Abstract
The post-translational modification of serine and threonine residues of proteins found in the numerous sub-cellular locations by O-linked N-acetylglucosamine (O-GlcNAc) is emerging as a key mediator of a number of cardiovascular pathophysiological processes. Early studies implicated increased protein O-GlcNAcylation as contributing to the cardiovascular complications associated with diabetes; whereas, subsequent studies demonstrated that acute increases in O-GlcNAc levels were protective against ischemia/reperfusion injury. There is now growing understanding that O-GlcNAc modification of proteins influences numerous cellular functions, including transcription, protein turnover, calcium handling, and bioenergetics. As a result, a more nuanced view of the role of protein O-GlcNAcylation in the cardiovascular system is emerging along with the recognition that it is required for normal cellular function and homeostasis. Consequently, the impact of changes in O-GlcNAc cycling due to stress or disease on the heart is complex and highly dependent on the specific context of these events. The goal of this review is to provide an overview of some of the more recent advances in our understanding of the role O-GlcNAcylation plays in mediating cardiovascular function and disease.
Introduction
The first reports suggesting that the post-translational modification (PTM) of serine and threonine residues of proteins by O-linked N-acetylglucosamine (O-GlcNAc) could play a role in the cardiovascular system occurred about 20 years ago in the late 1990s. These studies showed that the small heat shock protein (HSP) αB-crystallin was O-GlcNAcylated in the rat heart [1], that O-GlcNAc transferase (OGT) activity was higher in the heart than many other tissues [2], and that in vascular smooth muscle cells the transcription factor Sp1 was an O-GlcNAc target [3]. Interestingly, Yki-Järvinen et al. raised the possibility for the first time that O-GlcNAcylation of proteins in the heart may contribute to the effects of glucose toxicity [2]. Since those early studies, there has been a growing appreciation that O-GlcNAcylation plays a role in a wide range of (patho) physiological processes in the cardiovascular system including ischemic cardioprotection, hypertrophy, diabetic complications, hypertension and heart failure. There have been a number of detailed reviews on O-GlcNAcylation and the heart in the past few years [4–8]; therefore, the focus of this mini-review will be to highlight some of the more recent findings on O-GlcNAcylation and cardiovascular pathophysiology.
Ischemia/Reperfusion injury
While many of the earlier studies frequently emphasized the role of O-GlcNAcylation in mediating glucose toxicity in the heart and vascular system [7–9], a number of more recent studies have shown that increasing O-GlcNAc levels provided significant cardioprotection against ischemia/reperfusion (I/R) in both in vitro and in vivo model systems [5, 10, 11]. Pharmacological approaches for increasing O-GlcNAc levels include increasing its synthesis via activation of the hexosamine biosynthesis pathway (HBP) or through blocking its removal by inhibiting O-GlcNAcase (OGA). However, the mechanisms underlying O-GlcNAc-mediated cardioprotection remain to be identified and will be a critical goal for future studies.
Glucose-insulin-potassium (GIK) therapy has a long history as a potential therapeutic approach for the treatment of I/R injury. However, the adoption of GIK therapy in normal clinical practice remains controversial largely because of variable outcomes in clinical trials [12, 13], although there is more of a consensus of its utility in the setting of cardiac surgery [14, 15]. Another limitation is a lack of agreement as to the mechanism underlying the cardioprotective effects of GIK therapy. We have previously demonstrated, using a cell-based model of I/R injury in neonatal rat ventricular myocytes (NRVMs), that the protective effects of increased glucose delivery were mediated via increased HBP flux and ultimately increased O-GlcNAc levels [16]. Recently Chun et al. reported, that the combination of increased glucose and insulin in an NRVM model of I/R significantly reduced cell death, and this was also associated with increased O-GlcNAc levels [17]; unfortunately, however, they did not evaluate a direct causal relationship between the increase in O-GlcNAc and improved cell survival. Interestingly, in a double-blind randomized trial patients undergoing aortic valve replacement surgery that were assigned to GIK treatment had improved outcomes compared to the placebo control group, and this was associated with an increase in O-GlcNAc levels measured in biopsy samples [18]. While there are some limitations with this study [19], it provided the first evidence that GIK therapy in humans may exert its beneficial effect via increased O-GlcNAc levels.
