under low-dense conditions (lt = 100), Fig 10. In this case, the number of truncated steps is much lower, when compared to the super-dense limit. A μ = 2 Lévy search walk gives rise to an output distribution of step lengths which is still a truncated power-law, with best-fit value μ = 2.19 close to the original one. Indeed, the log-likelihood of the truncated power-law is −5571, with absolute value smaller than that of the exponential function (−5593). Also, the Akaike weight is 1 for the truncated power-law and 0 for the exponential, consistently with the findings in the low-dense regime.
Fig 10. Output distribution of step lengths for a μ = 2.0 Lévy searcher in a low-dense landscape (lt = 100).
In the sparse regime, the number of truncated moves due to targets encounters is relatively low and long steps are much more frequent, if compared to the super-dense limit (see inset). Statistical data inference (MLE and AIC methods) indicates that the output distribution of step lengths in the low-dense regime is actually a power-law (Lévy-like), with best-fit exponent μ = 2.19 close to the original one.
Discussion
The current debate on the emergentist vs. evolutionary origin of animal foraging is one of the most important issues in behavioral ecology [50, 51]. In its heart lies the paradigmatic Lévy flight foraging hypothesis, proposed more than a decade ago [1, 49]. Lévy strategies are robust [145, 152] in optimizing random search processes—or at least to yield advantageous outcomes—under conditions of scarce information availability and sparse targets, a common situation in actual ecosystems. In this sense, the evolutionary scenario states that organisms would have evolved via natural selection to exploit these advantageous distributions of move lengths.
On the other hand, in the emergentist view a Lévy foraging pattern would arise from complex interactions between the searcher and the environment. In other words, it would depend on the exact mechanisms through which the landscape features influence the individuals’ actions.
As in any scientific query, the full picture must rely on accurate empirical data together with suitable analysis based on solid conceptual interpretation [167]. Hence, theoretical arguments and proper models are essential in helping to identify the fundamental features of the process, leading to definite conclusions. Thus, statistical physics, and more specifically random search theory, can contribute in an important way to address the problem, exactly the approach followed in the present work.
We have studied through a reaction-diffusion algorithm the impact of landscape diversity on efficient search dynamics. Remarkably, the same optimal solution in the broad class of Lévy walks arose in a large collection of assorted environments, differing in diverse aspects such as the density of targets, the number and size of patches, the degree of heterogeneity, fragmentation and fractal dimension of the Lévy dusts, provided the targets density is sparse and the searcher’s information is restricted to its vicinity.
We have also reported that deviations from the Lévy search dynamics can be effectively observed in plentiful landscapes (e.g., from the actually measured step lengths distribution, Fig 9), thus indicating that Lévy search walks is certainly not ubiquitous in Nature (although common). In this case, the emergent optimal strategy becomes strongly driven by the encounter interactions with the complex environment. A number of non-optimal strategies perform almost as efficient as the optimal one, and the pressure towards greater resource rewards obtained through the correct choice of optimal or nearly optimal strategies is then reduced [109].
Interestingly, in this high-dense regime a Brownian distribution of step lengths emerges due to the frequent truncation of steps, even if a Lévy pdf is considered as the input distribution, i.e., the inherent dynamics. Indeed, the plentiful availability of resources naturally induces an interaction with the landscape through the finding and consumption of target sites (it is relevant to mention that these analyzes corroborate similar results and conclusions presented in [21]). Conversely, changes in the environment’s features towards a depletion regime can drastically decrease the targets encounters, and thus an already existing (“built-in”) search Lévy behavior—conceivably developed through natural evolution—would help to improve the chances of successful foraging without the necessity of drastic switching mechanisms.
At this point some words of caution are in order. Since the 1990s, the empirical demonstration of the occurrence of Lévy-type probability distributions in stochastic phenomena has notably grown, with a great diversity of examples raising both from biological as well as non-biological systems [1, 2]. In this sense, it seems clear that other mechanisms rather than the Lévy flight foraging hypothesis can be also responsible to generate such heavy-tailed distributions. Actually, depending on the specificities of the problem, even in biological systems Lévy distributions may arise in contexts outside the domain of efficient random searches. For instance, we mention that the flight distances by shearwaters follow a power-law pdf with exponent μ ≈ 3/2, which can be attributed to the olfactory maps the birds use for navigation [26], not related to search strategies. Also, the emergent-induced Lévy paths by bumblebees may arise as a consequence of the rejection of a great number of flowers previously marked by other individuals with repellent scent [65]. Other examples include the movement of seeds, jellyfish, and swarming bacteria, and even protein motors and DNA [60]. Therefore, in this sense it is thus clear that our results favoring an evolutionary view implied by the Lévy flight foraging hypothesis address the specific context of the evolved optimal probabilistic foraging strategies.
Certainly, a great deal of theoretical and experimental effort is still necessary before some key questions in foraging can be answered. In the context of the emergentist vs. evolutionary debate, our findings do not definitely overturn the emergentist hypothesis. Nonetheless, the robustness of Lévy walks—showing search efficacy in a wide variety of landscapes—favors the hypothesis of an evolutionary origin for different motor programs that could produce Lévy walk or similar statistical properties (see the section: Important features of efficient foraging strategies).
Note that in principle the emergent view supports specific (rather than general) optimal responses according to the landscape characteristics. But a caveat is that such dedicated strategies would not be plastic, hence not evolutionarily sustainable at long time scales. An alternative emergentist scenario would be the one in which proper (e.g., superdiffusive) movement features would arise modulated by searcher-environment interactions in each particular ecological network [168]. Despite existing a diverse range of putative generative mechanisms for Lévy walk signatures to emerge (as we have discussed above), a key point is that different environments may induce very different interaction mechanisms. Moreover, many of them might give rise to properties out of the Lévy (and alike) optimized regimes. Thus, without an universal evolutionary pressure it becomes more difficult to accept landscape-dedicated species-specific advection diffusion responses systematically yielding akin Lévy-like behaviors. For instance, it has been proposed that landscapes act as modulators [169], strongly affecting local patterns of biodiversity. The relations between a group of species in habitat A can be considerably distinct from those for the same species but in habitat B [170]. Also, trophic interactions (e.g., the predator-multiprey competition) can be modified by the presence of heterogeneities in the environment [171, 172]. Hence, the implausibility of retaining Lévy strategies in complete distinct environments without any underlying evolutionary pressure has motivated the idea of a general Hierarchical Lévy Flight Foraging Hypothesis [50], of which examples in [60] and [79] are potential concrete cases.
