Abstract
Background
Enhanced recovery pathways (ERPs) have been shown to aid in patient recovery and improve outcomes in many surgical settings. At present, there is limited data available regarding the use and feasibility of ERPs for patients undergoing microsurgical breast reconstruction. We sought to assess patient outcomes before and after the introduction of an ERP that was adopted by multiple surgeons at a single cancer center.
Methods
A multidisciplinary ERP was developed for patients undergoing deep inferior epigastric perforator (DIEP) or muscle-sparing free transverse rectus abdominis myocutaneous (TRAM) flap breast reconstruction. Core elements of the ERP included substituting intravenous patient-controlled analgesia with a multimodal pain regimen consisting of intravenous ketorolac and transversus abdominis plane blocks with liposomal bupivacaine, as well as the use of intraoperative goal-directed fluid management. Patients who underwent surgery between April and August 2015 using the ERP were compared with a historical control cohort. The primary endpoints were hospital length of stay (LOS) and total postoperative opioid consumption.
Results
In total, 91 consecutive patients were analyzed (ERP, 42; pre-ERP, 49). Mean hospital LOS was significantly shorter in the ERP group than in the pre-ERP group (4.0 vs. 5.0 days; p<0.0001). Total postoperative morphine equivalent consumption was also lower in the ERP group (46.0 vs. 70.5 mg; p=0.003). There was no difference in the incidence of 30-day complications between the groups (p=0.6).
Conclusions
The adoption of an ERP for DIEP and TRAM flap reconstruction by multiple surgeons significantly decreased opioid consumption and reduced LOS by 1 day.
Introduction
Microsurgical breast reconstruction has evolved markedly during the last 15 years. The introduction of muscle-sparing free transverse rectus abdominis myocutaneous (TRAM) flap and deep inferior epigastric perforator (DIEP) reconstruction has significantly contributed to improved patient outcomes. However, opioid-related adverse drug events, such as nausea and constipation, continue to be a problem in the postoperative setting for patients with breast cancer (1, 2). Recently, there has been an increased focus on using enhanced recovery pathways (ERPs) in reconstructive breast surgery to further improve outcomes, such as early mobilization, optimization of pain control, and earlier hospital discharge (3–5). These ERPs aim to achieve early postoperative recovery by maintaining preoperative organ function and reducing surgical stress during and after surgery. The main principles of ERPs include preoperative optimization of the surgical patient, preoperative nutrition, avoidance of prolonged fasting, goal-directed fluid management, standardized multimodal analgesic and anesthetic regimens, early resumption of diet, and early mobilization in the postoperative period (6–9). Compared with conventional care models, ERPs have been shown to reduce morbidity and decrease hospital length of stay (LOS) (10–13).
Despite the increasing amount of evidence showing the benefits of ERPs for many surgical populations (14–19), the adoption of this strategy for patients undergoing microsurgical breast reconstruction has been slow. Only a few reports of ERPs being used in microsurgical breast reconstruction are available (3–5, 8). These studies have typically focused on a homogeneous patient population or were limited to 1 or 2 microsurgeons. The purpose of this study was to assess how the introduction of a simple ERP for microsurgical breast reconstruction among a larger group of surgeons with a heterogenous patient population affects outcomes such as opioid consumption and hospital LOS.
Materials and Methods
Study design
Institutional Review Board approval was obtained prior to this beginning the study. Because this was a de-identified database review, the IRB waived the requirement for informed consent. We retrospectively studied perioperative data from patients undergoing microsurgical breast reconstruction using the ERP at our institution between April and August 2015. This group of patients was compared with a historical control group of consecutive patients who underwent immediate or delayed breast reconstruction with traditional postoperative care between June and December 2013 (pre-ERP). The historical control group was mostly treated using an opioid-based regimen with patient-controlled analgesia (PCA). Patients undergoing immediate or delayed breast reconstruction with a DIEP or muscle-sparing free TRAM flap were included in the study. For immediate reconstruction, patients undergoing either prophylactic or therapeutic mastectomies were included. All procedures were performed among 7 plastic surgeons at a single cancer center. Patients who underwent a simultaneous procedure, such as oophorectomy, were excluded. All data were retrieved retrospectively from the patients’ electronic medical records. The primary endpoint was LOS, defined as the number of days from initial admission to hospital discharge. Secondary endpoints were opioid consumption, pain (assessed by the Likert pain scale), and incidence of complications within 30 days of surgery.
