Abstract
Background
Driving is a key functional activity for many older adults, and changes in routine driving may be associated with emerging cognitive decline due to early neurodegenerative disease. Current methods for assessing driving such as self-report are inadequate for identifying and monitoring subtle changes in driving patterns that may be the earliest signals of functional change in developing mild cognitive impairment (MCI).
Objective
This proof of concept study aimed to establish the feasibility of continuous driving monitoring in a sample of cognitively normal and MCI older adults for an average of 206 days using an unobtrusive driving sensor and demonstrate that derived sensor-based driving metrics could effectively discriminate between MCI and cognitively intact groups.
Methods
Novel objective driving measures derived from 6 months of routine driving monitoring were examined in older adults with intact cognition (n = 21) and MCI (n = 7) who were enrolled in the Oregon Center for Aging and Technology (ORCATECH) longitudinal assessment program.
Results
Unobtrusive continuous monitoring of older adults’ routine driving using a driving sensor was feasible and well accepted. MCI participants drove fewer miles and spent less time on the highway per day than cognitively intact participants. MCI drivers showed less day-to-day fluctuations in their driving habits than cognitively intact drivers.
Conclusion
Sensor-based driving measures are objective, unobtrusive, and can be assessed every time a person drives his or her vehicle to identify clinically meaningful changes in daily driving. This novel methodology has the potential to be useful for the early detection and monitoring of changes in daily functioning within individuals.
Keywords: Aging, cognitive decline, mild neurocognitive disorder, technology
INTRODUCTION
Early detection and monitoring of functional change in prodromal Alzheimer’s disease (AD) is important for effective implementation and evaluation of disease modifying treatments as they become available. A modest delay in the onset or progression of mild cognitive impairment (MCI) due to AD would significantly reduce the number of people affected, costs associated with the disease, and negative patient and caregiver outcomes [1]. In the transition from normal aging to MCI, subtle changes emerge in the initiation and completion of everyday tasks such as device use, medication taking, and computer use [2–8]. Early signals of functional change are a prime target for early detection of MCI. However, conventional episodic assessment tools do not capture subtle intra-individual changes in carrying out cognitively mediated daily tasks [9]. Clinic-based assessments can also be taxing, time consuming, and costly. Practical, cost effective, and noninvasive assessment tools are needed that can reliably detect subtle yet meaningful changes in daily tasks. Advances in wireless technology, pervasive computing, and data analytics can now be applied to continuously monitor routine daily activities inside and outside one’s home to assess real-world cognitive and functional changes [10, 11].
Driving is a highly complex activity that requires intact attention, speed of information processing, visuospatial skills, memory, and executive functioning [12]. Driving is also a key functional activity for many older adults as it is often central for maintaining independence and personal agency [13]. Given that over 80% of individuals over age 65 drive on a regular basis [14], routine driving may be a particularly relevant functional activity to monitor in longitudinal natural history studies and clinical trials. Older drivers as a group tend to self-regulate their driving [15], and this may be due in part to normative age-related lifestyle (e.g., more flexible schedules) or physical (e.g., decreased visual acuity) changes. Accumulating evidence suggests that certain changes in routine driving may also be associated with emerging cognitive decline due to early neurodegenerative disease [12, 16]. Self-report, informant-report, and simulator studies have observed that older adults with MCI have more difficulty driving, drive less often, make more errors, and are less confident drivers compared to cognitively intact older adults [12, 16–18]. Although self-report is a common method of gathering information about one’s daily functioning, accuracy of self-reported driving behavior has been repeatedly questioned [19]. Self-report is not well suited for assessing subtle aspects of driving such as context (e.g., time of day, day of week, type of roadway), specific episodic behaviors (e.g., hard breaks, left turns), and variability in driving patterns that may be important for understanding clinically relevant changes in older adults’ driving due to early neurodegenerative disease.
While real-world driving behavior is routinely monitored by sensor-based tracking devices on a large scale for industry (e.g., usage based auto insurance, commercial fleets), these methods have not been adequately investigated with older adult or cognitively impaired populations for the detection or tracking of cognitive decline. In the scientific literature, most research using these methods has been focused on driving outcomes, crash risk, and/or teenage drivers [20–22]. The Canadian and Australian CANDRIVE study is a unique multi-center prospective cohort study tracking driving outcomes of older adult drivers via self-report, transportation records, and in a subset, passive sensing devices [23–25]. Another group has used commercially available, off the shelf devices to determine the optimal time interval needed to obtain accurate data on an actual route driven with a small sample of older adults [26]. The latter group used the same sensor technology to derive spatial, temporal, and behavioral measures of driving for 5 cognitively normal older adults over 5 months [27]. Unobtrusive vehicle tracking devices may offer more objective measures of real-world driving that are needed to advance the assessment of clinically meaningful functioning in aging and dementia research.
The present study, embedded within the Oregon Center for Aging and Technology (ORCATECH; http://www.ohsu.edu/xd/research/centers-institutes/orcatech/index.cfm) longitudinal assessment program, first aimed to establish the feasibility of continuous driving monitoring in a sample of cognitively normal and MCI older adults for six months using an inexpensive, commercially available driving sensor. We developed computing infrastructure and algorithms to derive meaningful metrics of driving activity from transmitted sensor data in the domains of driving frequency, distance, duration, context, and behavior. Given the cognitive demands of driving, our second aim was to demonstrate that the derived sensor-based driving metrics could effectively discriminate between MCI and cognitively intact participants. Based on previous findings using clinic-based driving assessment methods, we hypothesized that older adults with MCI would make fewer trips and drive shorter distances than cognitively normal older adults. Previous studies have demonstrated a relationship between neuropsychological tests and driving performance via self-report and simulator approaches. We aimed to extend those findings and increase knowledge of the cognitive correlates of real-world driving behaviors. Based on available literature, we expected that the sensor-based driving metrics would be most strongly correlated with cognitive tests in the domains of global cognition and executive functioning. As an exploratory analysis, we investigated the longitudinal trajectories of the sensor-based driving metrics of MCI and cognitively intact participants.