In conclusion, under the specific ecological assumptions assumed throughout our discussions, the emergentist hypothesis (in its more restricted form) seems to be a limited framework in face of the present theoretical analysis and when confronted with the available empirical data in the literature. Importantly, many of the points raised here eventually may be answered only through combining: accurate experimental data in controlled conditions, animals with restrained (and known) internal motivations, and well designed modeling [52, 167]. We hope that this work can stimulate progress and promote advances in the exciting field of foraging ecology.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by: Conselho Nacional de Desenvolvimento Científico e Tecnológico Research grant, CNPq 1435-2012 to MGEdL; Conselho Nacional de Desenvolvimento Científico e Tecnológico Research grant, CNPq 3545-2104 to EPR; Conselho Nacional de Desenvolvimento Científico e Tecnológico Research grant, CNPq 5380-2015 to GMV; Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (BR) FACEPE 12355 to EPR; Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior PhD scholarship Capes to MEW; and Conselho Nacional de Desenvolvimento Científico e Tecnológico postdoc fellowship CNPq to MCS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Viswanathan GM, da Luz MGE, Raposo EP, Stanley HE. The Physics of Foraging: An Introduction to Random Searches and Biological Encounters. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 2. Méndez V, Campos D, Bartumeus F. Stochastic Foundations in Movement Ecology: Anomalous Diffusion, Front Propagation and Random Searches. Berlin: Springer; 2014. [Google Scholar]
- 3. MacArthur RH, Pianka ER. On optimal use of a patchy environment. Amer Natur. 1966. November;100:603–9. 10.1086/282454 [DOI] [Google Scholar]
- 4. Pyke GH. Optimal Foraging Theory: A Critical Review. Annu Rev Ecol Syst. 1984;15:523–75. 10.1146/annurev.es.15.110184.002515 [DOI] [Google Scholar]
- 5. Kamil AC, Sargent TD (eds.) Foraging Behavior: Ecological, Ethnological, and Psychological Approaches. New York: Garland STPM Press; 1981. [Google Scholar]
- 6. Kamil AC, Krebs JR, Pulliam HR (eds.) Foraging Behavior. New York: Plenum Press; 1987. [Google Scholar]
- 7. Krebs JR, Davies NB. An Introduction to Behavioral Ecology. Oxford: Blackwell; 1993. [Google Scholar]
- 8. Stephens DW, Brown JS, Ydenberg RC (eds.) Foraging: Behavior and Ecology. Chicago: The University of Chicago Press; 2007. [Google Scholar]
- 9. Bartumeus F, Raposo EP, Viswanathan GM, da Luz MGE. Stochastic Optimal Foraging: Tuning Intensive and Extensive Dynamics in Random Searches. PLoS One. 2014. September;9(9):e106373 10.1371/journal.pone.0106373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sims DW, Southall EJ, Humphries NE, Hays GC, Bradshaw CJ, Pitchford JW, et al. Scaling laws of marine predator search behavior. Nature. 2008. February;451(7182):1098–102. 10.1038/nature06518 [DOI] [PubMed] [Google Scholar]
- 11. Focardi S, Montanaro P, Pecchioli E. Adaptive Lévy walks in foraging fallow deer. PLoS One. 2009. August;4(8):e6587 10.1371/journal.pone.0006587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Humphries NE, Queiroz N, Dyer JR, Pade NG, Musyl MK, Schaefer KM, et al. Environmental context explains Lévy and Brownian movement patterns of marine predators. Nature. 2010. June;465(7301):1066–9. 10.1038/nature09116 [DOI] [PubMed] [Google Scholar]
- 13. Franks NR, Richardson TO, Keir S, Inge SJ, Bartumeus F, Sendova-Franks AB. Ant search strategies after interrupted tandem runs. J Exp Biol. 2010. May;213(Pt 10):1697–708. 10.1242/jeb.031880 [DOI] [PubMed] [Google Scholar]
- 14. de Jager M, Weissing FJ, Herman PM, Nolet BA, van de Koppel J. Lévy walks evolve through interaction between movement and environmental complexity. Science. 2011. June;332(6037):1551–53. 10.1126/science.1201187 [DOI] [PubMed] [Google Scholar]
- 15. Hays GC, Bastian T, Doyle TK, Fossette S, Gleiss AC, Gravenor MB, et al. High activity and Lévy searches: jellyfish can search the water column like fish. Proc Biol Sci. 2012. February;279(1728):465–73. 10.1098/rspb.2011.0978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Humphries NE, Weimerskirch H, Queiroz N, Southall EJ, Sims DW. Foraging success of biological Lévy flights recorded in situ. Proc Natl Acad Sci USA. 2012. May;109(19):7169–74. 10.1073/pnas.1121201109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bazazi S, Bartumeus F, Hale JJ, Couzin ID. Intermittent motion in desert locusts: behavioral complexity in simple environments. PLoS Comput Biol. 2012;8(5):e1002498 10.1371/journal.pcbi.1002498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sims DW, Humphries NE, Bradford RW, Bruce BD. Lévy flight and Brownian search patterns of a free-ranging predator reflect different prey field characteristics. J Anim Ecol. 2012. March;81(2):432–42. 10.1111/j.1365-2656.2011.01914.x [DOI] [PubMed] [Google Scholar]
- 19. Reynolds AM. Distinguishing between Lévy walks and strong alternative models. Ecology. 2012. May;93(5):1228–33. 10.1890/11-1815.1 [DOI] [PubMed] [Google Scholar]
- 20. Reynolds AM. Olfactory search behavior in the wandering albatross is predicted to give rise to Lévy flight movement patterns. Anim Behav. 2012. May;83(5):1225–29. 10.1016/j.anbehav.2012.02.014 [DOI] [Google Scholar]
