Abstract
Purpose of Review
Injured skin in the mammalian fetus can heal regeneratively due to the ability of fetal fibroblasts to effectively reorganize the extracellular matrix (ECM). This process occurs without fetal fibroblasts differentiating into highly contractile myofibroblasts which cause scarring and fibrosis in adult wounds. Here, we provide a brief review of fetal wound healing and the evidence supporting a unique contractile phenotype in fetal fibroblasts. Furthermore, we discuss the biomechanical role of the ECM in driving myofibroblast differentiation in wound healing and the implications for new clinical modalities based on the biophysical properties of fetal fibroblasts.
Recent Findings
We and others have found that fetal fibroblasts are refractory to the environmental stimuli necessary for myofibroblast differentiation in adult wound healing including mechanical stress.
Summary
Understanding the biomechanical mechanisms that regulate the contractile phenotype of fetal fibroblasts may unlock new avenues for anti-scarring therapies that target myofibroblast differentiation of adult fibroblasts.
Keywords: wound healing, fetal, scarless, regeneration, myofibroblast, contractility
INTRODUCTION
Injuries to tissues resulting from traumas, surgeries, and diseases are a leading global health concern. In the United States alone, treatments for dermal wounds cost tens of billions of dollars in health care expenditures [1]. Wound healing and repair are important considerations when treating tissue injuries. Many therapies have been developed to regulate the wound healing process; however, injured tissues still heal imperfectly resulting in scar formation or fibrosis which can have significant consequences. For example, dermal scarring can range from cosmetic abnormalities to major body deformations and impaired physical function [2]. Therefore, there exists a pressing need for new clinical strategies to regulate tissue repair and drive healing towards a more regenerative outcome.
Nature provides examples of regeneration in the mammalian fetus (Fig. 1). Fetal tissues can heal scarlessly depending on wound size and gestational age [3]. This regenerative response was first discovered in the dermis [4] but has also been observed in the upper airway mucosa [5] and tendon [6]. While differences in the extracellular matrix (ECM) and inflammation of fetal wounds contribute to scarless healing [7], the intrinsic properties of fetal fibroblasts are also thought to play a crucial role in this regeneration [8]. In particular, this review will focus on the biochemical and biophysical properties of dermal fetal fibroblasts that indicate a unique contractile phenotype capable of appropriately contracting and remodeling the ECM leading to regenerative repair. Understanding the mechanisms that regulate these phenotypic characteristics of fetal fibroblasts may lead to new therapeutic targets with the potential for reducing scarring and fibrosis.
Fig. 1.
After traumatic injury to tissues such as the skin, adult wounds heal with imperfect repair and scar formation resulting in a loss of natural structure and function. In contrast, fetal dermal wounds heal in a regenerative manner and are virtually indistinguishable from non-injured tissue. In either case, the quality of healing is dictated by fibroblasts that are responsible for invading the wound bed, depositing new collagenous tissue, and contracting and remodeling this tissue. Since fibroblast activity in the wound bed determines healing outcomes, regenerative or scarless wound healing is highly dependent on the intrinsic properties of fetal fibroblasts.
THE ROLE OF FIBROBLASTS IN WOUND HEALING
Fibroblasts are responsible for the production and organization of new tissue in the wound bed. In post-natal or “adult’ wounds, fibroblasts differentiate into myofibroblasts which synthesize and deposit new collagenous ECM that is initially composed of type III collagen but is later dominated by type I collagen [9]. Myofibroblasts are characterized by mature focal adhesions and stress fibers containing α-smooth muscle actin (α-SMA) that generate large contractile forces via actomyosin contractility [10]. These forces are used to excessively contract and remodel the newly deposited ECM; this mechanical response is perpetuated in a positive feedback loop as mechanical stress continues to build in the ECM due to sustained cellular forces ultimately resulting in scarring and fibrosis [11]. α-SMA expression and collagen synthesis are regulated by the cytokine transforming growth factor-β1 (TGF-β1) which is the major promoter of myofibroblast differentiation [12]. In addition, the splice variant of fibronectin containing the extra domain A is upregulated in wounds and necessary for the conversion of fibroblasts to myofibroblasts [13].