A potential concern regarding the use of GIK therapy is the fact that hyperglycemia itself is linked to poorer outcomes in patients presenting with acute myocardial infarction (MI), which has raised the possibility that the variability in outcomes of GIK trials could be due to variable plasma glucose levels. To begin to address this issue, Yu et al. subjected dogs to an I/R protocol and randomly assigned them to four experimental groups: normal insulin/normal glucose (NI/NG), normal-insulin/hyperglycemia (NI/HG), high-insulin/normal glucose (HI/NG), and high insulin/hyperglycemia (HI/HG). They observed worse outcomes in both high glucose groups but improved function and decreased injury in the HI/NG group compared to all other groups. In parallel, cell-based studies with NRVMs showed that the HI/NG group resulted in increased tyrosine phosphorylation of Insulin receptor substrate-1 (IRS-1), which was suppressed in both HG groups. This decrease in IRS-1 phosphorylation was associated with increased O-GlcNAcylation of IRS-1 in the HG groups. They also reported that inhibition of the HBP in HG treated cells attenuated the O-GlcNAcylation of IRS-1, increased IRS-1 phosphorylation, and this partially reversed the adverse effects of HG on cell injury; however, they did not look at the effects of HBP inhibition in the HI/NG group [20]. The overall conclusions from this study were that O-GlcNAcylation of IRS-1 contributed to impaired cardioprotection associated with hyperglycemia. While the outcome of this study is contrary to the concept that increasing O-GlcNAc levels is cardioprotective, it does highlight the complexity surrounding both GIK and the effects of increased O-GlcNAc levels on the heart, particularly in the setting of I/R.
The strongest evidence supporting cardioprotection afforded by increasing O-GlcNAc levels are from studies that have either directly stimulated O-GlcNAc synthesis, by adding glucosamine or via inhibition of OGA. It is possible, therefore, that these approaches may be more effective than those focused on glucose and insulin. In this context it is somewhat surprising that given the early success of increasing O-GlcNAc levels in vitro and in vivo murine model systems of I/R [21–23], that this has not been translated into studies in larger animal models. One hurdle for continued studies has been defining the specific pathways contributing to the changes. In this regard a number of putative mechanisms have been proposed to account for the protective effects associated with acute increases in O-GlcNAc levels. Studies have indicated that increased O-GlcNAcylation may attenuate the initial calcium influx that occurs in reperfusion, thereby reducing the consequences of Ca2+ overload [24, 25]. Others have suggested that it might increase the tolerance of mitochondria to oxidative stress, possibly by direct modification of mitochondrial proteins such as voltage-dependent anion channel (VDAC) [26]. It has also been shown, although not in the heart, that increasing O-GlcNAc levels increases the production of HSPs [27]. More recently it has been proposed that O-GlcNAc-mediated protection of mouse embryonic stem cells (mESCs) against the hypoxia-induced apoptosis may occur due to increased expression of glycerol-3-phosphate acyltransferase-1 (GPAT1) and subsequent mechanistic target of rapamycin (mTOR) activation [28]. Taken together, these studies demonstrate that an increase in O-GlcNAc levels prior to I/R injury, hypoxia, or during reperfusion all improve functional recovery and that this might occur via a number of different mechanisms.
Hypertrophy and heart failure
Increased O-GlcNAc levels have been reported in human heart tissue from patients undergoing surgery for aortic stenosis relative to a control group [29]. The authors found similar observations in rat models of aortic constriction, hypertension, and MI-induced heart failure; however, the underlying cause of the increase in O-GlcNAc levels was unclear as there were no consistent changes in OGT and OGA protein levels across these various models. A detailed review by Mailleux et al. provides a thorough overview of potential anti- and pro-hypertrophic actions of O-GlcNAcylation in the heart [7]. Of note, past studies have both suggested that increased O-GlcNAc levels blunted hypertrophic signaling [30] in cardiomyocytes, while others have shown that enhanced O-GlcNAc signaling is required for activation of initial hypertrophic signaling [31]. Interestingly, c-Myc overexpression induced cardiac hypertrophy and increased O-GlcNAc levels [32]; whereas c-Myc knockout attenuated pressure overload-induced hypertrophy and decreased O-GlcNAc levels [33]. Increased cardiomyocyte size induced by intermittent hypoxia was associated with a time-dependent increase in cardiac O-GlcNAc levels; however, whether this played a causal role in the increased inflammatory response and increased apoptosis remains unclear [34]. Deletion of OGT in the adult rat heart accelerated the progression to heart failure following coronary artery ligation; however, it had no effect on cardiomyocyte size [35]. On the other hand, constitutive ablation of cardiomyocyte OGT resulted in marked increase in heart weight and increased cardiomyocyte size as well as increased embryonic and perinatal death [36]. Interestingly, even partial ablation of OGT in the heart resulted in a progressive dilated cardiomyopathy associated with cardiomyocyte degeneration and increased fibrosis [36]. OGT deletion in adult mice also resulted in progressive cardiac dysfunction as early as one month following deletion [36]. These studies show that both lowering and increasing O-GlcNAc can advance heart failure, supporting the concept that maintaining O-GlcNAc homeostasis is necessary for normal cardiomyocyte function.