- 21. de Jager M, Bartumeus F, Kölzsch A, Weissing FJ, Hengeveld GM, Nolet BA, et al. How superdiffusion gets arrested: ecological encounters explain shift from Lévy to Brownian movement. Proc Biol Sci. 2013. November 13;281(1774):20132605 10.1098/rspb.2013.2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sims DW, Reynolds AM, Humphries NE, Southall EJ, Wearmouth VJ, Metcalfe B, et al. Hierarchical random walks in trace fossils and the origin of optimal search behavior. Proc Natl Acad Sci USA. 2014. July;111(30):11073–8. 10.1073/pnas.1405966111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reynolds AM, Schultheiss P, Cheng K. Does the Australian desert ant Melophorus bagoti approximate a Lévy search by an intrinsic bi-modal walk? J Theor Biol. 2014. January;340:17–22. 10.1016/j.jtbi.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 24. Raichlen DA, Wood BM, Gordon AD, Mabulla AZ, Marlowe FW, Pontzer H. Evidence of Levy walk foraging patterns in human hunter-gatherers. Proc Natl Acad Sci U S A. 2014. January;111(2):728–33. 10.1073/pnas.1318616111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Focardi S, Cecere JG. The Lévy flight foraging hypothesis in a pelagic seabird. J Anim Ecol. 2014. March;83(2):353–64. 10.1111/1365-2656.12147 [DOI] [PubMed] [Google Scholar]
- 26. Reynolds AM, Cecere JG, Paiva VH, Ramos JA, Focardi S. Pelagic seabird flight patterns are consistent with a reliance on olfactory maps for oceanic navigation. Proc R Soc B 2015. July;282(1811):20150468 10.1098/rspb.2015.0468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murakami H, Niizato T, Tomaru T, Nishiyama Y, Gunji YP. Inherent noise appears as a Lévy walk in fish schools. Sci Rep. 2015. June;5:10605 10.1038/srep10605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reynolds AM, Bartumeus F, Kölzsch A, van de Koppel J. Signatures of chaos in animal search patterns. Sci Rep. 2016. March;6:23492 10.1038/srep23492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reynolds AM, Paiva VH, Cecere JG, Focardi S. Lévy patterns in seabirds are multifaceted describing both spatial and temporal patterning. Front Zool. 2016. June;13:29 10.1186/s12983-016-0160-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Humphries NE, Weimerskirch H, Sims DW. A new approach for objective identification of turns and steps in organism movement data relevant to random walk modelling. Methods Ecol Evol. 2013. October;4(10):930–38. [Google Scholar]
- 31. Edwards AM, Phillips RA, Watkins NW, Freeman MP, Murphy EJ, Afanasyev V, et al. Revisiting Lévy flight search patterns of wandering albatrosses, bumblebees and deer. Nature. 2007. October;449(7165):1044–48. 10.1038/nature06199 [DOI] [PubMed] [Google Scholar]
- 32. Benhamou S. How many animals really do the Lévy walk? Ecology. 2007. August;88(8):1962–9. 10.1890/06-1769.1 [DOI] [PubMed] [Google Scholar]
- 33. Reynolds AM, Smith AD, Menzel R, Greggers U, Reynolds DR, Riley JR. Displaced honey bees perform optimal scale-free search flights. Ecology. 2007. August;88(8):1955–61. 10.1890/06-1916.1 [DOI] [PubMed] [Google Scholar]
- 34. Edwards AM. Using likelihood to test for Lévy flight search patterns and for general power-law distributions in nature. J Anim Ecol. 2008. November;77(6):1212–22. 10.1111/j.1365-2656.2008.01428.x [DOI] [PubMed] [Google Scholar]
- 35. Bartumeus F. Behavioral intermittence, Lévy patterns, and randomness in animal movement. Oikos. 2009. April;118(4):488–94. 10.1111/j.1600-0706.2009.17313.x [DOI] [Google Scholar]
- 36. de Knegt HJ, Hengeveld GM, van Langevelde F, de Boer WF, Kirkman KP. Patch density determines movement patterns and foraging efficiency of large herbivores. Behav Ecol. 2007. September;18(6):1065–72. 10.1093/beheco/arm080 [DOI] [Google Scholar]
- 37. Planck MJ, Codling EA. Sampling rate and misidentification of Lévy and non-Lévy movement paths. Ecology. 2009. December;90(12):3546–53. 10.1890/09-0079.1 [DOI] [PubMed] [Google Scholar]
- 38. Clauset A, Shalizi CR, Newman MEJ. Power-Law Distributions in Empirical Data. SIAM Rev. 2009. November;51(4):661–703. 10.1137/070710111 [DOI] [Google Scholar]
- 39. Reynolds AM. Bridging the gulf between correlated random walks and Lévy walks: autocorrelation as a source of Lévy walk movement patterns. J R Soc Interface. 2010. December 6;7(53):1753–8. 10.1098/rsif.2010.0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Edwards AM. Overturning conclusions of Lévy flight movement patterns by fishing boats and foraging animals. Ecology. 2011. June;92(6):1247–57. 10.1890/10-1182.1 [DOI] [PubMed] [Google Scholar]
- 41. Edwards AM, Freeman MP, Breed GA, Jonsen ID. Incorrect likelihood methods were used to infer scaling laws of marine predator search behaviour. PLoS One. 2012;7(10):e45174 10.1371/journal.pone.0045174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stumpf MP, Porter MA. Mathematics. Critical truths about power laws. Science. 2012. February 10;335(6069):665–6. 10.1126/science.1216142 [DOI] [PubMed] [Google Scholar]
- 43. Jansen VAA, Mashanova A, Petrovskii S. Comment on “Lévy walks evolve through interaction between movement and environmental complexity”. Science. 2012. February 24;335(6071):918 [DOI] [PubMed] [Google Scholar]
- 44. Viswanathan GM, Raposo EP, Bartumeus F, Catalan J, da Luz MGE. Necessary criterion for distinguishing true superdiffusion from correlated random walk processes. Phys Rev E. 2005. July;72:011111 10.1103/PhysRevE.72.011111 [DOI] [PubMed] [Google Scholar]
- 45. Tromer RM, Barbosa MB, Bartumeus F, Catalan J, da Luz MG, Raposo EP, Viswanathan GM. Inferring Lévy walks from curved trajectories: A rescaling method. Phys Rev E. 2015. August;92(2):022147 10.1103/PhysRevE.92.022147 [DOI] [PubMed] [Google Scholar]