Enhanced Recovery Pathway (ERP)
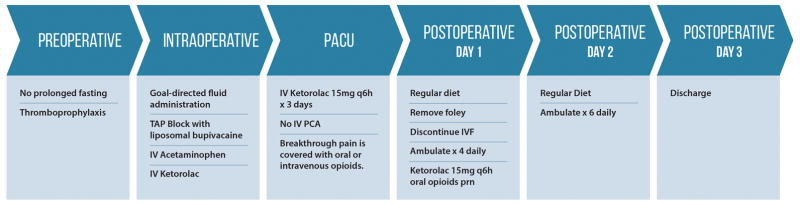
The ERP was implemented as a collaborative effort among the anesthesia, nursing, and plastic surgery teams. The main elements of this ERP included the following components. Preoperatively, patients received education to optimize surgery, including avoiding prolonged fasting (oral clear liquids until 2 h before hospital arrival time). Intraoperatively, opioid use was supplemented with nonopioid analgesia (intravenous acetaminophen and/or ketorolac), and transversus abdominis plane (TAP) blocks with liposomal bupivacaine were administered by the surgeon. Additionally, antiemetic drugs (ondansetron or dexamethasone) were administered, and intraoperative goal-directed fluid management was guided by hemodynamic parameters, including the pulse pressure variation method. Postoperatively, intravenous ketorolac (15 mg) was administered every 6 h for the first 3 days and then was transitioned to an oral equivalent for an additional 2 days. Breakthrough pain was covered with oral or intravenous opioids. PCA was eliminated in most patients. All patients were instructed to ambulate and start a regular diet on the first postoperative day, with the expectation of hospital discharge on postoperative day 3. Details of this protocol are illustrated in Figure 1.
Figure 1.
Elements of the enhanced recovery pathway
Surgical technique
All patients underwent either a DIEP or a muscle-sparing free TRAM flap, on the basis of intraoperative findings. The recipient vessels were the internal mammary artery and vein, with a portion of the third rib cartilage removed. Once recipient and donor site dissections were complete, 15 mg of intravenous ketorolac was given before anastomosis so that any potential bleeding would be controlled in the widely exposed operative field. In all patients who underwent a muscle-sparing free TRAM flap, wide-pore Prolene mesh was used to reinforce the abdominal wall. TAP blocks were administered bilaterally using liposomal bupivacaine: 20 mL of 1.3% (266 mg) liposomal bupivacaine was diluted with 180 mL of normal saline. Previously, an open technique was used involving direct dissection to the transversus abdominis fascia. We then used a sharp infiltration needle into the transversus abdominis plane under ultrasound guidance. Currently, a blind technique is used based on landmarks and tactile feedback, which can be administered more rapidly. An 18-gauge Tuohy needle is used to pierce the external and internal oblique fascia until resistance from the transversus abdominis fascia is met at the level of the anterior axillary line, approximately 2 cm below the costal margin. The Tuohy needle is curved and blunt, making it a safer choice for the blind technique, as the surgeon can feel the transversus abdominis fascia without easily penetrating it. Next, 40 mL of solution is introduced into the plane, after aspiration to ensure there is no intravascular injection. The remaining 120 mL of solution is used for supplemental injection around the fascial closure (within the rectus sheath itself) and along the inferior abdominal skin edge and drain sites. If a midline plication is performed superior to the umbilicus, direct injection into this area is important, as the TAP block will not cover the subxiphoid region. Closed suction drains were used followed by a standard layered skin closure. In this series, the mastectomy site was not injected. Flap monitoring was predominantly performed using a handheld Doppler and, in some cases, a T.Ox system device (ViOptix, Fremont, CA).
Statistical analysis
Fisher’s exact test was used to evaluate continuous variables in the ERP group, compared with the pre-ERP group. The Wilcoxon rank sum test was used to evaluate categorical variables. The two outcomes of interest were (1) hospital LOS and (2) total postoperative opioid consumption (in morphine equivalents). The correlation between LOS and total opioid consumption was evaluated using the Spearman correlation test. Exploratory examination indicated that the distribution of both outcomes were right-skewed. Therefore, the regression analyses of outcomes utilized the log-transformed versions of length of hospital stay and postoperative opioid consumption. Univariable linear regression analyses were performed to examine the relationship between each outcome of interest and ERP status. Multivariable regression models were constructed, starting with all variables with p<0.10 in univariable analyses. The secondary outcome of 30-day complications was compared between ERP status using Fisher’s exact test. All statistical tests were 2-tailed, and p<0.05 was considered to indicate statistical significance. All analyses were performed using Stata 13 (StataCorp, College Station, TX).
Results
In total, 91 patients were included in this study. Forty-two consecutive patients underwent DIEP or muscle-sparing free TRAM flap breast reconstruction with the ERP between April and August 2015. The historical cohort (pre-ERP group) consisted of 49 consecutive patients who underwent DIEP or muscle-sparing free TRAM flap reconstruction between June and December 2013. As shown in Table 1, patient demographics and preoperative characteristics were similar in both cohorts. There was no significant difference between the ERP and pre-ERP groups in the number of delayed and immediate reconstructions, type of procedure (DIEP or muscle-sparing TRAM), or laterality of reconstruction (Table 2).