METHODS
Participants
All participants provided written informed consent for this driving monitoring study (OHSU IRB# 12064) and were already enrolled in ongoing studies within the NIA-Layton Alzheimer’s Disease Center and ORCATECH. Clinical and cognitive data were collected as part of these longitudinal aging studies. Participants were recruited from the Portland, Oregon, metropolitan area through advertisement and presentations at local retirement communities. The clinical study protocols were approved by the Oregon Health & Science University Institutional Review Board (Life Laboratory IRB #2765; ADC IRB #725, ADNI #6923, AADAPt #1480, and CBDP #1639). Inclusion criteria were: 62 y and older, living independently (living with a companion or spouse was allowed, but not as caregiver), without dementia as evidenced by a Mini-Mental State Examination (MMSE) [28] score greater than 24 and a Clinical Dementia Rating (CDR) [29] scale score less than or equal to 0.5, in average health for age without poorly controlled medical illnesses, have a general class driver’s license and have been actively driving for at least 1 year, drive at least once per week, live in the local region for at least nine months/year, intend to drive for the next year, own or lease a vehicle with model year 1996 or newer, and be the primary driver of that vehicle (drive that vehicle >75% of the time). For the present study, we enrolled a convenience sample of 31 older adults; 28 participated in all or most of the driving study. The average number days in the driving study was 206 ± 36 (March-September 2016). Five participants had >8 months of driving recorded and one participant had only 4 months of data recorded. Two cognitively intact participants withdrew early - one due to a technical complaint and one gave up her car and stopped driving because of infrequent use and the availability of convenient alternative transportation options (e.g., streetcar).
Clinical assessment procedures
Participants received clinical assessments during annual visits in their homes using a standardized battery of tests including the MMSE and the Geriatric Depression Scale-15 [30]. Clinical and cognitive data from the annual visit closest to their enrollment into the driving study were used. Classification of MCI was defined by (1) CDR score of 0.5, (2) preserved general cognitive functions as confirmed by a score of 24 or above on the MMSE; (3) no significant change in functional abilities, as confirmed by two or fewer activities marked as dependent on the FAQ; and (4) absence of severe depression as confirmed by a score <5 on the 15-item GDS. Neuropsychological tests were administered to participants as part of their annual clinical exams. Tests in the memory domain included WMS-R [31] Logical Memory II Story A, WMS-R Visual Reproduction II, and CERAD [32] Word-List Recall. Language tests included Boston Naming Test [33] and category fluency (animals). Executive function tests included letter fluency (CFL), Trail Making Test [34] Part B, and Stroop [35] color-word conflict. Processing speed tests included WAIS-R [36] Digit Symbol, Trail Making Test Part A, and Stroop color naming. Working memory tests included WAIS-R Digits Backward, WAIS-III Letter Number Sequencing or WAIS-IV [37] Digit Sequencing, and MMSE item WORLD backward. Visual Perceptual/Construction tests included WAIS-R Block Design, WAIS-R Picture Completion, and WMS-R Visual Reproduction I. Cognitive domain z-scores were tabulated from 2–3 representative neuropsychological tests for each of five cognitive domains. The use of composite cognitive scores is a common procedure for increasing reliability of results and decreasing Type 1 errors from excessive multiple comparisons. It also has the advantage of minimizing floor and ceiling effects and other types of random variability. Although each test requires multiple cognitive skills, we classified the tests assessing related abilities into standard representative cognitive domains. Cognitive domain z-scores were calculated using group mean and standard deviations of the raw test scores from all cognitively intact subjects (CDR = 0) at study entry into the ORCATECH cohorts (n = 180). The individual participant scores were z-normalized, summed, and averaged. Use of CDR score for classification of MCI was chosen to avoid diagnostic circularity with the use of neuropsychological test scores in correlation analyses with the driving sensor data.
Driving assessment platform and sensor-based driving metrics
The driving assessment platform consisted of a commercially available passive sensing device (http://www.automatic.com) plugged into participants’ vehicle data port. Using this platform, raw data for each drive were automatically recorded, processed via cellular link (Bluetooth or 3 G wireless) through a study iPhone located in the car with the Automatic app, securely transmitted to a central data server, and uploaded to our research database. An open application programming interface was used to write software algorithms to read transmitted trip data and derive our variables of interest. Driving variables were calculated on a daily, weekly, and monthly basis in the domains of Frequency: Number of Trips; Duration: Total Time Driving; Distance: Total Mileage; Context: Time of First Trip, Time of Last Trip, Time Driving on Local Streets, Time Driving on Highways, Time Driving during Daylight, Time Driving After Dusk/At Night, Number of Unique Destinations; and Episodic Behavior: Number of Hard Breaks, Number of Hard Accelerations, Number of Left Turns, Number of Right Turns. Mean and variability (standard deviation) in the daily, weekly, and monthly driving variables were calculated using days with at least one drive. Participants completed a self-report driving habits questionnaire at study entry and a feedback survey at study exit. We tracked times when the driving sensor or app malfunctioned and excluded these data from analyses. Participants reported vacation time or family members driving their car to a study research assistant and we excluded those data from analyses.