- 46. Shlesinger MF, Klafter J. Lévy walks versus Lévy flights In: Stanley HE, Ostrowsky N. On Growth and Form, Nijhoff:Dordrecht; 1986, p. 279. [Google Scholar]
- 47. Levandowsky M, Klafter J, White BS. Feeding and Swimming Behavior in Grazing Microzooplankton. J Protozool. 1998. May;35(2):243–46. 10.1111/j.1550-7408.1988.tb04334.x [DOI] [Google Scholar]
- 48. Levandowsky M, Klafter J, White BS. Swimming behavior and chemosensory responses in the protistan microzooplankton as a function of the hydrodynamic regime. Bull Mar Sci. 1998. November;43(3):758–63. [Google Scholar]
- 49. Viswanathan GM, Buldyrev SV, Havlin S, da Luz MG, Raposo EP, Stanley HE. Optimizing the success of random searches. Nature. 1999. October 28;401(6756):911–4. 10.1038/44831 [DOI] [PubMed] [Google Scholar]
- 50. da Luz MGE, Raposo EP, Viswanathan GM. And yet it optimizes: Comment on “Liberating Lévy walk research from the shackles of optimal foraging” by A.M. Reynolds. Phys Life Rev. 2015. September;14:94–8. 10.1016/j.plrev.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 51. Pyke GH. Understanding movements of organisms: it’s time to abandon the Lévy foraging hypothesis. Methods Ecol Evol. 2015;6(1):1–16. 10.1111/2041-210X.12298 [DOI] [Google Scholar]
- 52. Bartumeus F. Behavioral ecology cannot turn its back on Lévy walk research: Comment on “Liberating Lévy walk research from the shackles of optimal foraging” by A.M. Reynolds. Phys Life Rev. 2015. September;14:84–6. 10.1016/j.plrev.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 53. Boyer D. What future for Lévy walks in animal movement research?: Comment on “Liberating Lévy walk research from the shackles of optimal foraging”, by A.M. Reynolds. Phys Life Rev. 2015. September;14:87–9. 10.1016/j.plrev.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 54. Cheng K. Answer (in part) blowing in the wind: Comment on “Liberating Lévy walk research from the shackles of optimal foraging” by A. Reynolds. Phys Life Rev. 2015. September;14:90–3. 10.1016/j.plrev.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 55. Focardi S. Do the albatross Lévy flights below the spandrels of St Mark?: Comment on “Liberating Lévy walk research from the shackles of optimal foraging” by A.M. Reynolds. Phys Life Rev. 2015. September;14:99–101. 10.1016/j.plrev.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 56. Humphries NE. Why Lévy Foraging does not need to be ‘unshackled’ from Optimal Foraging Theory: Comment on “Liberating Lévy walk research from the shackles of optimal foraging” by A.M. Reynolds. Phys Life Rev. 2015. September;14:102–4. 10.1016/j.plrev.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 57. MacIntosh AJJ. At the edge of chaos—error tolerance and the maintenance of Lévy statistics in animal movement: Comment on “Liberating Lévy walk research from the shackles of optimal foraging” by A.M. Reynolds. Phys Life Rev. 2015. September;14:105–7 10.1016/j.plrev.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 58. Miramontes O. Divorcing physics from biology? Optimal foraging and Lévy flights: Comment on “Liberating Lévy walk research from the shackles of optimal foraging” by A.M. Reynolds. Phys Life Rev. 2015. September;14:108–10. 10.1016/j.plrev.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 59. Sims DW. Intrinsic Lévy behavior in organisms—searching for a mechanism: Comment on “Liberating Lévy walk research from the shackles of optimal foraging” by A.M. Reynolds. Phys Life Rev. 2015. September;14:111–14 10.1016/j.plrev.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 60. Reynolds AM. Liberating Lévy walk research from the shackles of optimal foraging. Phys Life Rev. 2015. September;14:59–83. 10.1016/j.plrev.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 61. Reynolds AM. Venturing beyond the Lévy flight foraging hypothesis: Reply to comments on “Liberating Lévy walk research from the shackles of optimal foraging”. Phys Life Rev. 2015. September;14:115–9. 10.1016/j.plrev.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 62. Ramos-Fernandez G, Mateos JL, Miramontes O, Cocho G, Larralde H, Ayala-Orozco B. Lévy walk patterns in the foraging movements of spider monkeys (Ateles geoffroyi). Behav Ecol Sociobiol. 2004. January;55(3):223–30. 10.1007/s00265-003-0700-6 [DOI] [Google Scholar]
- 63. Boyer D, Ramos-Fernandez G, Miramontes O, Mateos JL, Cocho G, Larralde H, et al. Scale-free foraging by primates emerges from their interaction with a complex environment Proc Biol Sci. 2006. July; 273(1595): 1743–50. 10.1098/rspb.2005.3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guy AG, Bohan DA, Powers SJ, Reynolds AM. Avoidance of conspecific odour by carabid beetles: a mechanism for the emergence of scale-free searching patterns. Anim Behav. 2008. September;76(3):585–91. 10.1016/j.anbehav.2008.04.004 [DOI] [Google Scholar]
- 65. Reynolds AM. Lévy flight patterns are predicted to be an emergent property of a bumblebees’ foraging strategy. Behav Ecol Sociobiol. 2009. November;64:19–23. 10.1007/s00265-009-0813-7 [DOI] [Google Scholar]
- 66. Reynolds AM. Animals that randomly reorient at cues left by correlated random walkers do the Lévy walk. Amer Natur. 2010. May;175(5):607–13. 10.1086/651617 [DOI] [PubMed] [Google Scholar]
- 67. Reynolds AM. Effective leadership in animal groups when no individual has pertinent information about resource locations: How interactions between leaders and followers can result in Lévy walk movement patterns. Europhys Lett. 2013. April;102(1):18001 10.1209/0295-5075/102/18001 [DOI] [Google Scholar]