The fact that the effects of O-GlcNAcylation in the development of hypertrophy are unclear is likely due to its role in transcriptional regulation. For example, Sp1, which has been shown to have multiple O-GlcNAcylation sites, is involved in regulation of many cardiac genes including those involved in cardiomyocyte hypertrophy [37]. Moreover, O-GlcNAcylation of Sp1 has been reported to have multiple and sometimes opposing effects. On one hand Sp1 O-GlcNAcylation has been reported to be required for its transcriptional activity [38]; while others have shown inhibition of its transcriptional activation [39]. It has also been shown that insulin-induced O-GlcNAcylation of Sp1 triggers its nuclear translocation; where it is then subsequently phosphorylated and activates gene expression [38]. c-Myc one of the most studied members of the MYC family of transcription factors, has been shown to be O-GlcNAcylated at Thr58, a known phosphorylation site. Phosphorylation at Thr58 enhances c-Myc degradation; consequently, it has been proposed that O-GlcNAcylation may act to stabilize c-Myc and thus increase its transcriptional activity. Activation of the fetal gene program, which is characteristic of cardiac hypertrophy, is regulated by mammalian switch-independent 3A (mSin3A) and multiple histone deacetylases (HDAC) through interactions with the DNA binding proteins repressor element-1 silencing transcription factor (REST). Importantly, recent studies have shown that in mouse heart REST is an O-GlcNAc target and directly interacts with OGT [40] and that OGT also interacts with mSin3A as well as HDAC1/2 [41]. Moreover, these interactions can be modulated by diet, exercise, and diabetes. Recently, GATA4 and Mef2c have been shown to be O-GlcNAc targets and an increase in their respective O-GlcNAcylation has been implicated in liver X receptor-alpha (LXRα)-mediated anti-hypertrophic effects in the heart [42]. Much of the hypertrophic transcriptional machinery is dependent on an initial increase in cytosolic Ca2+ and the ER/SR protein Stromal interaction molecule-1 (STIM-1) has recently been implicated in this process [43]. Of note STIM1 is also an O-GlcNAc target and O-GlcNAcylation influences its function [44]. It is clear from these studies that a number of components involved in hypertrophic signaling are targets for O-GlcNAcylation, consistent with a role of O-GlcNAc modification in regulating cardiac remodeling.
Decreases in myofilament protein phosphorylation have been reported in both animal models and human heart failure [45–47]. A decrease in phosphorylation of Troponin T (TnT) at Ser207 and Ser208 has been reported in both myocardial and plasma samples following MI-induced heart failure. Specifically, in the failing rat heart there was an increase in TnT O-GlcNAcylation accompanied by a decrease in phosphorylation of Ser208 [48]. The authors confirmed the reciprocity between TnT phosphorylation and O-GlcNAcylation either by pharmacologically increasing O-GlcNAc levels by inhibiting OGA, or decreasing O-GlcNAc levels by knocking down OGT. Moreover, TnT O-GlcNAcylation was also increased when PKCε was inhibited, further emphasizing the cross-talk between phosphorylation and O-GlcNAcylation. Using mass spectrometry, they found that both Ser208 and Ser190 of TnT were O-GlcNAc targets and that O-GlcNAcylation of TnT at Ser190 inhibited phosphorylation of TnT at Ser208. While the functional consequences of O-GlcNAcylation of TnT have not been identified, increased myofilament O-GlcNAc levels in skinned rat trabeculae decreased Ca2+ sensitivity [49]. Thus, it is possible that changes in the balance between O-GlcNAcylation and phosphorylation of TnT may alter cardiomyocyte Ca2+ homeostasis contributing to the changes in adaptive cardiac remodeling.
Although an increase in myocardial O-GlcNAcylation is commonly observed in both hypertrophy and heart failure, how this occurs is not completely defined. Recently, Muthusamy et al. reported that in a coronary ligation model of heart failure at 5 and 28 days there was a decrease in OGA at both the mRNA and protein level. They also observed that microRNA (miR)-539 was upregulated in the failing heart at both 5 and 28 days. Interestingly, miR-539 bound to the OGA-3′UTR and this specific binding site is conserved between mouse, rat and human, providing a potential mechanism for regulation of OGA protein levels. They demonstrated that overexpression of miR-539 significantly decreased OGA expression and that subsequent inhibition of miR-539 reversed this effect. A potentially confounding factor in these studies was that overexpression of miR-539 also decreased OGT protein levels; since OGT does not contain target sites for miR-539 binding it is possible that the reduction in OGT is an adaptive response to decreased OGA levels [50]. This connected with other epigenetic mechanisms of gene regulation highlight a new area of research for cardiac O-GlcNAc biology [51].
Diabetes and the heart
Sustained elevation of protein O-GlcNAc levels has been widely recognized as contributing to the adverse effects of diabetes on the heart. Clark et al. reported that the effects of high glucose on cardiomyocyte Ca2+ handling could be mimicked by the addition of glucosamine or overexpression of OGT; whereas overexpression of OGA attenuated the effect of hyperglycemia [52]. A later study by the same group showed that overexpressing OGA in the heart in vivo improved contractile function in the hearts from diabetic mice, which was linked to a restoration of SERCA2a expression [53, 54]. Recently SERCA2a itself has been shown to be an O-GlcNAc target and the level of its O-GlcNAcylation is increased in response to diabetes [54]. As discussed above, O-GlcNAcylation of myofilaments decreased Ca2+ sensitivity; consistent with these findings recent studies have found that decreasing myofilament O-GlcNAc levels restored myofilament Ca2+ sensitivity associated with diabetes [55]. Interestingly, this same study reported that diabetes resulted in changes in the subcellular localization of both OGT and OGA; providing a new mechanism for O-GlcNAc specificity.