Table 1.
Baseline demographic and comorbidity data
| Pre-ERP (n=49) | ERP (n=42) | p | |
|---|---|---|---|
| Age at surgery, years | 51.0 (34.0, 65.1) | 50.0 (33.0, 72.0) | 0.6 |
| Body mass index (kg/m2) | 28.8 (20.8, 38.8) | 29.3 (21.9, 43.5) | 0.5 |
| ASA PS score | |||
| I | 3 (6) | 1 (2) | 0.8 |
| II | 22 (45) | 20 (48) | |
| III | 24 (49) | 21 (50) | |
| Chronic pain diagnosis | 11 (22) | 10 (24) | 1.0 |
| Chronic opioid use | 1 (2) | 1 (2) | 1.0 |
| History of nausea or vomiting | 18 (37) | 13 (31) | 0.7 |
| Smoking history | |||
| Never | 35 (71) | 28 (67) | 0.8 |
| Former | 13 (27) | 13 (31) | |
| Current | 1 (2) | 1 (2) | |
| Sleep apnea | 5 (10) | 2 (5) | 0.4 |
| COPD | 0 (0) | 0 (0) | NA |
| Asthma | 5 (10) | 3 (7) | 0.7 |
| Hypertension | 11 (22) | 9 (21) | 1.0 |
| Diabetes mellitus | 1 (2) | 0 (0) | 1.0 |
Data are median (25th, 75th percentile) or no. (%). ASA PS, American Society of Anesthesiologist Physical Status; COPD, chronic obstructive pulmonary disease; ERP, enhanced recovery program; NA, not applicable.
Table 2.
Reconstruction and flap data
| Pre-ERP (n=49) | ERP (n=42) | p | |
|---|---|---|---|
| Reconstruction timing | |||
| Delayed | 20 (41) | 12 (29) | 0.3 |
| Immediate | 29 (59) | 30 (71) | |
| Reconstruction type | |||
| DIEP | 28 (57) | 28 (67) | 0.11 |
| MS TRAM | 16 (33) | 14 (33) | |
| TRAM | 5 (10) | 0 (0) | |
| Laterality | |||
| Unilateral | 29 (59) | 21 (50) | 0.4 |
| Bilateral | 20 (41) | 21 (50) | |
Data are no. (%). DIEP, deep inferior epigastric artery perforator; ERP, enhanced recovery program; MS, muscle sparing; TRAM, transverse rectus abdominus myocutaneous.
Intraoperative data is shown in Table 3. Length of surgery was shorter in the ERP group than in the pre-ERP group (median hours, 7.2 vs. 8.8 [p=0.007] for unilateral procedures, and 9.1 vs. 10.6 [p=0.014] for bilateral procedures). Liposomal bupivacaine was not used in any of the patients in the pre-ERP group and was administered to all patients in the ERP group. The ERP group received a significantly lower amount of intravenous fluid than the pre-ERP group (3.85 vs. 5.55 L; p<0.0001).
Table 3.
Intraoperative data
| Pre-ERP (n=49) | ERP (n=42) | p | |
|---|---|---|---|
| Length of surgery, h | |||
| All patients | 9.8 (8.1, 10.9) | 8.4 (6.7, 9.1) | 0.001 |
| Unilateral | 8.8 (6.9, 10.1) | 7.2 (5.9, 8.4) | 0.007 |
| Bilateral | 10.6 (9.6, 11.4) | 9.1 (8.8, 9.5) | 0.014 |
| Use of liposomal bupivacaine infiltration | 0 (0) | 42 (100) | NA |
| Total intraoperative IV fluids, mL | 5500.0 (4500.0, 6750.0) | 3850.0 (3200.0, 4500.0) | <0.0001 |
| Estimated blood loss, mL | 150.0 (100.0, 200.0) | 112.5 (50.0, 200.0) | 0.033 |
| Ketorolac | 0 (0) | 17 (40) | NA |
| Acetaminophen, g | 1000.0 (1000.0, 1000.0) | 1000.0 (1000.0, 2000.0) | 0.005 |
| Intravenous morphine, mg | 21.8 (8.0, 47.0) | 21.5 (6.0, 55.0) | 0.98 |
Data are median (25th, 75th percentile) or no. (%). ERP, enhanced recovery program; NA, not applicable.