In contrast, scarless healing occurs in wounded skin of the mammalian fetus early in gestation and within the first two trimesters in humans [7]. Fetal dermal wounds heal much faster and regain their natural structure and function including skin appendages and sebaceous glands [14]. α-SMA-containing fibroblasts are not present in fetal wounds or are only transiently expressed [15], and these wounds close with minimal contraction [16]. Fetal wounds are characterized by a diminished inflammatory response resulting in decreased TGF-β1 expression and altered collagen production [17]. Fetal skin is composed of a higher ratio of type III to type I collagen which has also been reported in some fetal wounds as well [9, 18]. The newly deposited collagenous ECM in fetal wounds is organized in a fine reticular pattern similar to unwounded skin [19] resulting in the full restoration of tensile strength which does not occur with adult scars [20, 21]. In addition, higher levels of glycosaminoglycans such as hyaluronic acid are also produced in fetal wounds and have been found to be important in regenerative healing [7]. Despite these environmental differences, potential therapeutic strategies targeting cytokines such as TGF-β1 and ECM synthesis in adult wounds have yet to restrain fibrotic healing [22, 23].
THE FETAL FIBROBLAST
Pioneering transplantation studies performed at Stanford University in the early 1990’s have established the unique ability of fetal fibroblasts to facilitate scarless healing. Human fetal skin transplanted subcutaneously in adult nude mice and later wounded healed without scarring despite exposure to adult serum and inflammatory cells [8, 14]. The collagen in the regenerated skin was identified as human indicating that it was produced by the fetal fibroblasts. In contrast, the transplantation of adult sheep skin into the backs of fetal lambs and later wounded healed with scars despite exposure to amniotic fluid rich in growth factors and ECM components originally thought to be important for scarless repair [24, 25, 16]. These results suggested that scarless healing is an intrinsic property of fetal skin that is orchestrated by fetal fibroblasts. Therefore, the fetal fibroblast was concluded to be the key effector of scarless repair [8]. Intriguingly, a clinical study in which collagen constructs seeded with human fetal skin cells were transplanted into the burns of pediatric patients found that these wounds healed without retraction and with minimal scarring [26] further supporting a unique role for these cells in regenerative repair.
These findings are supported by in vitro studies showing differences in the molecular and cellular characteristics of fetal and adult fibroblasts [27–30]. For example, significant differences have been found in the expression of growth factors and ECM components by fetal fibroblasts as well as in TGF-β1 signaling [31–33]. While exogenous TGF-β1 increases whole tissue α-SMA expression and scarring in fetal wounds [34], α-SMA expression in fetal fibroblasts in vitro does not change on stress-free collagen gels or plastic despite similar TGF-β receptor and Smad mRNA levels when compared to adult fibroblasts [33, 35]. Fetal fibroblasts have also been shown to express lower levels of α1 and α3 integrins which are thought to affect their contractile capacity [36]. In fact, contractility of collagen gels has served as the primary in vitro surrogate for understanding differences in wound contraction that occurs in vivo [16, 25, 37, 33]. Consistent with other reports [16, 25], our work also found that fetal fibroblasts contract free floating collagen gels to a greater extent than their adult counterparts [38]. However, these gels lack tension and thus compact due to the migration of cells into the collagen matrix which is thought to mimic the early stages of wound healing in which fibroblasts migrate into the wound bed [39, 40]. These results parallel other studies we performed on two-dimensional surfaces and in three-dimensional collagen plugs in which fetal fibroblasts consistently migrated at a faster rate [41, 42]. In addition, we also found differential effects on migration and contraction due to a defective prostaglandin E2 (PGE2) pathway in fetal fibroblasts despite similar expression levels of the PGE2 receptors when compared to adult fibroblasts [41, 38, 43]. Therefore, fetal fibroblasts exhibit distinct phenotypic characteristics that may contribute to their ability to enable scarless wound healing such as increased migration and altered responses to soluble mediators.