The role of hyperglycemia and diabetes on cardiomyocyte mitochondrial function has received a substantial amount of attention and remains somewhat controversial. Hyperglycemia has been reported to increase O-GlcNAc levels on several mitochondrial complexes resulting in impaired mitochondrial function; in addition, an increase in O-GlcNAc levels mediated by hyperglycemia has also been implicated in increased mitochondrial fission through O-GlcNAcylation of dynamin-related protein 1 (DRP1) [56]. However, in contrast Dassanayaka et al. demonstrated that while hyperglycemia resulted in impaired complex 2-dependent respiration in cardiomyocytes these effects were independent of protein O-GlcNAcylation and more likely a consequence of osmotic stress [57]. A long-standing limitation in evaluating the impact of O-GlcNAcylation on mitochondrial function was that there was no consensus as to how mitochondrial proteins were being O-GlcNAcylated. Although a mitochondrial targeted isoform of OGT had been identified, there was no evidence of a mitochondrial OGA; it was also unclear whether UDP-GlcNAc, the essential sugar donor and substrate for OGT, could get into the mitochondria. However, in 2015 Banerjee et al. showed for the first time that a pyrimidine nucleotide carrier transported UDP-GlcNAc into mitochondria, and that cardiomyocyte mitochondria contain substantial OGA activity. Also of note, they reported a direct association of OGT with complex IV, which was reduced in mitochondria from diabetic hearts. This study demonstrated for the first time an active O-GlcNAc cycle in cardiac mitochondria and that it was altered in response to diabetes [58]. Subsequently, O-GlcNAcomic profiling identified more than 88 mitochondrial proteins as O-GlcNAc targets, with proteins involved in oxidative phosphorylation as major targets [59]. Further studies using comparative O-GlcNAc profiling of mitochondria from control and diabetic rat hearts identified many proteins that had site-specific alterations in O-GlcNAcylation in response to diabetes [60]. These recent insights into O-GlcNAcylation of mitochondrial proteins have provided a better understanding of the mechanisms by which elevated O-GlcNAc levels contribute to the adverse effects of diabetes, but will also usher in new insights into the cardioprotective mechanisms associated with acute increases in O-GlcNAcylation [61].
O-GlcNAcylation and vascular function
Protein O-GlcNAcylation also plays an important role in regulating vascular function including vasoconstriction, vasodilation, calcification, and vascular remodeling. Federici et al. reported that in human coronary arterial cells phosphorylation of endothelial nitric oxide synthase (eNOS) at serine 1177 was reduced in response to hyperglycemia in a HBP- and O-GlcNAc-dependent manner [62]. More recently, hyperglycemia was shown to impair vasodilation also via activation of the HBP along with an increase in eNOS O-GlcNAcylation and decreased Ser1177 phosphorylation [63]. Furthermore, Makino et al. demonstrated that in type 1 diabetic mice, endothelium-dependent induced overexpression of OGA reduced endothelial O-GlcNAc levels and restored endothelial-dependent vasodilation. While increased expression of endothelial OGA in the diabetic group did not significantly decrease eNOS O-GlcNAc levels there was significant reduction in O-GlcNAcylation of connexin 43, suggesting that this might contribute to the improved vascular function [64]. Overall, these studies suggest that increased O-GlcNAcylation could be a contributing factor to hypertension or microvascular disease, which is commonly associated with diabetes. Impaired vascular function was associated with elevated O-GlcNAc levels in rats fed a high fat diet and pharmacologically increasing O-GlcNAc levels in normal fed animals increased basilar artery contraction [65]. Similarly, diabetes significantly increases the risk for vascular calcification and it has been shown that increased O-GlcNAcylation promoted vascular calcification via increased expression of the osteogenic transcription factor Runx2 and increased activation of Akt [66].
Using the deoxycorticosterone acetate and salt (DOCA-salt) hypertensive rat model, Lima et al. provided evidence linking increased O-GlcNAc levels directly to hypertension. After 5 weeks of treatment, the DOCA-salt treated group had significantly increased blood pressure and their arteries contained a marked increase in overall protein O-GlcNAcylation and exhibited enhanced vasoconstriction in response to phenylephrine. Interestingly, increasing O-GlcNAc levels by OGA inhibition in normotensive animals also enhanced vasoreactivity. Phosphorylation of eNOS, Akt, and phosphoinositide-3 kinase (PI3K) were all decreased in the hypertensive animals and eNOS O- GlcNAcylation was increased [67]. Furthermore, studies by the same group suggested that the O-GlcNAc-mediated increase in vasoreactivity was due at least in part via activation of the RhoA/Rho kinase pathway [68].