Postoperative data is presented in Table 4. Hospital LOS was 1 day shorter in the ERP group than in the pre-ERP group (4.0 vs 5.0 days; p<0.0001). Almost all patients (98%) in the pre-ERP group used a PCA, compared with only 21% in the ERP group (p<0.0001). Among patients who required a PCA, the median duration of PCA use was shorter in the ERP group than in the pre-ERP group (24.9 vs. 41.0 hours; p=0.011). The proportion of patients using ketorolac postoperatively was lower in the pre-ERP group than in the ERP group (8% vs 71%; p<0.0001). Total postoperative intravenous morphine use was significantly lower in the ERP group (46.0 vs. 70.5 mg; p=0.003). Patients in the pre-ERP group had lower pain scores 18–24 h after surgery and 24–48 h after surgery (6.0 vs. 4.0 [p=0.02] and 6.0 vs. 5.0 [p=0.02], respectively). Pain scores for the remainder of hospital LOS were not significantly different between the two groups. As shown in Table 5, there were no significant differences in the proportions of patients with complications (p=0.2), readmissions (p=1.00), or reoperations (p=0.44) between the groups. There was a moderate correlation between the two outcomes of interest, LOS and postoperative opioid consumption (Spearman’s correlation rho = 0.343; p=0.0009).
Table 4.
Postoperative outcomes
| Pre-ERP (n=49) | ERP (n=42) | p | |
|---|---|---|---|
| Use of antiemetics | 28 (57) | 22 (52) | 0.7 |
| Hospital LOS | 5.0 (4.0, 6.0) | 4.0 (3.0, 5.0) | <0.0001 |
| Use of PCA | 48 (98) | 9 (21) | <0.0001 |
| PCA duration, h (n=57) | 41.0 (36.4, 61.8) | 24.9 (16.3, 37.7) | 0.011* |
| Ketorolac | 4 (8) | 30 (71) | <0.0001 |
| Intravenous morphine, mg | |||
| Total (n=91) | 70.5 (10.0, 312.0) | 46.0 (0.0, 132.5) | 0.003 |
| 4 h (n=91) | 3.0 (0.0, 7.0) | 2.5 (0.0, 10.0) | 0.9 |
| POD 0 (n=84) | 3.0 (2.0, 8.0) | 3.0 (1.0, 15.0) | 0.7 |
| POD 1 (n=88) | 30.0 (19.0, 47.8) | 18.0 (12.5, 34.0) | 0.03 |
| POD 2 (n=84) | 20.0 (10.5, 26.5) | 12.5 (5.0, 20.0) | 0.004 |
| POD 3 (n=70) | 10.0 (6.7, 20.0) | 10.0 (2.5, 17.5) | 0.5 |
| POD 4 (n=56) | 8.3 (5.0, 12.5) | 10.0 (2.5, 12.5) | 0.7 |
| Highest postoperative pain score | |||
| OR end/4h | 4.0 (0.0, 10.0) | 4.75 (0.0, 10.0) | 0.77 |
| 4 h–8 h | 3.0 (0.0, 8.0) | 3.0 (0.0, 9.0) | 0.93 |
| 8 h–12 h | 3.0 (0.0, 9.0) | 3.5 (0.0, 9.0) | 0.57 |
| 12 h–18 h | 4.0 (0.0, 8.0) | 4.5 (0.0, 8.0) | 0.67 |
| 18 h–24 h | 4.0 (0.0, 9.0) | 6.0 (2.0, 10.0) | 0.02 |
| 24 h–48 h | 5.0 (0.0, 10.0) | 6.0 (2.0, 8.0) | 0.02 |
| 48 h–72 h | 5.0 (0.0, 9.0) | 6.0 (3.0, 10.0) | 0.22 |
Data are median (range) or no. (%). ERP, enhanced recovery program; LOS, length of stay; OR, operating room; PCA, patient-controlled anesthesia; POD, postoperative day.
Does not account for LOS.
Table 5.
Complications, readmissions, and reoperations
| Pre-ERP (n=49) | ERP (n=42) | p | |
|---|---|---|---|
| Complication within 30 days | |||
| No | 38 (78) | 38 (90) | 0.2 |
| Yes | 11 (22) | 4 (10) | |
| Unplanned reoperations | |||
| No | 44 (90) | 40 (95) | 0.44 |
| Yes | 5 (10) | 2 (5) | |
| Hospital readmission | |||
| No | 48 (98) | 41 (98) | 1 |
| Yes | 1 (2) | 1 (2) | |
| Number of complications | |||
| 0 | 38 (78) | 38 (90) | 0.2 |
| 1 | 9 (18) | 4 (10) | |
| 2 | 2 (4) | 0 (0) | |
| Complication | |||
| Breast cellulitis | 1 (2) | 0 (0) | NA |
| Breast hematoma | 4 (8) | 2 (5) | |
| Mastectomy flap necrosis | 2 (4) | 1 (2) | |
| Deep vein thrombosis | 3 (6) | 0 (0) | |
| Pulmonary embolism | 1 (2) | 0 (0) | |
| Pneumonia | 2 (4) | 0 (0) | |
| Flap loss | 0 (0) | 1 (2) | |
ERP, enhanced recovery program; NA, not applicable.