ECM BIOMECHANICS AND CELLULAR CONTRACTILITY
The mechanical state of the ECM is known to play a fundamental role in driving pathological conditions in a number of conditions and diseases by regulating cellular phenotypes [44]. The tension and stiffness that develop in granulation tissue promote myofibroblast differentiation and subsequent fibrotic ECM reorganization of adult dermal wounds [45]. TGF-β1 is expressed early in the healing process but does not induce myofibroblast differentiation until a mechanical threshold is reached in the ECM [10]. In fact, latent TGF-β1 bound to the ECM must first be activated through conformational changes of the latency associated peptide caused by integrin-mediated cellular forces [46]. Similar findings in vitro have shown that ECM rigidity is a significant factor in promoting TGF-β1-induced myofibroblast differentiation [47]. Interestingly, fetal skin and wounds are more compliant than their adult counterparts [48, 20] which is thought to be due to less cross-linking of a more immature ECM composed of higher levels of type III collagen [21]. This notion is supported by an in vivo study using a type III collagen-deficient mouse model that found increases in myofibroblasts, contraction, and scarring during dermal wound repair [49]. While β1 integrins are responsible for binding fibrillar collagens and transducing cellular forces [50], types I and III collagen have different integrin-binding motifs that have been shown to affect fibroblast adhesion [51, 52]. Since the strength of cellular adhesion is critical for force generation [53], these ECM components may play an important role in determining myofibroblast differentiation. Furthermore, other integrins and ECM components may contribute to this process such as ανβ3 and fibronectin [53, 54]. Therefore, the mechanical nature of the fetal wound environment may not be conducive to myofibroblast differentiation.
To gain insight into whether mechanical stress can promote cellular contractility in fetal fibroblasts, we began to look at the biomechanical role of the ECM in adult versus fetal wound healing in vitro [38, 43]. In particular, we developed our own version of an attached type I collagen gel model as a 3-D dermal equivalent (Fig. 2a) [43]. These models have been shown to approximate granulation tissue due to biological and mechanical similarities [55], and fibroblasts will differentiate into myofibroblasts once stress develops in the gels [56]. Interestingly, fetal fibroblasts contracted the anchored collagen gels to a greater extent than adult fibroblasts at an early time point when tension was not yet present [43] similar to free floating collagen gels (Fig. 2b) [38]. However, once the gels were under noticeable tension, we found no difference between adult and fetal fibroblast contraction (Fig. 2b) [43]. At longer time points, adult fibroblasts had significantly surpassed fetal fibroblasts in their ability to contract the collagen gels (Figs. 2b–c) [43]. At the transition point in which mechanical tension was present, adult fibroblasts exhibited more prominent actin stress fibers that were rich in α-SMA (Fig. 2d) [43]. Despite differences in contraction, fetal fibroblasts were able to reorganize the anchored collagen gels to the same extent as adult fibroblasts suggesting that cellular forces exerted by untransformed fibroblasts are sufficient for ECM remodeling [43, 57].
Fig. 2.
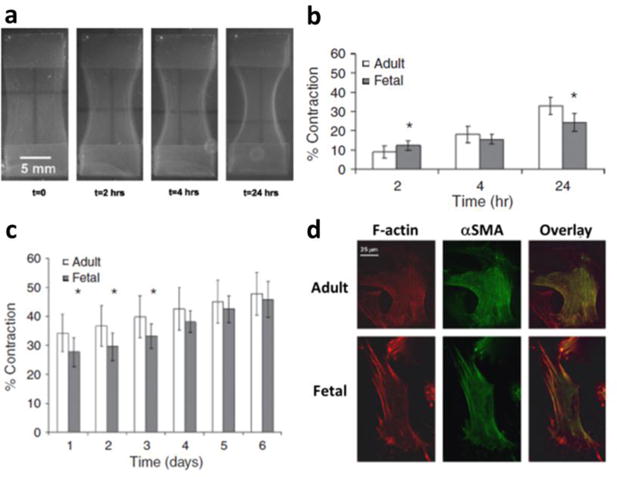
Anchored collagen gel contraction by human dermal adult and fetal fibroblasts. (A) Anchored collagen gel contraction over time by adult fibroblasts where contraction is calculated based on the change in area of the collagen gel between the stainless steel anchors (top and bottom). As the gels contract, they become taut between the anchors within 4 hours. (B) While fetal fibroblast contraction is initially greater, adult fibroblast contraction increases at a faster rate once mechanical tension develops in the collagen gel (4 hours) and becomes greater than fetal fibroblast contraction even at (C) later time points. (D) This difference in contractile ability coincides with the expression of prominent stress fibers containing α-SMA (yellow in overlay) throughout the cytoplasm of adult but not fetal fibroblasts at 4 hours suggesting a myofibroblast phenotype. Reprinted from Parekh A, Sandulache VC, Singh T, Cetin S, Sacks MS, Dohar JE, and Hebda PA, Prostaglandin E2 differentially regulates contraction and structural reorganization of anchored collagen gels by human adult and fetal dermal fibroblasts, Wound Repair and Regeneration, 2009, 17(1), 88–98 [43] with permission from John Wiley & Sons, Inc