In addition to being associated with impaired vascular function, a number of studies have also suggested that protein O-GlcNAcylation can have a protective role in the vasculature. For example, Oparil and colleagues reported that pharmacologically increasing O-GlcNAc levels acutely with glucosamine and or inhibition of OGA, following endoluminal damage reduced neutrophil and monocyte infiltration, resulting in a 50% reduction in neointima formation in carotid arteries [69]. Subsequent studies indicated that the potential decrease in vascular remodeling was mediated through increased NFκB p65 O- GlcNAcylation and reduced expression of inflammatory mediators [70]. The same group later showed that acutely increasing O-GlcNAc levels in rat aortic rings with either glucosamine or thiamet G attenuated TNFα-induced endothelial dysfunction, at least in part by suppression of inducible NOS (iNOS) expression [71].
Future Implications
The above discussion demonstrates that the role of O-GlcNAcylation in regulating cardiovascular function is complex and that as most studies focus on its role in cardiovascular pathophysiology, the traditional view is that changes in O-GlcNAc levels are classified as either “good” or “bad” (Figure 1). On the other hand, as we begin to understand more about the cellular functions regulated by protein O-GlcNAcylation it is increasingly clear that a more accurate concept would be that O-GlcNAc modification of cardiovascular proteins is a dynamic process that is critical in maintaining normal cardiomyocyte function. This is reinforced by the observations that O-GlcNAcylation of proteins plays a role in regulating fundamental cellular processes ranging from transcription to metabolism; there is also emerging evidence to suggest that protein O-GlcNAcylation also contributes to regulation of autophagy, epigenetics, and mitochondrial biogenesis [4, 51, 61]. Consequently, the context in which O-GlcNAc levels are changed are as specific as the stimuli, cell type or disease state with regard to its effect on cell function. Another consideration with regard to differential responses to changes in O-GlcNAc levels is the duration and extent to which levels are increased. There are also distinct sub-cellular pools of OGT and OGA in the nucleus, cytoplasm and mitochondria, which are likely going to respond differently to a particular stimulus.
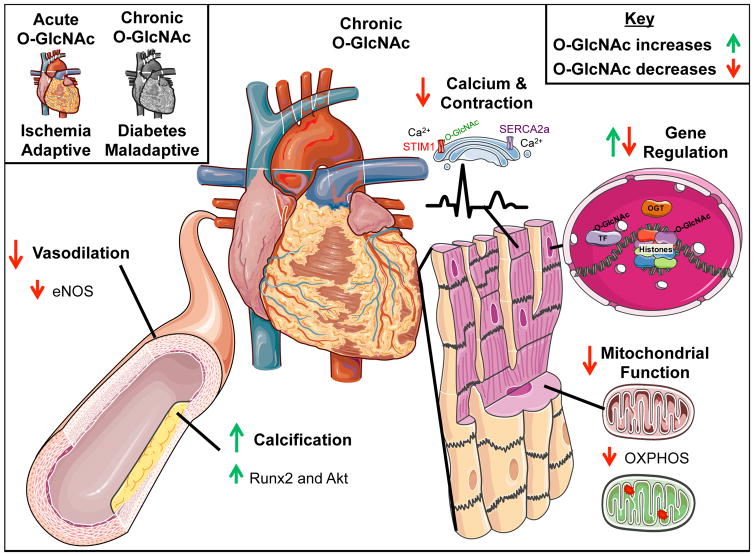
Figure 1. O-GlcNAcylation in Cardiovascular Function and Disease.
Cellular signaling includes the protein post-translational modification (PTM) and regulation of proteins by O-linkage of N-acetylglucosamine (O-GlcNAc). Within the cardiovascular system this PTM confers both protection against ischemic injury and contributes to stem cell growth while in excess O-GlcNAc contributes to pathologic hypertrophy, contractile dysfunction, and decreased mitochondrial capacity. These changes occur throughout the cell in the nucleus to regulate gene expression, to mitochondria to decrease oxidative phosphorylation, within other membrane proteins to disrupt autophagy, and on contractile and Ca2+ handling proteins. Please see text for additional details. OGT, O-GlcNAc transferase; OXPHOS, oxidative phosphorylation; TF, transcription factor. This figure was produced using free images modified from Servier Medical Art (www.servier.com).
Despite the growing appreciation of the importance of O-GlcNAcylation in the cardiovascular system, our understanding of the mechanisms regulating O-GlcNAc turnover as well as the transcriptional regulation of OGT and OGA remain remarkably limited and represent important areas for future research. Moreover, little is known about the mechanisms regulating the activities of OGT and OGA and our knowledge of what controls the flux of glucose through the HBP is also remarkably limited. However, it was recently reported that spliced X-box binding protein 1 (Xbp1s) a key signal transducer of the unfolded protein response (UPR) directly controls transcription of the HBP[72]. Our knowledge of O-GlcNAcylation in human cardiovascular system is also underdeveloped. For example, as noted above Lunde et al., reported an increase in global cardiac O-GlcNAcylation in patients with symptomatic aortic stenosis [29]. They attributed the increase in cardiac O-GlcNAc to increased flux through the HBP; however, O-GlcNAc may be increased in response to stress stimuli, inflammatory cytokines or other factors that can cause aberrant signaling. The complexity of O-GlcNAcylation in regulating cardiovascular function makes it a difficult target for therapy; however, the fact that global changes improve function in the diabetic heart or decrease injury from I/R, suggest that as we develop a better understanding of the regulation of O-GlcNAcylation in the cardiovascular system there is the potential for developing novel therapeutics targeting O-GlcNAc turnover.