Results of univariable linear regression analyses of both LOS and opioid consumption are shown in Table 6. All elements of ERP that were introduced (i.e. liposomal bupivacaine, postoperative ketorolac, goal directed fluid management) as well as a shorter length of surgery, were significantly associated with reduced LOS and less postoperative opioid consumption. Older age was an additional factor that predicted somehwat less postoperative opioid consumption (p=0.001).
Table 6.
Univariable regression analysis of LOS and total post operative opioid consumption
| LOS* | Postoperative opioid consumption** | |||||
|---|---|---|---|---|---|---|
| Variable | Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value |
| Liposomal bupivacaine TAP block | −0.23 | −0.33, −0.14 | <0.0001 | −0.66 | −1.04, −0.28 | 0.001 |
| Age | 0.00 | −0.01, 0.01 | 0.9 | −0.04 | −0.06, −0.01 | 0.001 |
| History of pain | 0.06 | −0.07, 0.18 | 0.4 | −0.17 | −0.64, 0.31 | 0.5 |
| History of nausea/vomiting | 0.08 | −0.03, 0.19 | 0.2 | 0.42 | 0.00, 0.83 | 0.049 |
| History of opioid use | −0.14 | −0.50, 0.21 | 0.4 | −1.14 | −2.49, 0.21 | 0.10 |
| BMI | 0.01 | −0.00, 0.02 | 0.13 | −0.01 | −0.05, 0.03 | 0.7 |
| MS-TRAM vs. DIEP | 0.11 | 0.01, 0.22 | 0.039 | 0.29 | −0.11, 0.70 | 0.2 |
| Intraop Morphine (per 10 mg) | 0.05 | 0.02, 0.08 | 0.001 | 0.08 | −0.03, 0.20 | 0.2 |
| IV Fluids (liters) | 0.07 | 0.04, 0.10 | <0.0001 | 0.18 | 0.06, 0.30 | 0.004 |
| OR Duration (hours) | 0.02 | 0.01, 0.04 | 0.001 | 0.08 | 0.02, 0.13 | 0.006 |
| Post-op Ketorolac | −0.18 | −0.28, −0.08 | 0.001 | −0.83 | −1.21, −0.45 | <0.0001 |
| LOS | -- | -- | -- | 0.28 | 0.12, 0.44 | 0.001 |
LOS was log-transformed;
Post-operative opioid consumption was log-transformed after adding 1
Multivariable models of both outcomes are presented in Table 7. With respect to LOS, after adjusting for intraoperative IV morphine level, procedure type (TRAM vs. DIEP), and length of surgery, the use of liposomal bupivacaine TAP blocks was associated with 16% shorter length of stay (coefficient = −0.18, p=0.0001). Post-operative opioid consumption was also reduced by 49% with the use of liposomal bupivacaine TAP blocks (coefficient = −0.68, p=0.0003), after adjusting for intraoperative IV morphine, age at surgery, length of surgery, and laterality (unilateral or bilateral). Higher intraoperative IV morphine (mg) is associated with a marginally longer LOS (coefficient = 0.03 per 10 mg increase, p=0.015). Older age at surgery was associated with less postoperative opioid consumption (coefficient = −0.04, p=0.001). Similarly, unilateral procedures were associated with a 37% reduction in opioid consumption compared to bilateral reconstructions (coefficient = −0.47, p=0.014).
Table 7.
Multivariable regression analysis of LOS and total postoperative opioid consumption
| LOS* | Postoperative opioid consumption** | |||||
|---|---|---|---|---|---|---|
| Variable | Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value |
| Lipisomal bupivacaine vs none | −0.18 | −0.27, −0.09 | 0.0001 | −0.68 | −1.04, −0.32 | 0.0003 |
| Intra-op Morphine (per 10 mg) | 0.03 | 0.01, 0.06 | 0.015 | −0.06 | −0.17, 0.05 | 0.3 |
| OR Duration (hours) | 0.01 | −0.00, 0.03 | 0.050 | 0.05 | −0.00, 0.10 | 0.075 |
| MS-TRAM vs DIEP | 0.09 | 0.00, 0.18 | 0.048 | |||
| Age | −0.04 | −0.06, −0.01 | 0.001 | |||
| Unilateral vs. Bilateral | −0.47 | −0.84, −0.10 | 0.014 | |||
LOS was log-transformed
Post operative opioid consumption was log-transformed after adding 1
Discussion
ERPs have been integrated into the treatment of patients undergoing colorectal surgery, among numerous other surgical populations (14–20), with the ultimate goal of enhancing patient recovery. These multidisciplinary surgical care pathways have been shown to improve recovery, reduce LOS, and optimize health outcomes and resource utilization (8). We believe that ERPs that use a multidisciplinary approach and multimodal pain management to care for patients represent the future of plastic surgery.