THERAPEUTIC PERSPECTIVE
Initial therapeutic strategies targeting scarring and fibrosis have primarily focused on inflammation and collagen deposition [58]; however, there are currently no accepted anti-fibrotic therapies [59]. While these approaches have typically been designed to indirectly affect myofibroblasts, their lack of translational success suggests that new clinical avenues must be sought for directly targeting myofibroblasts to combat fibrotic healing. An alternative approach is to interfere with the contractile forces generated by myofibroblasts that lead to excessive ECM contraction and remodeling [10, 23]. Myofibroblast differentiation and force generation are ultimately a result of fibroblasts sensing increased mechanical stress in granulation tissue [10, 45]. From this perspective, potential methods include blocking specific integrins to interfere with the transduction of mechanical signals from the ECM to the cell thus reducing cellular force generation, contraction and remodeling, and/or activation of latent TGF-β1 [23]. Similarly, targeting molecules in integrin-based signaling pathways that respond to mechanical stress such as focal adhesion kinase may also impede myofibroblast differentiation and/or activity as well as other downstream profibrotic pathways [60–62]. In addition, altering the ECM mechanical environment can modulate myofibroblast contractile activity such as by interfering with cross-linking [63] or by offloading the mechanical stress in granulation tissue [64].
Careful consideration must be given to any potential therapies regarding the degree to which fibroblast activity is impeded since some activity is necessary for proper wound healing to occur. Coming from a fetal wound healing perspective, we have advocated for reducing adult fibroblast contractile forces to a “fetal level” in an attempt to promote regenerative healing [41, 38, 43]. Evidence from our work suggests that the unique contractile phenotype of fetal fibroblasts could serve as a biomechanical benchmark for fibroblast activity. While we and others have found some signaling defects in fetal fibroblasts which have been well documented [27–30], intrinsic biochemical variations in fetal fibroblasts could be further explored and potentially exploited in adult fibroblasts to compromise their fibrotic behavior. In particular, fetal fibroblasts appear to exhibit distinct responses to mechanical factors, thus we feel strongly that this phenotype warrants further exploration in an attempt to uncover new therapeutic targets that likely exist in mechanical signaling pathways. In addition, molecular methods aimed at reprogramming adult fibroblasts could be combined with bioengineered scaffolds to provide a mechanical environment more favorable for scarless healing [65, 29]. Alternatively, cell therapies using fetal fibroblasts could also be utilized to take advantage of their innate ability for regenerative repair [66–69] given their early but promising clinical results [26]. While the use of fetal cells and tissues is highly controversial and now significantly limited [70], human dermal fetal fibroblasts may hold the key for regeneration and warrant consideration for future work in this understudied area of wound healing.
CONCLUSIONS
While the properties of the fetal wound environment contribute to scarless healing, fetal fibroblasts exhibit distinct biochemical and biophysical characteristics that are necessary for regenerative repair. In particular, fetal fibroblasts exhibit a unique contractile phenotype that facilitates arrangement of the ECM in a manner that recapitulates its natural structure and function in the dermis. This cellular behavior is conserved in vitro, in vivo, and ex vivo indicating that fetal fibroblasts have intrinsic differences in their molecular makeup when compared to their adult counterparts. While more studies are necessary, fetal fibroblasts may not be able to differentiate into myofibroblasts due to altered biomechanical responses that reduce their contractility. Understanding the molecular mechanisms that regulate the contractile phenotype of fetal fibroblasts may reveal new therapeutic targets in mechanical signaling pathways to minimize scarring and fibrosis caused by adult fibroblast activity.