Acknowledgments
Funding
Work in the authors’ laboratories is supported by ADA grant 1-16-PDF-024 (HEC), NIH grants HL122975 (JCC) and HL133011 (ARW) and a UAB School of Medicine Multi-investigator Planning grant (JCC).
Abbreviations
- PTM
post-translational modification
- O-GlcNAc
O-linked N-acetylglucosamine
- OGT
O-GlcNAc transferase
- HSP
heat shock protein
- Sp1
specificity protein 1
- HBP
hexosamine biosynthesis pathway
- OGA
O-GlcNAcase
- GIK
glucose-insulin-potassium
- I/R
ischemia/reperfusion
- NRVM
neonatal rat ventricular myocyte
- MI
myocardial infarction
- NI
normal insulin
- NG
normal glucose
- HG
hyperglycemia
- HI
high insulin
- IRS-1
insulin receptor substrate-1
- VDAC
voltage dependent anion channel
- mESCs
mouse embryonic stem cells
- GPAT1
glycerol-3- phosphate acyltransferase-1
- mTOR
mechanistic target of rapamycin
- mSin3A
mammalian switch-independent 3A
- HDAC
histone deacetylases
- REST
repressor element-1 silencing transcription factor
- Mef2c
myocyte enhancer factor 2C
- LXRα
liver X receptor-alpha
- STIM1
Stromal Interaction molecule-1
- TnT
Troponin T
- PKCε
protein kinase C epsilon
- UTR
untranslated region
- miR
microRNA
- SERCA2a
sarco/endoplasmic reticulum Ca2+-ATPase
- DRP1
dynamin-related protein 1
- UDP-GlcNAc
uridine diphosphate N-acetylglucosamine
- eNOS
endothelial nitric oxide synthase
- DOCA-salt
deoxycorticosterone acetate and salt
- Akt
protein kinase B
- PI3K
phosphoinositide-3 kinase
- RhoA
Ras homolog gene family, member A
- NF-κB p65
nuclear factor kappa-light-chain-enhancer of activated B cells p65 subunit
- TNFα
tumor necrosis factor alpha
- iNOS
inducible nitric oxide synthase
- Runx2
Runt related transcription factor 2
- Xbp1s
X-box binding protein 1s
- UPR
Unfolded protein response
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with this manuscript.
References
- 1.Roquemore EP, et al. Dynamic O-GlcNAcylation of the small heat shock protein αB-crystallin. Biochemistry. 1996;35(11):3578–3586. doi: 10.1021/bi951918j. [DOI] [PubMed] [Google Scholar]
- 2.Yki-Järvinen H, et al. UDP-N-acetylglucosamine transferase and glutamine: fructose 6-phosphate amidotransferase activities in insulin-sensitive tissues. Diabetologia. 1997;40(1):76–81. doi: 10.1007/s001250050645. [DOI] [PubMed] [Google Scholar]
- 3.Han I, Kudlow JE. Reduced O glycosylation of Sp1 is associated with increased proteasome susceptibility. Molecular and Cellular Biology. 1997;17(5):2550–8. doi: 10.1128/mcb.17.5.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wende AR. Post-translational modifications of the cardiac proteome in diabetes and heart failure. Proteomics Clinical Applications. 2016;10(1):25–38. doi: 10.1002/prca.201500052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dassanayaka S, Jones SP. O-GlcNAc and the cardiovascular system. Pharmacol Ther. 2014;142(1):62–71. doi: 10.1016/j.pharmthera.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marsh SA, Collins HE, Chatham JC. Protein O-GlcNAcylation and cardiovascular (patho)physiology. Journal of Biological Chemistry. 2014;289(50):34449–34456. doi: 10.1074/jbc.R114.585984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mailleux F, et al. O-GlcNAcylation, enemy or ally during cardiac hypertrophy development? Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2016 doi: 10.1016/j.bbadis.2016.08.012. In Press. [DOI] [PubMed] [Google Scholar]
- 8.Banerjee PS, Lagerlöf O, Hart GW. Roles of O-GlcNAc in chronic diseases of aging. Molecular Aspects of Medicine. 2016;51:1–15. doi: 10.1016/j.mam.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 9.McLarty JL, Marsh SA, Chatham JC. Post-translational protein modification by O-linked N-acetyl-glucosamine: Its role in mediating the adverse effects of diabetes on the heart. Life sciences. 2013;92(11):621–627. doi: 10.1016/j.lfs.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatham JC, Marchase RB. The role of protein O-linked β-N-acetylglucosamine in mediating cardiac stress responses. Biochimica et biophysica acta. 2010;1800(2):57. doi: 10.1016/j.bbagen.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laczy B, et al. Inhibition of O-GlcNAcase in perfused rat hearts by NAG-thiazolines at the time of reperfusion is cardioprotective in an O-GlcNAc-dependent manner. American Journal of Physiology - Heart and Circulatory Physiology. 2010;299(5):H1715–H1727. doi: 10.1152/ajpheart.00337.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin PY, et al. Glucose–insulin–potassium therapy in patients with acute coronary syndrome: a meta-analysis of randomized controlled trials. BMC Cardiovascular Disorders. 2014;14(1):169. doi: 10.1186/1471-2261-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao YT, et al. Comparison of glucose-insulin-potassium and insulin-glucose as adjunctive therapy in acute myocardial infarction: a contemporary meta-analysis of randomised controlled trials. Heart. 2010;96(20):1622–6. doi: 10.1136/hrt.2010.194563. [DOI] [PubMed] [Google Scholar]