Statistical analysis
Mean and day-to-day variability (standard deviation) of daily driving metrics were calculated over the entire study period as well as percentage of all days with a drive and percentage of days with ≥ 20 miles driven. Chi-square test or Fisher’s exact test (for small cell sizes) were used to examine cross-sectional group differences in categorical variables. For each continuous variable, the histogram was visually inspected and a goodness-of-fit test was used to determine the normality of the distribution. Two-sample t-test or Wilcoxon rank sum test (its nonparametric counterpart) were used to examine group differences in continuous variables. Spearman’s r correlation was used to examine cognitive correlates of driving measures. Longitudinal generalized mixed effects regression models were used to explore trajectories of driving activity in cognitively normal and MCI participants over time. Self-reported and objective driving round trips and miles per week were categorized into binary variables and compared via chi-square test. The prevalence of discordance (underestimation) of self-report versus objective driving was examined by cognitive status via chi-square test. Given the small sample size in this pilot study, analyses were not adjusted for covariates. Analyses were performed using SAS software 9.4 (Cary, NC).
RESULTS
Descriptive statistics
Our sample included 28 participants (21 cognitively intact, 7 MCI) with a mean age of 82 years (Table 1). Our sample was 62% female, well educated (mean = 15.6 years), with low levels of depression (mean GDS score = 0.8). The average MMSE score was 28.6. Our participants lived in both retirement communities (50%) and in single-family homes. Nineteen of 28 (68%) lived alone. There were no significant group differences in type of residence (e.g., retirement center versus single-family home). MCI and cognitively intact participants’ residences were spread throughout the metro area with no trend toward urban over suburban or rural in either group. There were 16 unique zip codes in the sample of 28 participants. There were no significant group differences in age, sex, education, global cognitive screening score, or depression (p’s > 0.05).
Table 1.
Participant characteristics (mean and standard deviation or percentages are presented)
| Variable | Total | Intact | MCI | p-value |
|---|---|---|---|---|
| N | 28 | 21 | 7 | |
| Age at baseline (y) | 82.0 (7.5) | 82.0 (6.3) | 81.8 (11.0) | 0.92 |
| Gender (% female) | 61% | 71% | 29% | 0.08 |
| Education (y) | 15.6 (3.4) | 15.5 (3.4) | 15.9 (3.4) | 0.98 |
| MMSE | 28.6 (1.5) | 28.8 (1.5) | 28.0 (1.6) | 0.20 |
| Global cognitive z-score | 0.1 (0.7) | 0.2 (0.6) | −0.4 (0.9) | 0.05 |
| Executive function z-score | 0.3 (1.0) | 0.4 (0.9) | −0.3 (1.0) | 0.08 |
| Working memory z-score | −0.4 (1.3) | −0.3 (1.2) | −0.8 (1.4) | 0.44 |
| Attention z-score | −0.02 (0.76) | 0.0 (0.8) | −0.1 (0.8) | 0.73 |
| Memory z-score | 0.1 (1.0) | 0.3 (0.7) | −0.8 (1.3) | 0.06 |
| Visuospatial z-score | 0.4 (0.8) | 0.6 (0.7) | −0.4 (0.7) | <0.01** |
| Geriatric Depression Scale | 0.8 (1.1) | 0.5 (0.8) | 1.6 (1.4) | 0.06 |
| Functional Assessment Questionnaire | 1.0 (2.1) | 0.4 (1.1) | 3.0 (3.5) | 0.05 |
| Reside in a retirement community (%) | 50% | 62% | 14% | 0.08 |
| Living alone (%) | 68% | 76% | 43% | 0.17 |
| Miles from nearest freeway | 1.8 (1.7) | 1.9 (1.9) | 1.6 (1.0) | 0.77 |
p < 0.01.
Self-report driving habits questionnaire
On the self-report driving questionnaire administered at study entry, 39% of the sample reported driving five or more times per week. Seventy-three percent reported driving 3 or more times per week during daylight compared to 26% reporting nighttime driving with the same frequency. Participants in our sample reported driving more often in familiar areas than in unfamiliar areas, with 73% driving in familiar areas 3 or more times per week, versus 13% in unfamiliar areas with the same frequency. 50% reported driving on local streets 3+ times per week, while 45% reported driving on highways 3+ times per week. Sixteen percent reported using a cell phone while driving at least one time per week, and 23% reported using a GPS system while driving at least one time per week. Sixty-five percent of the sample reported driving 20+ miles per week. There were no significant group differences in the self-report driving habits items.
Sensor-based driving data
MCI and Cognitively Intact participants took close to the same number of trips per day; however, Cognitively Intact older adults showed greater variability in their daily driving distance and time (Table 2). They also drove more miles and spent more time driving on the highway per day than MCI participants. There were no significant group differences in time spent driving during daytime or nighttime, number of left or right-hand turns, driving over 70 mph or number of hard breaks or accelerations per day.
Table 2.