However, to date, only a few ERPs have been described in the plastic surgery literature (3–5, 21, 22). Batdorf et al. initially described a comprehensive ERP for microsurgical breast reconstruction (3). Although the ERP group in their study had a lower BMI and underwent more DIEP procedures, the findings were very compelling and made an important contribution to the use of ERPs for this patient population. Davidge et al. (21) examined patients undergoing TRAM flap breast reconstruction. They identified the use of multimodal analgesia, lower American Society of Anesthesiologists class, and surgery >6 months after implementation of the pathway as predictors of shorter time to discharge. Bonde et al. (5) reported outcomes among patients undergoing unilateral breast reconstruction and found a decrease in mean LOS, from 7.4 to 6.2 days. They continued to improve on their fast-track protocol with the identification of specific factors that prolonged hospital stay. A more recent paper by the same group (22) demonstrated a dramatic reduction in mean LOS, to 3.1 days, among 16 consecutive patients. These patients mostly underwent delayed, unilateral DIEP procedures; their outcomes continued to improve as the ERP implementation efforts continued.
Whereas promising results have been reported with the use of ERPs for breast reconstruction, the particular practice environment may also influence outcomes. Previous studies have introduced ERPs into practices composed of 1 or 2 microsurgeons. Introducing ERPs into practices comprising a larger, heterogenous group of surgeons performing a variety of surgical techniques may result in a wider range of outcomes. The aim of this study was to determine whether the implementation of a basic ERP into such an environment would reduce hospital LOS and overall opioid consumption. The core elements of this ERP include multimodal analgesia, goal-directed fluid management, an emphasis on early ambulation, and a regular diet the morning after surgery (Figure 1). In this study, we found that the ERP was effective even when implemented among several surgeons with different practice patterns.
The current ERP at our institution is based on combined ketorolac and liposomal bupivacaine TAP block administration without PCA. This is a fundamental departure from the traditional opioid-centered model, where patients often receive a PCA for 2 or 3 days. Opioids are very effective at treating pain and remain an important component of postoperative pain relief, but sole reliance on narcotics is associated with opioid-related adverse drug events. These include nausea, constipation, and obtundation, which can lead to serious consequences in high-risk patients. In this study, patients in the pre-ERP group, who received a PCA-centered regimen, had higher total morphine equivalents and longer LOS, which significantly contribute to higher health care costs (23–25). In addition, patients using a PCA are often reluctant to give it up, and the extra line and intravenous pole may interfere with early postoperative ambulation and recovery. Similar to Batdorf et al., we noted a decrease in PCA use in the ERP group, from 98% to 21%. This reduction was made possible by the administration of liposomal bupivacaine TAP blocks and ketorolac. Although concerns about bleeding with ketorolac use exist, there were no differences in complications between the ERP and pre-ERP groups, similar to the findings of other studies (26, 27). However, this data is confounded by selection bias, as patients who bled more easily during surgery were generally not given ketorolac.
One question we asked was: did the addition of ketorolac to the liposomal bupivacaine reduce opioid consumption or length of stay? All of the patients in the ERP group received liposomal bupivacaine TAP blocks, and 71% received scheduled postoperative ketorolac. Only 8% of patients in the pre-ERP group (n=4) received postoperative ketorolac. In this study, liposomal bupivacaine was the only variable that was significantly correlated with a reduction in both hospital LOS and total postoperative opioid consumption. The addition of ketorolac did not further decrease LOS, but it did significantly reduce opioid consumption, compared with liposomal bupivacaine alone (p=0.016).
By the use of goal-directed fluid management, we also noted a significant decrease in intraoperative fluid administration in the ERP group. Clinical outcomes can be adversely affected by inadequate or excessive fluid administration. Euvolemia is important in microsurgery, as previous studies have reported increased flap complications with excessive fluid administration (28, 29).
ERP implementation significantly reduced hospital LOS, from 5.0 to 4.0 days, with no increase in postoperative complications. In addition, this pathway also significantly decreased total postoperative opioid consumption by 35%, with significant reductions seen on postoperative days 1 and 2. Interestingly, although patients in the ERP group had lower opioid consumption, they reported higher amounts of pain 18–48 h after surgery, compared with the pre-ERP group. Patients in the ERP group were out of bed and walking earlier than patients in the pre-ERP group, which may have affected pain scores. Additionally, pain scores are inherently subjective, interpreted differently among individuals, and are dependent on the activity of the patient at the time of the assessment (30, 31). Opioid consumption is a more objective endpoint and reflects the patient’s need for pain control. Substituting PCA with liposomal bupivacaine and ketorolac in the ERP may mitigate the adverse drug events seen with high levels of opioid consumption and lead to a reduction in length of hospitalization.
There are several confounding factors that should be mentioned. The operative time in the ERP group was 1 hour shorter than that in the pre-ERP group. This finding is likely a result of the recent trend at our institution to perform microsurgical cases with 2 microsurgeons, to reduce the patient’s time under general anesthesia. Additionally, a patient’s preoperative expectations regarding hospital LOS may influence this variable, although this was not formally evaluated in the study. Finally, this study was retrospective in nature and limited by the sample size.