- 68. Reynolds AM. Lévy flight movement patterns in marine predators may derive from turbulence cues. Proc Math Phys Eng Sci. 2014. November;470(2171):20140408 10.1098/rspa.2014.0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bartumeus F. Lévy processes in animal movement: an evolutionary hypothesis. Fractals. 2007. June;15(2):151–62. 10.1142/S0218348X07003460 [DOI] [Google Scholar]
- 70. Reynolds AM, Smith AD, Reynolds DR, Carreck NL, Osborne JL. Honeybees perform optimal scale-free searching flights when attempting to locate a food source. J Exp Biol. 2007. November;210(Pt 21):3763–70. 10.1242/jeb.009563 [DOI] [PubMed] [Google Scholar]
- 71. Reynolds AM, Rhodes CJ. The Lévy flight paradigm: random search patterns and mechanisms. Ecology. 2009. April;90(4):877–87. 10.1890/08-0153.1 [DOI] [PubMed] [Google Scholar]
- 72. Preston MD, Pitchford JW, Wood AJ. Evolutionary optimality in stochastic search problems. J R Soc Interface. 2010. September;7(50):1301–10. 10.1098/rsif.2010.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Reynolds AM. On the origin of bursts and heavy tails in animal dynamics. Physica A. 2011. January;390(2):245–49. 10.1016/j.physa.2010.09.020 [DOI] [Google Scholar]
- 74. Reynolds AM. Signatures of active and passive optimized Lévy searching in jellyfish J R Soc Interface. 2014. August;11(99):20140665 10.1098/rsif.2014.0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Humphries NE, Sims DW. Optimal foraging strategies: Lévy walks balance searching and patch exploitation under a very broad range of conditions. J Theor Biol. 2014. October;358:179–93. 10.1016/j.jtbi.2014.05.032 [DOI] [PubMed] [Google Scholar]
- 76. Kölzsch A, Alzate A, Bartumeus F, de Jager M, Weerman EJ, Hengeveld GM, et al. Experimental evidence for inherent Lévy search behaviour in foraging animals. Proc Biol Sci. 2015. May;282(1807):20150424 10.1098/rspb.2015.0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Benhamou S, Collet J, Ultimate failure of the Lévy Foraging Hypothesis: Two-scale searching strategies outperform scale-free ones even when prey are scarce and cryptic. J Theor Biol. 2015. December;387:221–7. 10.1016/j.jtbi.2015.09.034 [DOI] [PubMed] [Google Scholar]
- 78. Gutiérrez ED, Cabrera JL. A neural coding scheme reproducing foraging trajectories. Sci Rep. 2015. December;5:18009 10.1038/srep18009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Namboodiri VMK, Levy JM, Mihalas S, Sims DW, Shuler MGH. Rationalizing spatial exploration patterns of wild animals and humans through a temporal discounting framework. Proc Natl Acad Sci U S A. 2016. August;113(31):8747–52. 10.1073/pnas.1601664113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. da Luz MGE, Raposo EP, Viswanathan GM. Subjective expectation of rewards can change the behavior of smart but impatient foragers. Proc Natl Acad Sci U S A. 2016. August;113(31):8571–3 10.1073/pnas.1609369113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wosniack ME, Santos MC, Pie MR, Marques MC, Raposo EP, Viswanathan GM, da Luz MGE. Unveiling a mechanism for species decline in fragmented habitats: fragmentation induced reduction in encounter rates. J R Soc Interface. 2013. November 20;11(91):20130887 10.1098/rsif.2013.0887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Niebuhr BB, Wosniack ME, Santos MC, Raposo EP, Viswanathan GM, da Luz MGE, Pie MR. Survival in patchy landscapes: the interplay between dispersal, habitat loss and fragmentation. Sci Rep. 2015. July;5:11898 10.1038/srep11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mogul JC. Emergent (mis)behavior vs. complex software systems. EuroSys’06: Proceedings of the 1st ACM SIGOPS/EuroSys European Conference on Computer Systems. New York: ACM; 2006.
- 84. Fiksen Ø, Jørgensen C, Kristiansen T, Vikebø F, Huse G. Linking behavioural ecology and oceanography: larval behaviour determines growth, mortality and dispersal. Mar Ecol Prog Ser. 2007;347:195–205. 10.3354/meps06978 [DOI] [Google Scholar]
- 85. Resnick M. Turtles, Termites, and Traffic Jams. Cambridge-MA: MIT Press; 2000. [Google Scholar]
- 86. Mitchell M, Newman M. Complex systems theory and Evolution in Encycolopedia of Evolution Pagel M. (ed.) New York: Oxford University Press; 2002. [Google Scholar]
- 87. Noble AE, Machta J, Hastlings A. Emergent long-range synchronization of oscilatting ecological populations without external forcing described by Ising universality. Nat Commun. 2015;6:6664 10.1038/ncomms7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Moran PAP. The statistical analysis of the Canadian Lynx cycle. 2. Synchronization and meteorology. Aust. J. Zool. 1953; 1:291–298. [Google Scholar]
- 89. Goldwyn EE, Hastings A. The roles of the Moran effect and dispersal in synchronizing oscillating populations J Theor Biol. 2011;289:237–246. 10.1016/j.jtbi.2011.08.033 [DOI] [PubMed] [Google Scholar]
- 90. Keeling MJ, Wilson HB, Pacala SW. Deterministic limits to stochastic spatial models of natural enemies. Am Nat. 2002;159:57–80. 10.1086/324119 [DOI] [PubMed] [Google Scholar]
- 91. Gould SJ, Eldredge N. Punctuated equilibria; the tempo and mode of evolution reconsidered. Paleobiology. 1977;3:115–151. 10.1017/S0094837300005224 [DOI] [Google Scholar]
- 92. Raup DM, Sepkoski JJ. Periodic extinction of families and genera. Science. 1986;231:833–836. 10.1126/science.11542060 [DOI] [PubMed] [Google Scholar]
- 93. Lieberman BS, Melott AL. Considering the case for biodiversity cycles: reexamining the evidence for periodicity in the fossil record. PLos One. 1997;2:e759 10.1371/journal.pone.0000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Solé RV, Manrubia SC, Benton M, Bak P. Self-similarity of extinction statistics in the fossil record. Nature. 1997;388:764–767. 10.1038/41996 [DOI] [Google Scholar]