Summary driving characteristics per day (mean and standard deviation or percentages are presented)
| Variable | Total | Intact | MCI | p-value |
|---|---|---|---|---|
| N | 28 | 21 | 7 | |
| Mean # of (one-way) trips per day | 4.2 (1.0) | 4.1 (0.9) | 4.7 (1.4) | 0.19 |
| Day-to-day variability in # of trips | 2.1 (0.6) | 2.1 (0.5) | 2.3 (0.8) | 0.49 |
| Mean distance driven per day (miles) | 20 (13) | 22 (13) | 14 (11) | 0.06 |
| Day-to-day variability in distance driven | 26 (17) | 31 (17) | 13 (12) | 0.01* |
| Mean time driven per day (h) | 0.9 (0.4) | 0.9 (0.4) | 0.8 (0.4) | 0.26 |
| Day-to-day variability in time driven | 0.7 (0.3) | 0.8 (0.3) | 0.5 (0.2) | <0.01** |
| Mean first clock start time of driving per day | 11.06 (1.2) | 11.18 (1.2) | 10.36 (1.4) | 0.43 |
| Day-to-day variability in first start time (h) | 2.8 (0.8) | 2.8 (0.6) | 2.7 (1.1) | 0.78 |
| Mean last clock start time of driving per day | 15.4 (1.6) | 15.2 (1.4) | 15.9 (1.9) | 0.33 |
| Day-to-day variability in last start time (h) | 3.2 (0.6) | 3.2 (0.6) | 3.2 (0.8) | 0.79 |
| Mean # of days monitored | 206 (36) | 208 (38) | 201 (33) | 0.70 |
| % of days at least one trip was taken out of all days monitored | 52% | 49% | 60% | 0.21 |
| % of driving days with ≥20 miles driven | 26% | 27% | 21% | 0.51 |
| Mean time of highway driving per day (s) | 450 (506) | 543 (533) | 172 (288) | 0.01* |
| Mean time of nighttime driving per day (s) | 227 (323) | 191 (194) | 337 (571) | 0.81 |
| Mean # left turns per day | 8.2 (3.2) | 7.9 (3.3) | 9.2 (3.0) | 0.35 |
| Mean # right turns per day | 9.9 (3.9) | 9.6 (4.1) | 10.9 (3.0) | 0.43 |
| Mean time driving over 70 mph from day (s) | 38 (60) | 44 (62) | 21 (53) | 0.24 |
| Mean # of hard breaks per day | 1.2 (0.9) | 1.3 (0.9) | 0.7 (0.6) | 0.09 |
| Mean # of hard accelerations per day | 0.8 (0.8) | 0.8 (0.9) | 0.7 (0.7) | 0.83 |
| Self-reported driving habits | ||||
| Drives five or more round trips per week (%) | 39% | 33% | 57% | 0.38 |
| Drives 20 or more miles per week (%) | 64% | 67% | 57% | 0.67 |
| Drives on highway three or more times per week (%) | 43% | 43% | 43% | 1.0 |
p < 0.05;
p < 0.01; p = 0.05/23 = 0.0022 after adjusting for multiple comparisons.
Associations between driving data and cognitive test scores
We examined correlations between global and cognitive domain z-scores, self-report. and sensor driving variables. More sensor monitored driving (distance, time, and highway) and variability in daily driving was associated with higher cognitive scores (Table 3). Executive functioning was the individual cognitive domain most strongly associated with the sensor driving variables (Table 4). There were no significant associations between self-reported driving (round trips, distance, and highway) variables and cognitive test scores.
Table 3.
Spearman’s r correlations between 6-month sensor driving summary variables (per day) and global cognitive z-scores; n = 28
| Spearman’s r | p-value | |
|---|---|---|
| Variable | ||
| Mean # of trips per day | 0.09 | 0.65 |
| Day-to-day variability in # of trips | 0.00 | 1.00 |
| Mean distance driven per day (miles) | 0.51 | <0.01** |
| Day-to-day variability in distance driven | 0.47 | 0.01* |
| Mean time driven per day (min) | 0.39 | 0.04* |
| Day-to-day variability in time driven | 0.42 | 0.03* |
| Mean first clock start time of driving per day | 0.16 | 0.41 |
| Day-to-day variability in first start time | 0.44 | 0.02* |
| Mean last clock start time of driving per day | 0.15 | 0.44 |
| Day-to-day variability in last start time | 0.20 | 0.30 |
| % of days at least one trip was taken out of all days monitored | −0.26 | 0.19 |
| % of driving days ≥20 miles | 0.44 | 0.02* |
p < 0.05;
p < 0.01; p = 0.0042 after adjusting for multiple comparisons.
Table 4.
Spearman’s r correlations (p-values) between driving summary variables (per day) and domain-specific cognitive z-scores; n = 28
| Executive function | Attention | Memory | Working memory | Visuospatial | |
|---|---|---|---|---|---|
| Variable | |||||
| Mean distance driven per day (miles) | 0.54 | 0.49 | 0.38 | NS | NS |
| 0.003** | <0.01** | 0.045* | |||
| Day-to-day variability in distance driven | 0.49 | NS | 0.42 | NS | 0.40 |
| <0.01** | 0.03* | <0.05* | |||
| Mean time driven per day (min) | 0.49 | NS | NS | NS | NS |
| <0.01** | |||||
| Day-to-day variability in time driven | 0.42 | 0.38 | 0.38 | NS | NS |
| 0.03* | <0.05* | <0.05* | |||
| Day-to-day variability in first start time | NS | NS | 0.47 | NS | NS |
| 0.02 | |||||
| % of driving days ≥ 20 miles | 0.49 | 0.44 | NS | NS | NS |
| <0.01** | 0.02* | ||||
| Mean time of highway driving per day (s) | 0.59 | 0.46 | 0.43 | 0.40 | 0.50 |
| 0.001** | 0.02* | 0.02* | 0.04* | 0.01* | |
p < 0.05;
p < 0.01.
NS, not significant; p = 0.0014 after adjusting for multiple comparisons.
Comparisons between objective-sensor and self-report driving data
Thirty-nine percent of the sample self-reported driving five or more round trips per week. However, objective data showed that a significant number of participants underestimated the number of trips per week. In fact, 75% of the cohort actually drives five or more round trips per week. There was no difference in those who underestimated their round trips according to diagnostic group or global cognitive z-score. Sixty-five percent of the sample self-reported driving 20 or more miles per week on average. However, objective data showed that 100% of the sample actually drives 20 or more miles per week on average (lowest = 24). There was no difference in the 35% who underestimated their miles per week according to diagnostic group or cognitive scores. Despite limitations of self-report, correlation analyses between self-report and corresponding objective driving variables revealed that self-reported round trips per week were significantly correlated with actual sensor-measured round trips per week, p < 0.0001. However self-reported distance driven and highway driving were not correlated with the corresponding sensor driving metrics.