Acknowledgments
Support provided by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R03AR066875 to AP. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
The authors declare they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
Papers of particular interest with historical significance have been highlighted as •.
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17(1–2):113–25. doi: 10.2119/molmed.2009.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cass DL, Bullard KM, Sylvester KG, Yang EY, Longaker MT, Adzick NS. Wound size and gestational age modulate scar formation in fetal wound repair. J Pediatr Surg. 1997;32(3):411–5. doi: 10.1016/s0022-3468(97)90593-5. [DOI] [PubMed] [Google Scholar]
- 4.Burrington JD. Wound healing in the fetal lamb. J Pediatr Surg. 1971;6(5):523–8. doi: 10.1016/0022-3468(71)90373-3. [DOI] [PubMed] [Google Scholar]
- 5.Dohar JE, Klein EC, Betsch JL, Hebda PA. Fetal airway wound repair: a new frontier. Arch Otolaryngol Head Neck Surg. 1998;124(1):25–9. doi: 10.1001/archotol.124.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31(10):1143–52. doi: 10.1114/1.1616931. [DOI] [PubMed] [Google Scholar]
- 7.Wilgus TA. Regenerative healing in fetal skin: a review of the literature. Ostomy Wound Manage. 2007;53(6):16–31. quiz 2–3. [PubMed] [Google Scholar]
- 8 •.Lorenz HP, Lin RY, Longaker MT, Whitby DJ, Adzick NS. The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg. 1995;96(6):1251–9. doi: 10.1097/00006534-199511000-00002. discussion 60–1. This study was critical for establishing the role of human dermal fetal fibroblasts in scarless healing in vivo. [DOI] [PubMed] [Google Scholar]
- 9.Knight KR, Lepore DA, Horne RS, Ritz M, Hurley JV, Kumta S, et al. Collagen content of uninjured skin and scar tissue in foetal and adult sheep. Int J Exp Pathol. 1993;74(6):583–91. [PMC free article] [PubMed] [Google Scholar]
- 10.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43(1):146–55. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, et al. Mechanotransduction and fibrosis. J Biomech. 2014;47(9):1997–2005. doi: 10.1016/j.jbiomech.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. The Journal of cell biology. 1993;122(1):103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. The Journal of cell biology. 1998;142(3):873–81. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, Adzick NS. Scarless wound repair: a human fetal skin model. Development. 1992;114(1):253–9. doi: 10.1242/dev.114.1.253. [DOI] [PubMed] [Google Scholar]
- 15.Haynes JH, Krummel TM, Schatzki PF, Dunn JD, Flood LC, Cohen IK, et al. Histology of the open fetal rabbit wound. Surg Forum. 1989;40:558–60. [Google Scholar]
- 16.Krummel TM, Ehrlich HP, Nelson JM, Michna BA, Thomas BL, Haynes JH, et al. In vitro and in vivo analysis of the inability of fetal rabbit wounds to contract. Wound Repair Regen. 1993;1(1):15–21. doi: 10.1046/j.1524-475X.1993.10106.x. [DOI] [PubMed] [Google Scholar]
- 17.Dostal GH, Gamelli RL. Fetal wound healing. Surg Gynecol Obstet. 1993;176(3):299–306. [PubMed] [Google Scholar]
- 18.Hallock GG, Rice DC, Merkel JR, DiPaolo BR. Analysis of collagen content in the fetal wound. Ann Plast Surg. 1988;21(4):310–5. doi: 10.1097/00000637-198810000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, et al. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg. 1990;25(1):63–8. doi: 10.1016/s0022-3468(05)80165-4. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 20.Julia MV, Albert A, Morales L, Miro D, Sancho MA, Garcia X. Wound healing in the fetal period: the resistance of the scar to rupture. J Pediatr Surg. 1993;28(11):1458–62. doi: 10.1016/0022-3468(93)90430-s. doi:0022-3468(93)90430-S [pii] [DOI] [PubMed] [Google Scholar]