- 95. Newman CM, Cohen JE, Kipnis C. Neo-Darwinian evolution implies punctuated equilibria. Nature. 1985;315:400–401. 10.1038/315400a0 [DOI] [Google Scholar]
- 96. Simons AW. The continuity of microevolution and macroevolution. J Evol Biol. 2002;15:688–701. 10.1046/j.1420-9101.2002.00437.x [DOI] [Google Scholar]
- 97. Reznick DN, Ricklefs RE. Darwin’s bridge between microevolution and macroevolution. Nature. 2009;457:837–842. 10.1038/nature07894 [DOI] [PubMed] [Google Scholar]
- 98. Hull P. Life in the aftermath of mass extinctions. Curr Biol. 2015;25:R941–R952. 10.1016/j.cub.2015.08.053 [DOI] [PubMed] [Google Scholar]
- 99. Brussate SL, et al. The extinction of the dinosaurs. Biol Rev Camb Philos Soc. 2015;90:628–642. 10.1111/brv.12128 [DOI] [PubMed] [Google Scholar]
- 100. Zee PC, terHorst CP, Schreiber SJ. Rapid evolution slows extinctions in food webs. bioRxiv: http://biorxiv.org/content/early/2017/02/11/107763. [Google Scholar]
- 101. West-Eberhard MJ. Developmental Plasticity and Evolution. Oxford: Oxford University Press; 2003. [Google Scholar]
- 102. Norazlimi NA, Ramli R. The relationships between morphological characteristics and foraging behavior in four selected species of shorebirds and water birds utilizing tropical mudflats. The Sci World J. 2015;105296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Barbosa A, Moreno E. Evolution of foraging strategies in shorebirds: an ecomorphological approach. The Auk. 1999;116(3):712–725. 10.2307/4089332 [DOI] [Google Scholar]
- 104. Naish D. The fossil record of bird behavior. J Zool. 2014. April;292(4):268–280. 10.1111/jzo.12113 [DOI] [Google Scholar]
- 105. Mayr G. The origins of crown group birds: molecules and fossils. Palaeontology 2014. March;57(2):231–242. 10.1111/pala.12103 [DOI] [Google Scholar]
- 106. da Silveira NS, Niebhur BBS, Muylaert RL, Ribeiro MC, Pizo MA. Effects of land cover on the movement of frugivorous birds in a heterogeneous landscape. Plos One. 2016;11(6):e0156688 10.1371/journal.pone.0156688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brown CT, Liebovitch LS, Glendon R. Lévy flights in Dobe Ju/’hoansi foraging patterns. Hum Ecol. 2007;35(1):129–138. 10.1007/s10745-006-9083-4 [DOI] [Google Scholar]
- 108. Raichlena DA, Woodb BM, Gordonc AD, Mabullad AZP, Marlowee FW, Pontzerf H. Evidence of Lévy walk foraging patterns in human hunter—gatherers. Proc Natl Acad Sci USA. 2014;111:728–733. 10.1073/pnas.1318616111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sotelo-López SA, Santos MC, Raposo EP, Viswanathan GM, da Luz MGE. Conditions under which a superdiffusive random-search strategy is necessary. Phys Rev E. 2012. September;86:031133 10.1103/PhysRevE.86.031133 [DOI] [PubMed] [Google Scholar]
- 110. Faustino CL, da Silva LR, da Luz MGE, Raposo EP, Viswanathan GM. Search dynamics at the edge of extinction: Anomalous diffusion as a critical survival state. EPL. 2007. January;77:30002 10.1209/0295-5075/77/30002 [DOI] [Google Scholar]
- 111. Bartumeus F, Catalan J, Viswanathan GM, Raposo EP, da Luz MGE. The influence of turning angles on the success of non-oriented animal searches. J Theor Biol. 2008. May;252(1):43–55. 10.1016/j.jtbi.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 112. Mantegna RN, Stanley HE. Stochastic process with ultraslow convergence to a Gaussian: the truncated Lévy flight, Phys Rev Lett. 1994. November;73(22):2946–49. 10.1103/PhysRevLett.73.2946 [DOI] [PubMed] [Google Scholar]
- 113. Lucas JR. Time scale and diet choice decisions; in Behavioural Mechanisms of Food Selection NATO ASI Series (Series G: Ecological Sciences), vol 20 Hughes RB (ed) pp. 165–185. Berlin: Springer; 1990. [Google Scholar]
- 114. Heath JP, Gilchrist HG, Ydenberg RC. Interactions between rate processes with different timescales explain counterintuitive foraging patterns of arctic wintering eiders. Proc R Soc B. 2010;277:3179–3186. 10.1098/rspb.2010.0812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hughes BD, Shlesinger MF, Montroll EW. Random walks with self-similar clusters. Proc Nat Acad Sci. 1981. June;78(6):3287–3291 10.1073/pnas.78.6.3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lubashevsky I, Friedrich R, Heuer A. Continuous-time multidimensional Markovian description of Lévy walks. Phys Rev E. 2009;80:031148 10.1103/PhysRevE.80.031148 [DOI] [PubMed] [Google Scholar]
- 117. Siegle P, Goychuk I, Talkner P, Hanggi P. Markovian embedding of non-Markovian superdiffusion. Phys Rev E. 2010;81:011136 10.1103/PhysRevE.81.011136 [DOI] [PubMed] [Google Scholar]
- 118. Campos D, Bartumeus F, Raposo EP, Méndez V. First-passage times in multiscale random walks: The impact of movement scales on search efficiency. Phys Rev E. 2015;92:052702 10.1103/PhysRevE.92.052702 [DOI] [PubMed] [Google Scholar]
- 119. Plank MJ, James A. Optimal foraging: Lévy pattern or process? J R Soc Interface. 2008;5:1077–1086. 10.1098/rsif.2008.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Benhamou S. Of scales and stationarity in animal movements. Ecol Lett. 2014. March;17(3):261–272. 10.1111/ele.12225 [DOI] [PubMed] [Google Scholar]
- 121. Reynolds AM. Mussels realize Weierstrassian Lévy walks as composite correlated random walks. Sci Rep. 2014. March;4:4409 10.1038/srep04409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Santos MC, Raposo EP, Viswanathan GM, da Luz MGE. Can collective searches profit from Lévy walks strategies? J Phys A. 2009;42(43):434017 10.1088/1751-8113/42/43/434017 [DOI] [Google Scholar]