Exploratory longitudinal analysis of sensor-monitored driving data
There were no significant differences in the longitudinal slope (or change) in daily driving metrics over the study period between the intact and MCI participants (Table 5, Figs. 1 and 2).
Table 5.
Mixed effects models to examine changes in driving metrics over time according to cognitive status
| Covariate | Model 1: Daily Total Driving Distance
|
Model 2: Daily Total Driving Time
|
||
|---|---|---|---|---|
| Coefficient | p-value | Coefficient | p-value | |
| MCI versus intact | −0.26 | 0.37 | −0.08 | 0.63 |
| Time, days | 0.0006 | 0.20 | 0.0003 | 0.31 |
| MCI * time | −0.001 | 0.24 | −0.001 | 0.08 |
Driving variables log-transformed to obtain normal distribution.
Fig. 1.
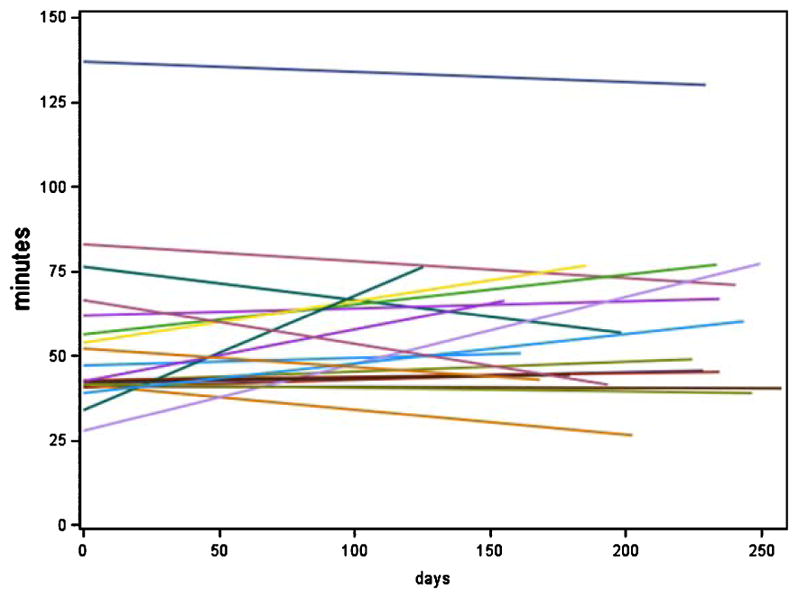
Total daily driving (in min) over time by cognitive group. Displays data from cognitively intact group.
Fig. 2.
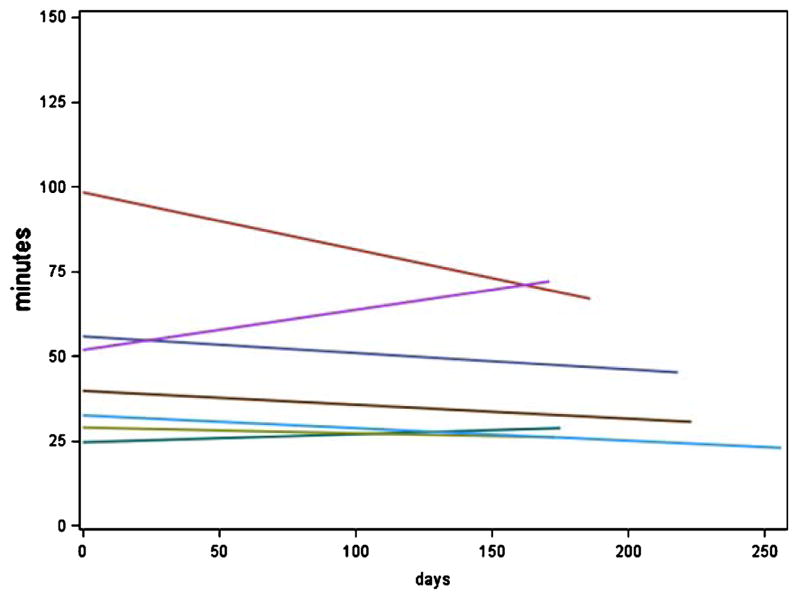
Total daily driving (in min) over time by cognitive group. Displays data from the MCI group. The y-axis shows the total daily driving in min (numbers are predicted min obtained from the mixed-effects models) and the x-axis shows days.
Study feedback exit survey
Approximately 90% of participants reported that participating in the study required almost no time or very little time. 89% strongly agreed that they did not mind having the cell phone and driving sensor in their car, 76% reported that they never/rarely had problems with the devices in their car, and 83% reported they would likely participate in a future driving monitoring study (all participants except the one who dropped out due to technical issues).
DISCUSSION
Unobtrusive continuous monitoring of older adults’ routine driving using a driving sensor easily installed in the data port of the participant’s vehicle was feasible and well accepted in our sample. Over an average 206-day monitoring period, participants drove an average of 20 miles per day and spent almost 1 h per day driving. On average, participants spent less than 4 min per day driving at night, less than 1 min per day driving over 70 mph, 7.5 min per day highway driving, had a first drive per day at 11 am, and a last drive per day at 3:30 pm.