- 21.Lovvorn HN, 3rd, Cheung DT, Nimni ME, Perelman N, Estes JM, Adzick NS. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg. 1999;34(1):218–23. doi: 10.1016/s0022-3468(99)90261-0. [DOI] [PubMed] [Google Scholar]
- 22.Gharaee-Kermani M, Phan SH. Role of cytokines and cytokine therapy in wound healing and fibrotic diseases. Curr Pharm Des. 2001;7(11):1083–103. doi: 10.2174/1381612013397573. [DOI] [PubMed] [Google Scholar]
- 23.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, Adzick NS. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg. 1994;219(1):65–72. doi: 10.1097/00000658-199401000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wider TM, Yager JS, Rittenberg T, Hugo NE, Ehrlich HP. The inhibition of fibroblast-populated collagen lattice contraction by human amniotic fluid: a chronologic examination. Plast Reconstr Surg. 1993;91(7):1287–93. doi: 10.1097/00006534-199306000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Hohlfeld J, de Buys Roessingh A, Hirt-Burri N, Chaubert P, Gerber S, Scaletta C, et al. Tissue engineered fetal skin constructs for paediatric burns. Lancet. 2005;366(9488):840–2. doi: 10.1016/S0140-6736(05)67107-3. [DOI] [PubMed] [Google Scholar]
- 27.Lo DD, Zimmermann AS, Nauta A, Longaker MT, Lorenz HP. Scarless fetal skin wound healing update. Birth Defects Res C Embryo Today. 2012;96(3):237–47. doi: 10.1002/bdrc.21018. [DOI] [PubMed] [Google Scholar]
- 28.Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol. 2012;2012:698034. doi: 10.5402/2012/698034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu MS, Maan ZN, Wu JC, Rennert RC, Hong WX, Lai TS, et al. Tissue engineering and regenerative repair in wound healing. Ann Biomed Eng. 2014;42(7):1494–507. doi: 10.1007/s10439-014-1010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walraven M, Gouverneur M, Middelkoop E, Beelen RH, Ulrich MM. Altered TGF-beta signaling in fetal fibroblasts: what is known about the underlying mechanisms? Wound Repair Regen. 2014;22(1):3–13. doi: 10.1111/wrr.12098. [DOI] [PubMed] [Google Scholar]
- 31.Gosiewska A, Yi CF, Brown LJ, Cullen B, Silcock D, Geesin JC. Differential expression and regulation of extracellular matrix-associated genes in fetal and neonatal fibroblasts. Wound Repair Regen. 2001;9(3):213–22. doi: 10.1046/j.1524-475x.2001.00213.x. doi:wrr213 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Broker BJ, Chakrabarti R, Blynman T, Roesler J, Wang MB, Srivatsan ES. Comparison of growth factor expression in fetal and adult fibroblasts: a preliminary report. Arch Otolaryngol Head Neck Surg. 1999;125(6):676–80. doi: 10.1001/archotol.125.6.676. [DOI] [PubMed] [Google Scholar]
- 33.Moulin V, Tam BY, Castilloux G, Auger FA, O’Connor-McCourt MD, Philip A, et al. Fetal and adult human skin fibroblasts display intrinsic differences in contractile capacity. J Cell Physiol. 2001;188(2):211–22. doi: 10.1002/jcp.1110. [DOI] [PubMed] [Google Scholar]
- 34.Lanning DA, Diegelmann RF, Yager DR, Wallace ML, Bagwell CE, Haynes JH. Myofibroblast induction with transforming growth factor-beta1 and -beta3 in cutaneous fetal excisional wounds. J Pediatr Surg. 2000;35(2):183–7. doi: 10.1016/s0022-3468(00)90007-1. discussion 7–8. doi:S0022-3468(00)90007-1 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Colwell AS, Krummel TM, Longaker MT, Lorenz HP. Fetal and adult fibroblasts have similar TGF-beta-mediated, Smad-dependent signaling pathways. Plast Reconstr Surg. 2006;117(7):2277–83. doi: 10.1097/01.prs.0000224299.16523.76. [DOI] [PubMed] [Google Scholar]