- 14.Straus S, et al. Glucosa-Insulin-Potassium (GIK) Solution Used with Diabetic Patients Provides Better Recovery After Coronary Bypass Operations. Med Arh. 2013;67(2):84–87. doi: 10.5455/medarh.2013.67.84-87. [DOI] [PubMed] [Google Scholar]
- 15.Shim JK, et al. Myocardial protection by glucose–insulin–potassium in acute coronary syndrome patients undergoing urgent multivessel off-pump coronary artery bypass surgery. British Journal of Anaesthesia. 2013;110(1):47–53. doi: 10.1093/bja/aes324. [DOI] [PubMed] [Google Scholar]
- 16.Lu XT, Zou L, Chatham JC. In cardiomyocytes LPS-induced activation of IκBα and COX-2 expression are mediated in part by the hexosamine biosynthesis pathway. The FASEB Journal. 2012;26(1 Supplement):1136.7. [Google Scholar]
- 17.Chun WJ, et al. Glucose-insulin-potassium solution protects ventricular myocytes of neonatal rat in an in vitro coverslip ischemia/reperfusion model. Korean Circ J. 2015;45(3):234–241. doi: 10.4070/kcj.2015.45.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell NJ, et al. Glucose-insulin-potassium reduces the incidence of low cardiac output episodes after aortic valve replacement for aortic stenosis in patients with left ventricular hypertrophy: results from the Hypertrophy, Insulin, Glucose, and Electrolytes (HINGE) trial. Circulation. 2011;123(2):170–7. doi: 10.1161/CIRCULATIONAHA.110.945170. [DOI] [PubMed] [Google Scholar]
- 19.Taegtmeyer H, Khalaf KI. Letter by Taegtmeyer and Khalaf Regarding Article, “Glucose-Insulin-Potassium Reduces the Incidence of Low Cardiac Output Episodes After Aortic Valve Replacement for Aortic Stenosis in Patients With Left Ventricular Hypertrophy: Results From the Hypertrophy, Insulin, Glucose, and Electrolytes (HINGE) Trial”. Circulation. 2011;124(14):e385–e385. doi: 10.1161/CIRCULATIONAHA.111.028795. [DOI] [PubMed] [Google Scholar]
- 20.Yu Q, et al. Effective glycaemic control critically determines insulin cardioprotection against ischaemia/reperfusion injury in anaesthetized dogs. Cardiovasc Res. 2014;103(2):238–47. doi: 10.1093/cvr/cvu132. [DOI] [PubMed] [Google Scholar]
- 21.Vibjerg Jensen R, et al. Ischemic preconditioning increases myocardial O-GlcNAc glycosylation. Scand Cardiovasc J. 2013;47(3):168–74. doi: 10.3109/14017431.2012.756984. [DOI] [PubMed] [Google Scholar]
- 22.Ngoh GA, et al. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40(3):895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2. Am J Physiol Cell Physiol. 2008;294(6):C1509–C1520. doi: 10.1152/ajpcell.00456.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. American Journal of Physiology - Heart and Circulatory Physiology. 2007;293(3):H1391–H1399. doi: 10.1152/ajpheart.00285.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, et al. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. Journal of Molecular and Cellular Cardiology. 2006;40(2):303–312. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Jones SP, et al. Cardioprotection by N-Acetylglucosamine Linkage to Cellular Proteins. Circulation. 2008;117(9):1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 27.Groves JA, et al. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress & Chaperones. 2013;18(5):535–558. doi: 10.1007/s12192-013-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HJ, et al. Glycerol-3-phosphate acyltransferase-1 upregulation by O-GlcNAcylation of Sp1 protects against hypoxia-induced mouse embryonic stem cell apoptosis via mTOR activation. Cell Death Dis. 2016;7:e2158. doi: 10.1038/cddis.2015.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunde IG, et al. Cardiac O-GlcNAc signaling is increased in hypertrophy and heart failure. Physiological Genomics. 2012;44(2):162–172. doi: 10.1152/physiolgenomics.00016.2011. [DOI] [PubMed] [Google Scholar]
- 30.Marsh SA, Dell’Italia LJ, Chatham JC. Activation of the hexosamine biosynthesis pathway and protein O-GlcNAcylation modulate hypertrophic and cell signaling pathways in cardiomyocytes from diabetic mice. Amino Acids. 2011;40(3):819–828. doi: 10.1007/s00726-010-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Facundo HT, et al. O-GlcNAc signaling is essential for NFAT-mediated transcriptional reprogramming during cardiomyocyte hypertrophy. American Journal of Physiology - Heart and Circulatory Physiology. 2012;302(10):H2122–H2130. doi: 10.1152/ajpheart.00775.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Olson AK, et al. C-Myc Induced Compensated Cardiac Hypertrophy Increases Free Fatty Acid Utilization for the Citric Acid Cycle. Journal of molecular and cellular cardiology. 