- 123. da Luz MGE, Buldyrev SV, Havlin S, Raposo EP, Stanley HE, Viswanathan GM. Improvements in the statistical approach to random Lévy flight searches. Physica A. 2001;295:89–92. 10.1016/S0378-4371(01)00058-9 [DOI] [Google Scholar]
- 124. Reynolds AM. Selection pressures give composite correlated random walks Lévy walk characteristics. J Theor Biol. 2013. September;332:117–122. 10.1016/j.jtbi.2013.04.035 [DOI] [PubMed] [Google Scholar]
- 125. Nolting BC, Hinkelman TM, Brassil CE, Tenhumberg B. Composite random search strategies based on non-directional sensory cues. Ecol Complex. 2015. June;22:126–138. 10.1016/j.ecocom.2015.03.002 [DOI] [Google Scholar]
- 126. de Jager M, Weissing FJ, Herman PM, Nolet BA, van de Koppel J. Response to Comment on “Lévy walks evolve through interaction between movement and environmental complexity”. Science. 2012. February;335(6071):918 10.1126/science.1215903 [DOI] [PubMed] [Google Scholar]
- 127. Bartumeus F, Levin SA. Fractal reorientation clocks: Linking animal behavior to statistical patterns of search. Proc Natl Acad Sci USA. 2008;105(49):19072–19077. 10.1073/pnas.0801926105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Reynolds AM, Ouellette NT. Swarm dynamics may give rise to Lévy flights. Sci Rep. 2016;6:30515 10.1038/srep30515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lihoreau M, Ings TC, Chittka L, Reynolds AM. Signatures of a globally optimal searching strategy in the three-dimensional foraging flights of bumblebees. Sci Rep. 2016;6:30401 10.1038/srep30401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Scanlon TM, Caylor KK, Levin SA, Rodriguez-Iturbe I. Positive feedbacks promote power-law clustering of Kalahari vegetation. Nature. 2007. September;449(7159):209–12. 10.1038/nature06060 [DOI] [PubMed] [Google Scholar]
- 131. Kéfi S, Rietkerk M, Alados CL, Pueyo Y, Papanastasis VP, Elaich A, et al. Spatial vegetation patterns and imminent desertification in Mediterranean arid ecosystems. Nature. 2007. September;449(7159):213–17. 10.1038/nature06111 [DOI] [PubMed] [Google Scholar]
- 132. Russell RW, Hunt GL, Coyle KO, Cooney RT. Foraging in a fractal environment: spatial patterns in a marine predator-prey system. Landsc Ecol. 1992. September;7(3):195–209. 10.1007/BF00133310 [DOI] [Google Scholar]
- 133. Hoddle MS. The effect of prey species and environmental complexity on the functional response of Franklinothrips orizabensis: a test of the fractal foraging model. Ecol Entomol. 2003. June;28(3):309–18. 10.1046/j.1365-2311.2003.00518.x [DOI] [Google Scholar]
- 134. Palmer MW. Fractal geometry: a tool for describing spatial patterns of plant communities. Vegetatio. 1988. May;75(1):91–102. 10.1007/BF00044631 [DOI] [Google Scholar]
- 135. Milne BT. Spatial aggregation and neutral models in fractal landscapes. Amer Natur. 1992. January;139(1):32–57. 10.1086/285312 [DOI] [Google Scholar]
- 136. Ritchie ME. Scale-dependent foraging and patch choice in fractal environments. Evol Ecol. 1998. March;12(3):309–30. 10.1023/A:1006552200746 [DOI] [Google Scholar]
- 137. Kitchell JA. Deep-sea foraging pathways: an analysis of randomness and resource exploitation. Paleobiology. 1979. April;5(2):107–25. 10.1017/S0094837300006400 [DOI] [Google Scholar]
- 138. Hagadorn JW, Schellenberg SA, Bottjer DJ. Paleoecology of a large Early Cambrian bioturbator. Lethaia. 2000. September;33(3):142–56. 10.1080/00241160025100026 [DOI] [Google Scholar]
- 139. With KA, King AW. Extinction thresholds for species in fractal landscapes. Conserv Biol. 1999. April;13(2):314–326. 10.1046/j.1523-1739.1999.013002314.x [DOI] [Google Scholar]
- 140. With KA, King AW. Dispersal success on fractal landscapes: a consequence of lacunarity thresholds. Landsc Ecol. 1999. February;14(1):73–82. 10.1023/A:1008030215600 [DOI] [Google Scholar]
- 141. With KA, Pavuk DM. Habitat area trumps fragmentation effects on arthropods in an experimental landscape system. Landsc Ecol. 2011. August;26(7):1035–48. 10.1007/s10980-011-9627-x [DOI] [Google Scholar]
- 142. Fauchald P. Foraging in a hierarchical patch system. Amer Natur. 1999. June;153(6):603–613. 10.1086/303203 [DOI] [Google Scholar]
- 143. Fauchald P, Erikstad KE, Skarsfjord H. Scale-dependent predator-prey interactions: the hierarchical spatial distribution of seabirds and prey. Ecology. 2000. March;81(3):773–83. 10.2307/177376 [DOI] [Google Scholar]
- 144. Johnson CG, Boyce MS, Mulders R, Gunn A, Gau RJ, Cluff HD, Case RL. Quantifying patch distribution at multiple spatial scales: applications to wildlife-habitat models. Landsc Ecol. 2004. December;19(8):869–82. 10.1007/s10980-005-0246-2 [DOI] [Google Scholar]
- 145. Wosniack ME, Santos MC, Raposo EP, Viswanathan GM, da Luz MGE. Robustness of optimal random searches in fragmented environments. Phys Rev E. 2015. May;91(5):052119 10.1103/PhysRevE.91.052119 [DOI] [PubMed] [Google Scholar]
- 146. Ferreira AS, Raposo EP, Viswanathan GM, da Luz MGE. The influence of the environment on Lévy random search efficiency: Fractality and memory effects. Physica A. 2012. June;391(11):3234–3246. 10.1016/j.physa.2012.01.028 [DOI] [Google Scholar]
- 147. Miramontes O, Boyer D, Bartumeus F. The effects of spatially heterogeneous prey distributions on detection patterns in foraging seabirds. PLoS One. 2012;7(4):e34317 10.1371/journal.pone.0034317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Anderson TM, McNaughton SJ, Ritchie ME. Scale-dependent relationships between the spatial distribution of a limiting resource and plant species diversity in an African grassland ecosystem. Oecologia 2004. April;139(2):277–287. 10.1007/s00442-004-1499-1 [DOI] [PubMed] [Google Scholar]