In summary, implementing an ERP in microsurgical breast reconstruction is a fundamental step toward making autologous reconstruction a more palatable option for patients, with several significant benefits. As microsurgical success rates and cosmetic outcomes have improved and surgical times have become shorter, the final hurdle in breast reconstructive surgery has been the perioperative patient experience. The results in this study demonstrate that an ERP can improve the perioperative patient experience by reducing hospital LOS and total opioid consumption. Additionally, this study was a collaboration between the anesthesia, nursing, and plastic surgery teams and reflects the increasing trend of multidisciplinary integration in plastic surgery care pathways to minimize the burden of recovery for our patients. Although these results are promising, more studies are needed to determine the most effective elements in an ERP.
Acknowledgments
Financial Support: MSK Cancer Center Support Grant/Core Grant (P30 CA008748), Pacira Pharmaceuticals Research Grant
Footnotes
Financial Disclosures: None of the authors has financial interests in any of the products, devices, or drugs mentioned in this manuscript.
Conflicts of Interests: Dr. Afonso is on the Health Outcomes Advisory Board at Pacira Pharmaceuticals and received an educational grant to help defray travel costs to present at the 2016 Miami Breast Cancer Conference. The authors have no other conflicts of interest to declare.
Ethical Approval: The study was approved by Memorial Sloan Kettering Cancer Center Intuitional Review Board, reference number 16-289.
List of Products, Devices, Drugs, etc, Mentioned in Manuscript: Acetaminophen, anti–inflammatory agents (dexamethasone), NSAIDs (ketorolac), EXPAREL (bupivacaine liposome injectable suspension, Pacira Pharmaceuticals, Parsippany, NJ), other local anesthetics (bupivacaine HCl), epinephrine, normal sterile saline, lactated Ringer’s solution, intravenous patient-controlled analgesia, opioids (fentanyl, oxycodone, hydromorphone, morphine), 5-hydroxytryptamine type 3 receptor antagonists (ondansetron), low molecular weight heparin, antibiotics, handheld Doppler T.Ox (ViOptix)
Author roles: Anoushka Afonso: study design, manuscript preparation, Sabine Oskar: data collection, Kay See Tan: statistical analysis, Joseph Disa: patient accrual, manuscript edits, Babak Mehrara: patient accrual, manuscript edits. Jihan Ceyhan: implementing postop ERP, Joseph Dayan: designed the enhanced recovery pathway, patient accrual, manuscript preparation.
References
- 1.Nelson JA, Fischer JP, Pasick C, et al. Chronic pain following abdominal free flap breast reconstruction: a prospective pilot analysis. Ann Plast Surg. 2013;71:278–282. doi: 10.1097/SAP.0b013e31828637ec. [DOI] [PubMed] [Google Scholar]
- 2.Gartner R, Kroman N, Callesen T, Kehlet H. Multimodal prevention of pain, nausea and vomiting after breast cancer surgery. Minerva Anestesiol. 2010;76:805–813. [PubMed] [Google Scholar]
- 3.Batdorf NJ, Lemaine V, Lovely JK, et al. Enhanced recovery after surgery in microvascular breast reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:395–402. doi: 10.1016/j.bjps.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Hainsworth AJ, Lobo CR, Williams P, et al. ‘23 h Model’ for breast surgery: an early experience. Breast. 2013;22:898–901. doi: 10.1016/j.breast.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Bonde C, Khorasani H, Eriksen K, Wolthers M, Kehlet H, Elberg J. Introducing the fast track surgery principles can reduce length of stay after autologous breast reconstruction using free flaps: A case control study. J Plast Surg Hand Surg. 2015;49:367–371. doi: 10.3109/2000656X.2015.1062387. [DOI] [PubMed] [Google Scholar]
- 6.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 7.Kehlet H, Slim K. The future of fast-track surgery. Br J Surg. 2012;99:1025–1026. doi: 10.1002/bjs.8832. [DOI] [PubMed] [Google Scholar]
- 8.Adamina M, Kehlet H, Tomlinson GA, Senagore AJ, Delaney CP. Enhanced recovery pathways optimize health outcomes and resource utilization: a meta-analysis of randomized controlled trials in colorectal surgery. Surgery. 2011;149:830–840. doi: 10.1016/j.surg.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630–641. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- 10.Langelotz C, Spies C, Muller JM, Schwenk W. “Fast-track”-rehabilitation in surgery, a multimodal concept. Acta Chir Belg. 2005;105:555–559. doi: 10.1080/00015458.2005.11679780. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasa S, Sammour T, Kahokehr A, Hill AG. Enhanced Recovery After Surgery (ERAS) protocols must be considered when determining optimal perioperative care in colorectal surgery. Ann Surg. 2010;252:409. doi: 10.1097/SLA.0b013e3181e9d947. author reply 409–410. [DOI] [PubMed] [Google Scholar]