Although MCI and cognitively intact participants took a similar number of trips (drives) per day, the MCI group drove fewer miles and spent less time on the highway per day than the cognitively intact group. MCI drivers showed less day-to-day fluctuations in their driving habits than cognitively intact drivers, and there was a trend toward declining in their time per day spent driving over the study. MCI participants’ residences were spread across multiple locations throughout the metro area, making it less likely that these individuals lived in one particular area that had less access to certain kinds of roads. Future prospective longitudinal studies will examine whether individual changes in these particular aspects of driving are strong predictors of developing MCI. Our results demonstrate that intact global cognition and executive functioning play a role in increased day-to-day variability in driving duration and distance. This could be manifested behaviorally in cognitively intact older adults’ greater comfort in initiating and navigating trips that may be novel, unplanned, complex, and outside of a structured routine. There were no significant group differences in time spent driving during daytime or nighttime, number of left or right-hand turns, time spent driving over 70 mph, number of hard breaks. or accelerations per day in our sample. These metrics reflect subtle aspects of episodic driving behavior and context, and given a larger sample size and longer period of monitoring, may differentiate MCI from cognitively intact older adult drivers.
The power of continuous sensor based activity monitoring for detecting subtly developing cognitive decline lies in its inherent ability to measure changes in variability, which may be missed with episodic assessments that yield far fewer data points over time. It has been shown that in the early stage of neurodegenerative disease, individuals begin to develop changes in variability (day-by-day performance fluctuations) in daily tasks. One theory is that increases or decreases in variability may reflect the magnitude of cognitive reserve compensating for an underlying pathological process [38]. The ability to complete high level daily tasks typically does not become impaired outright with early cognitive decline, but there may be a period of increased or decreased variability early on as cognitive reserve diminishes. During this early period, the initiation and execution of complex daily tasks gradually becomes less efficient and effective until compensatory mechanisms are exhausted and noticeable functional impairment develops.
Previous studies using self-report and simulator assessment approaches have observed that older adults with MCI have more difficulty driving, drive less often, and make more errors than cognitively intact older adults [12]. Results from the current study are congruent with these findings, providing objective evidence by capturing real-world driving behavior while also adding a more precise understanding of specific driving behaviors. For example, while MCI older adults drove less on the highway and drove fewer miles, they did not take fewer trips overall. Our observation of reduced variability in driving patterns of MCI older adults is broadly consistent with previous findings of MCI older adults’ self-reported increased driving difficulty. Indeed, a subjective sense of discomfort and increased trouble with driving could translate to more restricted (or simplification of) driving patterns. Our methodology did not capture driving errors per se, although subtle aspects of episodic driving behavior such as hard breaks, hard accelerations, and driving under or over the speed limit may differentiate MCI from cognitively intact older adult drivers given a larger sample size and longer period of monitoring.
Continuous sensor based monitoring yielded driving measures that were objective and more closely tied to cognition than self-report. Notably, the majority of participants underestimated their driving frequency and mileage via self-report and there was no difference in CDR score or global cognitive domain z-score between people who underestimated their driving and those who did not. These data show that unreliable self-report of driving activity isn’t simply a function of poorer cognitive functioning and provides further support for objective assessment of functional activities like driving to augment self-report across the spectrum of normal cognitive aging to MCI. Despite the fact that many participants underestimated their driving frequency and mileage, self-reported round trips per week were significantly correlated with actual sensor-measured round trips per week. Thus, while self-report data for more subtle, fine-grained aspects of driving should be interpreted with caution, broader, more rudimentary aspects of driving activity may be reliably assessed by self-report. Advantages of sensor-based driving measures are that they are objective, unobtrusive, and can be assessed every time a person drives his or her vehicle to identify clinically meaningful changes in daily driving with high precision and accuracy.
There were challenges in carrying out this proof of concept study that will serve to inform and refine the methods and technology for future larger scale studies. The commercial driving sensor was not compatible with vehicles older than 1996 or electric cars, eliminating four individuals who were otherwise interested and eligible for study participation. Three individuals who shared a vehicle equally with a partner were ineligible because the driving sensor was unable to differentiate between drivers. This is a current limitation of the technology as applied to clinical aging research and researchers are actively investigating how to enable individual driver identification through solutions such as person-specific key fobs, or wearable sensors that communicate wirelessly with the driving sensor. Recently, using only automatic sensor generated measures similar to those in our study, it was found that one could identify with 99% accuracy who was specifically driving a vehicle solely based on the characteristics of the sensor data, suggesting that the driver identity question will be less of an issue going forward [39], The smartphone and monthly cellular data plans required by the driving sensor to process the raw trip data for each participant were costly and time intensive for research staff. When iOS updates occurred or when cars were parked in underground garages, data transmission was occasionally interrupted causing the driving sensor and app to need to be re-connected by the participant or study staff. When this occurred, driving data was stored locally on the sensor for up to 8 h of driving and was retrievable by study staff. Future studies will take advantage of now less expensive off the shelf driving sensors with integrated 3 G wireless capability to eliminate the need for a smartphone or monthly data plan to process the transmitted data. This will greatly streamline data collection and processing and increase the cost-effectiveness of this approach. Compared to traditional clinic-based test batteries, refined driving monitoring methods will require little to no extra effort or action by the participant outside of normal routines, which could improve participant satisfaction and minimize attrition in longitudinal research protocols. Precision assessment and analysis of continuous driving data will cost less in the long-term and yield more precise fine-grained measures of ‘real’ function (which are inherently meaningful), allow for measurement of day-to-day variability (impossible to capture with infrequent episodic clinic testing), and will add incremental utility to conventional clinic measures. The wireless technology and software algorithms used in this approach are readily scalable to reach a large number of older adults.
Despite the challenges faced in this proof of concept study, about 90% of our sample said participating in the study took little to almost none of their time and over 80% would likely participate in another driving study in the future. In our convenience sample, there were minimal concerns about privacy or data security, consistent with studies showing that older adults are generally open to activity monitoring and data sharing with family or medical providers especially for maintaining health and independence [40, 41]. Future clinical implications of this technology include the ability to provide older adults, families, and medical professionals with objective information about driving patterns to stimulate discussions and inform decision making around driving restrictions or cessation.