- 36.Moulin V, Plamondon M. Differential expression of collagen integrin receptor on fetal vs. adult skin fibroblasts: implication in wound contraction during healing. Br J Dermatol. 2002;147(5):886–92. doi: 10.1046/j.1365-2133.2002.04975.x. doi:4975 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Coleman C, Tuan TL, Buckley S, Anderson KD, Warburton D. Contractility, transforming growth factor-beta, and plasmin in fetal skin fibroblasts: role in scarless wound healing. Pediatr Res. 1998;43(3):403–9. doi: 10.1203/00006450-199803000-00016. [DOI] [PubMed] [Google Scholar]
- 38.Parekh A, Sandulache VC, Lieb AS, Dohar JE, Hebda PA. Differential regulation of free-floating collagen gel contraction by human fetal and adult dermal fibroblasts in response to prostaglandin E2 mediated by an EP2/cAMP-dependent mechanism. Wound Repair Regen. 2007;15(3):390–8. doi: 10.1111/j.1524-475X.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 39.Ehrlich HP, Rajaratnam JB. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell. 1990;22(4):407–17. doi: 10.1016/0040-8166(90)90070-p. [DOI] [PubMed] [Google Scholar]
- 40.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. The Journal of cell biology. 1994;124(4):401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandulache VC, Parekh A, Li-Korotky HS, Dohar JE, Hebda PA. Prostaglandin E2 differentially modulates human fetal and adult dermal fibroblast migration and contraction: implication for wound healing. Wound Repair Regen. 2006;14(5):633–43. doi: 10.1111/j.1743-6109.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 42.Sandulache VC, Parekh A, Dohar JE, Hebda PA. Fetal dermal fibroblasts retain a hyperactive migratory and contractile phenotype under 2-and 3-dimensional constraints compared to normal adult fibroblasts. Tissue Eng. 2007;13(11):2791–801. doi: 10.1089/ten.2006.0412. [DOI] [PubMed] [Google Scholar]
- 43.Parekh A, Sandulache VC, Singh T, Cetin S, Sacks MS, Dohar JE, et al. Prostaglandin E2 differentially regulates contraction and structural reorganization of anchored collagen gels by human adult and fetal dermal fibroblasts. Wound Repair Regen. 2009;17(1):88–98. doi: 10.1111/j.1524-475X.2008.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature reviews. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45 •.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159(3):1009–20. doi: 10.1016/S0002-9440(10)61776-2. This study was critical for establishing that mechanical tension in granulation tissue regulated myofibroblast activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87(8-9):601–15. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154(3):871–82. doi: 10.1016/s0002-9440(10)65334-5. doi:S0002-9440(10)65334-5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21(12):3250–61. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 49.Volk SW, Wang Y, Mauldin EA, Liechty KW, Adams SL. Diminished type III collagen promotes myofibroblast differentiation and increases scar deposition in cutaneous wound healing. Cells Tissues Organs. 2011;194(1):25–37. doi: 10.1159/000322399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin GL, Cohen DM, Desai RA, Breckenridge MT, Gao L, Humphries MJ, et al. Activation of beta 1 but not beta 3 integrin increases cell traction forces. FEBS Lett. 2013;587(6):763–9. doi: 10.1016/j.febslet.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JK, Xu Y, Xu X, Keene DR, Gurusiddappa S, Liang X, et al. A novel binding site in collagen type III for integrins alpha1beta1 and alpha2beta1. J Biol Chem. 2005;280(37):32512–20. doi: 10.1074/jbc.M502431200. [DOI] [PubMed] [Google Scholar]
- 52.Yang L, Tsai CM, Hsieh AH, Lin VS, Akeson WH, Sung KL. Adhesion strength differential of human ligament fibroblasts to collagen types I and III. J Orthop Res. 1999;17(5):755–62. doi: 10.1002/jor.1100170521. [DOI] [PubMed] [Google Scholar]