- 12.Lassen K, Soop M, Nygren J, et al. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144:961–969. doi: 10.1001/archsurg.2009.170. [DOI] [PubMed] [Google Scholar]
- 13.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–1928. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 14.Labgaa I, Jarrar G, Joliat GR, et al. Implementation of Enhanced Recovery (ERAS) in Colorectal Surgery Has a Positive Impact on Non-ERAS Liver Surgery Patients. World J Surg. 2016;40:1082–1091. doi: 10.1007/s00268-015-3363-3. [DOI] [PubMed] [Google Scholar]
- 15.Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations - Part I. Gynecol Oncol. 2016;140:313–322. doi: 10.1016/j.ygyno.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Schatz C. Enhanced Recovery in a Minimally Invasive Thoracic Surgery Program. AORN J. 2015;102:482–492. doi: 10.1016/j.aorn.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Coyle MJ, Main B, Hughes C, et al. Enhanced recovery after surgery (ERAS) for head and neck oncology patients. Clin Otolaryngol. 2016;41:118–126. doi: 10.1111/coa.12482. [DOI] [PubMed] [Google Scholar]
- 18.Di Rollo D, Mohammed A, Rawlinson A, Douglas-Moore J, Beatty J. Enhanced recovery protocols in urological surgery: a systematic review. Can J Urol. 2015;22:7817–7823. [PubMed] [Google Scholar]
- 19.Arumainayagam N, McGrath J, Jefferson KP, Gillatt DA. Introduction of an enhanced recovery protocol for radical cystectomy. BJU Int. 2008;101:698–701. doi: 10.1111/j.1464-410X.2007.07319.x. [DOI] [PubMed] [Google Scholar]
- 20.Arsalani-Zadeh R, ElFadl D, Yassin N, MacFie J. Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg. 2011;98:181–196. doi: 10.1002/bjs.7331. [DOI] [PubMed] [Google Scholar]
- 21.Davidge KM, Brown M, Morgan P, Semple JL. Processes of care in autogenous breast reconstruction with pedicled TRAM flaps: expediting postoperative discharge in an ambulatory setting. Plast Reconstr Surg. 2013;132:339e–344e. doi: 10.1097/PRS.0b013e31829ace62. [DOI] [PubMed] [Google Scholar]
- 22.Bonde CT, Khorasani H, Elberg J, Kehlet H. Perioperative Optimization of Autologous Breast Reconstruction. Plast Reconstr Surg. 2016;137:411–414. doi: 10.1097/01.prs.0000475749.40838.85. [DOI] [PubMed] [Google Scholar]
- 23.Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate Enhanced Recovery After Surgery pathways. Can J Anaesth. 2015;62:203–218. doi: 10.1007/s12630-014-0275-x. [DOI] [PubMed] [Google Scholar]
- 24.Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41:400–406. doi: 10.1345/aph.1H386. [DOI] [PubMed] [Google Scholar]
- 25.Oderda GM, Evans RS, Lloyd J, et al. Cost of opioid-related adverse drug events in surgical patients. J Pain Symptom Manage. 2003;25:276–283. doi: 10.1016/s0885-3924(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Chang DW, Koutz C, et al. Incidence of hematoma associated with ketorolac after TRAM flap breast reconstruction. Plast Reconstr Surg. 2001;107:352–355. doi: 10.1097/00006534-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Gobble RM, Hoang HL, Kachniarz B, Orgill DP. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741–755. doi: 10.1097/01.prs.0000438459.60474.b5. [DOI] [PubMed] [Google Scholar]
- 28.Zhong T, Neinstein R, Massey C, et al. Intravenous fluid infusion rate in microsurgical breast reconstruction: important lessons learned from 354 free flaps. Plast Reconstr Surg. 2011;128:1153–1160. doi: 10.1097/PRS.0b013e318221da56. [DOI] [PubMed] [Google Scholar]
- 29.Booi DI. Perioperative fluid overload increases anastomosis thrombosis in the free TRAM flap used for breast reconstruction. Eur J Plast Surg. 2011;34:81–86. doi: 10.1007/s00238-010-0466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaeschke R, Singer J, Guyatt GH. A comparison of seven-point and visual analogue scales. Data from a randomized trial. Control Clin Trials. 1990;11:43–51. doi: 10.1016/0197-2456(90)90031-v. [DOI] [PubMed] [Google Scholar]
- 31.Kemp J, Despres O, Dufour A. Unreliability of the visual analog scale in experimental pain assessment: a sensitivity and evoked potentials study. Pain Physician. 2012;15:E693–699. [PubMed] [Google Scholar]