The current conclusions should be interpreted with caution. This is a relatively homogenous unbalanced (3:1 healthy to MCI ratio) convenience sample of predominately Caucasian (80%), well-educated community dwelling volunteers living in a metropolitan area who have low levels of depression and health co-morbidities and who are enrolled in ongoing aging studies. This reduces the generalizability of our findings to more diverse samples of older adults. Our sample size was small which limited our power to detect cross-sectional differences between diagnostic groups. Due to the small sample size and relatively brief monitoring period, statistical models were not adjusted for covariates and we were unable to examine prediction of MCI incidence. Through sensor-based assessment of daily driving it is possible to derive many new ecologically relevant metrics of driving activity from the large volume of data collected and processed. This first proof of concept study provided an opportunity to examine each of these metrics to learn about which driving metrics might be best at discriminating MCI versus intact cognition in a small sample. With this in mind, we chose to report preliminary data without adjusting for multiple comparisons. Larger, adequately powered studies will be carried out in the future to provide more definitive data. Future prospective longitudinal studies will be aimed at replicating these results with larger and more heterogeneous samples of older adults. These studies will identify the sensor-based driving metrics and within-person changes most predictive of MCI inception and progression to evaluate driving-related functional changes that could be a harbinger of conversion to dementia. Time series analysis techniques may be used to analyze trajectories of routine driving, especially with larger samples in future studies. Upcoming work will also combine additional cognitively mediated functional domains that can add to the predictive value of this approach (e.g., medication adherence, computer use, financial management).
Acknowledgments
This work was supported by the Alzheimer’s disease Research Fund of the Oregon Charitable Checkoff Program, administered by the Oregon Partnership for Alzheimer’s Research and National Institutes of Health grants AG024978, AG024059, and AG023477, P30AG008017, AG042191 and NIA U2CAG054397.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0116r4).
References
- 1.Alzheimer’s Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Dodge HH, Mattek N, Gregor M, Bowman M, Seelye A, Ybarra O, Asgari M, Kaye J. Social markers of mild cognitive impairment: Proportion of word counts in free conversational speech. Curr Alzheimer Res. 2015;12:513–519. doi: 10.2174/1567205012666150530201917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seelye A, Hagler S, Mattek N, Howieson DB, Wild K, Dodge HH, Kaye JA. Computer mouse movement patterns: A potential marker of mild cognitive impairment. Alzheimers Dement (Amst) 2015;1:472–480. doi: 10.1016/j.dadm.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaye JA, Austin J, Dodge HH, Mattek N, Riley T, Seelye A, Sharma N, Wild K. Ecologically valid assessment of life activities: Unobtrusive continuous monitoring with sensors. Alzheimers Dement. 2016;12(Suppl):P374. [Google Scholar]
- 5.Seelye A, Mattek N, Howieson DB, Austin D, Wild K, Dodge HH, Kaye JA. Embedded online questionnaire measures are sensitive to identifying mild cognitive impairment. Alzheimer Dis Assoc Disord. 2016;30:152–159. doi: 10.1097/WAD.0000000000000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin J, Klein K, Mattek N, Kaye J. Variability in medication taking is associated with cognitive function in nondemented older adults. Alzheimers Dement (Amst) 2017;6:210–213. doi: 10.1016/j.dadm.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayes TL, Larimer N, Adami A, Kaye JA. Medication adherence in healthy elders: Small cognitive changes make a big difference. J Aging Health. 2009;21:567–580. doi: 10.1177/0898264309332836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaye J, Mattek N, Dodge HH, Campbell I, Hayes T, Austin D, Hatt W, Wild K, Jimison H, Pavel M. Unobtrusive measurement of daily computer use to detect mild cognitive impairment. Alzheimers Dement. 2014;10:10–17. doi: 10.1016/j.jalz.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaytor N, Schmitter-Edgecombe M. The ecological validity of neuropsychological tests: A review of the literature on everyday cognitive skills. Neuropsychol Rev. 2003;13:181–197. doi: 10.1023/b:nerv.0000009483.91468.fb. [DOI] [PubMed] [Google Scholar]
- 10.Kaye JA, Maxwell SA, Mattek N, Hayes TL, Dodge H, Pavel M, Jimison HB, Wild K, Boise L, Zitzelberger TA. Intelligent systems for assessing aging changes: Home-based, unobtrusive, and continuous assessment of aging. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i180–i190. doi: 10.1093/geronb/gbq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyons BE, Austin D, Seelye A, Petersen J, Yeargers J, Riley T, Sharma N, Mattek N, Wild K, Dodge H, Kaye JA. Pervasive computing technologies to continuously assess Alzheimer’s disease progression and intervention efficacy. Front Aging Neurosci. 2015;7:102. doi: 10.3389/fnagi.2015.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hird MA, Egeto P, Fischer CE, Naglie G, Schweizer TA. A systematic review and meta-analysis of on-road simulator and cognitive driving assessment in Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 2016;53:713–729. doi: 10.3233/JAD-160276. [DOI] [PubMed] [Google Scholar]
- 13.Johnson DA, Frank O, Pond D, Stocks N. Older people with mild cognitive impairment – their views about assessing driving safety. Aust Fam Physician. 2013;42:317–320. [PubMed] [Google Scholar]
- 14.AAA Foundation. Understanding Older Drivers: An Examination of Medical Conditions, Medication Use, and Travel Behavior. https://www.aaafoundation.org/sites/default/files/Medication%20and%20Travel%20Behaviors%20-%20FINAL%20FTS%20FORMAT%20copy.pdf.