- 53.Roca-Cusachs P, Gauthier NC, Del Rio A, Sheetz MP. Clustering of alpha(5)beta(1) integrins determines adhesion strength whereas alpha(v)beta(3) and talin enable mechanotransduction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(38):16245–50. doi: 10.1073/pnas.0902818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chopra A, Murray ME, Byfield FJ, Mendez MG, Halleluyan R, Restle DJ, et al. Augmentation of integrin-mediated mechanotransduction by hyaluronic acid. Biomaterials. 2014;35(1):71–82. doi: 10.1016/j.biomaterials.2013.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carlson MA, Longaker MT. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen. 2004;12(2):134–47. doi: 10.1111/j.1067-1927.2004.012208.x. [DOI] [PubMed] [Google Scholar]
- 56.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends in cell biology. 2003;13(5):264–9. doi: 10.1016/s0962-8924(03)00057-6. doi:S0962892403000576 [pii] [DOI] [PubMed] [Google Scholar]
- 57.Huang C, Akaishi S, Ogawa R. Mechanosignaling pathways in cutaneous scarring. Arch Dermatol Res. 2012;304(8):589–97. doi: 10.1007/s00403-012-1278-5. [DOI] [PubMed] [Google Scholar]
- 58.Leung A, Crombleholme TM, Keswani SG. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr. 2012;24(3):371–8. doi: 10.1097/MOP.0b013e3283535790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryu JH, Daniels CE. Advances in the management of idiopathic pulmonary fibrosis. F1000 Med Rep. 2010;2:28. doi: 10.3410/M2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, et al. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2011;18(1):148–52. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paterno J, Vial IN, Wong VW, Rustad KC, Sorkin M, Shi Y, et al. Akt-mediated mechanotransduction in murine fibroblasts during hypertrophic scar formation. Wound Repair Regen. 2011;19(1):49–58. doi: 10.1111/j.1524-475X.2010.00643.x. [DOI] [PubMed] [Google Scholar]
- 62.Rustad KC, Wong VW, Gurtner GC. The role of focal adhesion complexes in fibroblast mechanotransduction during scar formation. Differentiation. 2013;86(3):87–91. doi: 10.1016/j.diff.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, et al. Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med. 2010;16(9):1009–17. doi: 10.1038/nm.2208. [DOI] [PubMed] [Google Scholar]
- 64.Gurtner GC, Dauskardt RH, Wong VW, Bhatt KA, Wu K, Vial IN, et al. Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg. 2011;254(2):217–25. doi: 10.1097/SLA.0b013e318220b159. [DOI] [PubMed] [Google Scholar]
- 65.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 66.Hebda PA, Dohar JE. Transplanted fetal fibroblasts: survival and distribution over time in normal adult dermis compared with autogenic, allogenic, and xenogenic adult fibroblasts. Otolaryngol Head Neck Surg. 1999;121(3):245–51. doi: 10.1016/S0194-5998(99)70179-8. [DOI] [PubMed] [Google Scholar]
- 67.Sandulache VC, Zhou Z, Sherman A, Dohar JE, Hebda PA. Impact of transplanted fibroblasts on rabbit skin wounds. Arch Otolaryngol Head Neck Surg. 2003;129(3):345–50. doi: 10.1001/archotol.129.3.345. [DOI] [PubMed] [Google Scholar]
- 68.Sandulache VC, Dohar JE, Hebda PA. Fibroblast transplantation in the airway: implications for subglottic stenosis. Arch Otolaryngol Head Neck Surg. 2005;131(12):1090–6. doi: 10.1001/archotol.131.12.1090. [DOI] [PubMed] [Google Scholar]
- 69.Sandulache VC, Dohar JE, Hebda PA. Adult-fetal fibroblast interactions: effects on cell migration and implications for cell transplantation. Cell Transplant. 2005;14(5):331–7. doi: 10.3727/000000005783983025. [DOI] [PubMed] [Google Scholar]
- 70.Wadman M. The truth about fetal tissue research. Nature. 2015;528(7581):178–81. doi: 10.1038/528178a. [DOI] [PubMed] [Google Scholar]