- 149. Ritchie ME. Scale, Heterogeneity, and the Structure and Diversity of Ecological Communities. Princeton: Princeton University Press; 2009. [Google Scholar]
- 150. Zhao K, Jurdak R, Liu J, Westcott D, Kusy B, Parry H, Sommer P, McKeown A. Optimal Lévy-flight foraging in a finite landscape. Proc R Soc Interf. 2015. January;12:20141158 10.1098/rsif.2014.1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Santos MC, Raposo EP, Viswanathan GM, da Luz MGE. Optimal random searches of revisitable targets: Crossover from superdifffusive to ballistic random walks. Europhy Lett. 2004:67:734–740. 10.1209/epl/i2004-10114-9 [DOI] [Google Scholar]
- 152. Raposo EP, Buldyrev SV, da Luz MG, Santos MC, Stanley HE, Viswanathan GM. Dynamical Robustness of Lévy Search Strategies. Phys Rev Lett. 2003. December 12;91(24):240601 10.1103/PhysRevLett.91.240601 [DOI] [PubMed] [Google Scholar]
- 153. Wosniack ME, Raposo EP, Viswanathan GM, da Luz MGE. Efficient search of multiple types of targets. Phys Rev E 2015;92:062135 10.1103/PhysRevE.92.062135 [DOI] [PubMed] [Google Scholar]
- 154. Reynolds AM et al. Evidence for a pervasive’idling-mode’ activity template in flying and pedestrian insects. R Soc Op Sci. 2015;(2):150085 10.1098/rsos.150085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rhee I, Shin M, Hong S, Lee K, Kim SJ, Chong S. On the Lévy-walk nature of human mobility. IEEE/ACM Trans Netw. 2011;19(3):630–43. 10.1109/TNET.2011.2120618 [DOI] [Google Scholar]
- 156. Reynolds AM. Beating the odds in the aerial lottery: passive dispersers select conditions at takeoff that maximize their expected fitness on landing. Am Nat. 2013. April;181(4):555–61. 10.1086/669677 [DOI] [PubMed] [Google Scholar]
- 157. Monserud JH, Schwartz DK. Interfacial Molecular Searching Using Forager Dynamics. Phys Rev Lett. 2016;116:098303 10.1103/PhysRevLett.116.098303 [DOI] [PubMed] [Google Scholar]
- 158. Ariel G, Rabani A, Benisty S, Partridge JD, Harshey RM, Be’er A. Swarming bacteria migrate by Lévy Walk. Nat Commun. 2015;6:8396 10.1038/ncomms9396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Viswanathan GM, Afanasyev V, Buldyrev SV, Havlin S, da Luz MGE, Raposo EP, Stanley HE. Lévy flights search patterns of biological organisms. Physica A 2001. June;295(1–2):85–88. 10.1016/S0378-4371(01)00057-7 [DOI] [Google Scholar]
- 160. Helical Lévy walks: Adjusting searching statistics to resource availability in microzooplankton. Proc Nat Acad Sci 2003. October;100(22):12771–12775. 10.1073/pnas.2137243100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Raposo EP, Bartumeus F, da Luz MG, Ribeiro-Neto PJ, Souza TA, Viswanathan GM. How landscape heterogeneity frames optimal diffusivity in searching processes. PLoS Comput Biol. 2011. November;7(11):e1002233 10.1371/journal.pcbi.1002233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Reynolds AM. Balancing the competing demands of harvesting and safety from predation: Lévy walk searches outperform composite Brownian walk searches but only when foraging under the risk of predation. Physica A. 2010. November;389:4740–4746. 10.1016/j.physa.2010.06.027 [DOI] [Google Scholar]
- 163. Faustino CL, Lyra ML, Raposo EP, Viswanathan GM, da Luz MGE. The universality class of random searches in critically scarce environments. EPL. 2012. March;97:50005 10.1209/0295-5075/97/50005 [DOI] [Google Scholar]
- 164. Abe MS, Shimada M. Lévy walks suboptimal under predation risk. Plos Comput Biol. 2015;11(11):e1004601 10.1371/journal.pcbi.1004601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Buldyrev SV, Havlin S, Kazakov AY, da Luz MGE, Raposo EP, Stanley HE, et al. Average time spent by Lévy flights and walks on an interval with absorbing boundaries. Phys Rev E. 2001. October;64(4 Pt 1):041108 10.1103/PhysRevE.64.041108 [DOI] [PubMed] [Google Scholar]
- 166. Wosniack ME, Raposo EP, Viswanathan GM, da Luz MGE. A parallel algorithm for random searches. Comp Phys Commun. 2015;196:390–397. 10.1016/j.cpc.2015.07.014 [DOI] [Google Scholar]
- 167. Bartumeus F, Campos D, Ryu WS, Lloret-Cabot R, Méndez V, Catalan J. Foraging success under uncertainty: search tradeoffs and optimal space use Ecol Lett. 2016;19(11):1299–1313. 10.1111/ele.12660 [DOI] [PubMed] [Google Scholar]
- 168. Hagen M, Kissling WD, Rasmussen C, Carstensen DW, Dupont YL, Kaiser-Bunbury CN, et al. Biodiversity, Species Interactions and Ecological Networks in a Fragmented World. Adv Ecol Reserch. 2012;46:89:210. [Google Scholar]
- 169. Shachak M, Boeken B, Groner E, Kadmon R, Lubin Y, Meron E, et al. Woody Species as Landscape Modulators and Their Effect on Biodiversity Patterns. BioScience. 2008;58(3):209–221. 10.1641/B580307 [DOI] [Google Scholar]
- 170. Danielson BJ. Communities in a landscape: the influence of habitat heterogeneity on the interactions between species. Am Nat. 1991. November;138(5):1105–20. 10.1086/285272 [DOI] [Google Scholar]
- 171. Fortin D, Buono P-L, Schmitz OJ, Courbin N, Losier C, St-Laurent M-G, et al. A spatial theory for characterizing predator-multiprey interactions in heterogeneous landscapes. Proc R Soc B. 2015. August;282(1812):20150973 10.1098/rspb.2015.0973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Wosniack ME, da Luz MGE, Schulman LS. Punctuated equilibrium as an emergent process and its modified thermodynamic characterization. J Theor Biol. 2017. January;412:113–122. 10.1016/j.jtbi.2016.10.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.