- 15.Baldock MR, Mathias JL, McLean AJ, Berndt A. Self-regulation of driving and its relationship to driving ability among older adults. Accid Anal Prev. 2006;38:1038–1045. doi: 10.1016/j.aap.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 16.Hird MA, Vesely KA, Fischer CE, Graham SJ, Naglie G, Schweizer TA. Investigating simulated driving errors in amnestic single- and multiple-domain mild cognitive impairment. J Alzheimers Dis. 2017;56:447–452. doi: 10.3233/JAD-160995. [DOI] [PubMed] [Google Scholar]
- 17.Devlin A, McGillivray J, Charlton J, Lowndes G, Etienne V. Investigating driving behaviour of older drivers with mild cognitive impairment using a portable driving simulator. Accid Anal Prev. 2012;49:300–307. doi: 10.1016/j.aap.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Rapoport MJ, Naglie G, Weegar K, Myers A, Cameron D, Crizzle A, Korner-Bitensky N, Tuokko H, Vrkljan B, Bedard M, Porter MM, Mazer B, Gelinas I, Man-Son-Hing M, Marshall S. The relationship between cognitive performance, perceptions of driving comfort and abilities, and self-reported driving restrictions among healthy older drivers. Accid Anal Prev. 2013;61:288–295. doi: 10.1016/j.aap.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard RA, Myers AM, Porter MM. Correspondence between self-reported and objective measures of driving exposure and patterns in older drivers. Accid Anal Prev. 2010;42:523–529. doi: 10.1016/j.aap.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Farmer CM, Kirley BB, McCartt AT. Effects of in-vehicle monitoring on the driving behavior of teenagers. J Safety Res. 2010;41:39–45. doi: 10.1016/j.jsr.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Carney C, McGehee DV, Lee JD, Reyes ML, Raby M. Using an event-triggered video intervention system to expand the supervised learning of newly licensed adolescent drivers. Am J Public Health. 2010;100:1101–1106. doi: 10.2105/AJPH.2009.165829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montgomery J, Kusano KD, Gabler HC. Age and gender differences in time to collision at braking from the 100-Car Naturalistic Driving Study. Traffic Inj Prev. 2014;15(Suppl 1):S15–S20. doi: 10.1080/15389588.2014.928703. [DOI] [PubMed] [Google Scholar]
- 23.Marshall SC, Man-Son-Hing M, Bédard M, Charlton J, Gagnon S, Gélinas I, Koppel S, Korner-Bitensky N, Langford J, Mazer B, Myers A, Naglie G, Polgar J, Porter MM, Rapoport M, Tuokko H, Vrkljan B, Woolnough A. Protocol for Candrive II/Ozcandrive, a multicentre prospective older driver cohort study. Accid Anal Prev. 2013;61:245–252. doi: 10.1016/j.aap.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Marshall SC, Man-Son-Hing M, Charlton J, Molnar LJ, Koppel S, Eby DW. The Candrive/Ozcandrive prospective older driver study: Methodology and early study findings. Accid Anal Prev. 2013;61:233–235. doi: 10.1016/j.aap.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Marshall SC, Wilson KG, Man-Son-Hing M, Stiell I, Smith A, Weegar K, Kadulina Y, Molnar FJ. The Canadian Safe Driving Study-Phase I pilot: Examining potential logistical barriers to the full cohort study. Accid Anal Prev. 2013;61:236–244. doi: 10.1016/j.aap.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Babulal GM, Addison A, Ghoshal N, Stout SH, Vernon EK, Sellan M, Roe CM. Development and interval testing of a naturalistic driving methodology to evaluate driving behavior in clinical research. F1000Res. 2016;5:1716. doi: 10.12688/f1000research.9150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Babulal GM, Traub CM, Webb M, Stout SH, Addison A, Carr DB, Ott BR, Morris JC, Roe CM. Creating a driving profile for older adults using GPS devices and naturalistic driving methodology. F1000Res. 2016;5:2376. doi: 10.12688/f1000research.9608.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 30.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. Wechsler Memory Scale-revised. Psychological Corporation; New York: 1987. [Google Scholar]
- 32.Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24:641–652. [PubMed] [Google Scholar]
- 33.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- 34.Armitage SG. An analysis of certain psychological tests used for the evaluation of brain injury. Psychology Monographs. 1946;60:1–48. [Google Scholar]
- 35.Jensen AR, Rohwer WD., Jr The Stroop color-word test: A review. Acta Psychol (Amst) 1966;25:36–93. doi: 10.1016/0001-6918(66)90004-7. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-revised. Psychological Corporation; New York: 1981. [Google Scholar]
- 37.Wechsler D. Manual for the Wechsler Adult Intelligence Scale. 4. Pearson; San Antonio, Texas: 2008. [Google Scholar]
- 38.Dodge HH, Mattek NC, Austin D, Hayes TL, Kaye JA. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology. 2012;78:1946–1952. doi: 10.1212/WNL.0b013e318259e1de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enev M, Takakuwa A, Koscher K, Kohno T. Automobile driver fingerprinting. Proceedings on Privacy Enhancing Technologies. 2016;1:34–51. [Google Scholar]
- 40.Boise L, Wild K, Mattek N, Ruhl M, Dodge HH, Kaye J. Willingness of older adults to share data and privacy concerns after exposure to unobtrusive in-home monitoring. Gerontechnology. 2013;11:428–435. doi: 10.4017/gt.2013.11.3.001.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Claes V, Devriendt E, Tournoy J, Milisen K. Attitudes and perceptions of adults of 60 years and older towards in-home monitoring of the activities of daily living with contactless sensors: An explorative study. Int J Nurs Stud. 2015;52:134–148. doi: 10.1016/j.ijnurstu.2014.05.010. [DOI] [PubMed] [Google Scholar]