2013;55:156–164. doi: 10.1016/j.yjmcc.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ledee D, et al. c-Myc Alters Substrate Utilization and O-GlcNAc Protein Posttranslational Modifications without Altering Cardiac Function during Early Aortic Constriction. PLoS ONE. 2015;10(8):e0135262. doi: 10.1371/journal.pone.0135262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo X, et al. Alterations in left ventricular function during intermittent hypoxia: Possible involvement of O-GlcNAc protein and MAPK signaling. Int J Mol Med. 2015;36(1):150–8. doi: 10.3892/ijmm.2015.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson LJ, et al. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(41):17797–802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson LJ, et al. Cardiomyocyte Ogt is essential for postnatal viability. Am J Physiol Heart Circ Physiol. 2014;306(1):H142–H153. doi: 10.1152/ajpheart.00438.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sack MN, et al. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth a program. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(12):6438–6443. doi: 10.1073/pnas.94.12.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majumdar G, et al. Insulin stimulates and diabetes inhibits O-linked N-acetylglucosamine transferase and O-glycosylation of Sp1. Diabetes. 2004;53(12):3184–3192. doi: 10.2337/diabetes.53.12.3184. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, et al. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci U S A. 2001;98(12):6611–6. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medford HM, et al. Consuming a Western diet for two weeks suppresses fetal genes in mouse hearts. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2014;306(8):R519–R526. doi: 10.1152/ajpregu.00253.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox EJ, Marsh SA. Exercise and diabetes have opposite effects on the assembly and O-GlcNAc modification of the mSin3A/HDAC1/2 complex in the heart. Cardiovascular Diabetology. 2013;12:101–101. doi: 10.1186/1475-2840-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cannon MV, et al. Cardiac LXRα protects against pathological cardiac hypertrophy and dysfunction by enhancing glucose uptake and utilization. EMBO Molecular Medicine. 2015;7(9):1229–1243. doi: 10.15252/emmm.201404669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hulot JS, et al. Critical Role for Stromal Interaction Molecule 1 in Cardiac Hypertrophy. Circulation. 2011;124(7):796–805. doi: 10.1161/CIRCULATIONAHA.111.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu-Mauldin X, et al. Modification of STIM1 by O-linked N-Acetylglucosamine (O-GlcNAc) Attenuates Store-operated Calcium Entry in Neonatal Cardiomyocytes. Journal of Biological Chemistry. 2012;287(46):39094–39106. doi: 10.1074/jbc.M112.383778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Velden J, et al. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovascular Research. 2006;69(4):876–887. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 46.Hamdani N, et al. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovascular Research. 2013;97(3):464–471. doi: 10.1093/cvr/cvs353. [DOI] [PubMed] [Google Scholar]
- 47.van der Velden J, et al. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovascular Research. 2003;57(1):37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 48.Dubois-Deruy E, et al. Interplay between troponin T phosphorylation and O-N-acetylglucosaminylation in ischaemic heart failure. Cardiovascular Research. 2015;107(1):56–65. doi: 10.1093/cvr/cvv136. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez-Correa GA, et al. O-linked GlcNAc modification of cardiac myofilament proteins: a novel regulator of myocardial contractile function. Circ Res. 2008;103(12):1354–8. doi: 10.1161/CIRCRESAHA.108.184978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muthusamy S, et al. MicroRNA-539 is up-regulated in failing heart, and suppresses O-GlcNAcase expression. Journal of Biological Chemistry. 2014;289(43):29665–29676. doi: 10.1074/jbc.M114.578682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis BA, Hanover JA. O-GlcNAc and the Epigenetic Regulation of Gene Expression. Journal of Biological Chemistry. 2014;289(50):34440–34448. doi: 10.1074/jbc.R114.595439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clark RJ, et al. Diabetes and the Accompanying Hyperglycemia Impairs Cardiomyocyte Calcium Cycling through Increased Nuclear O-GlcNAcylation. Journal of Biological Chemistry. 2003;278(45):44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 53.Hu Y, et al. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96(9):1006–13. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 54.De Blasio MJ, et al. Abstract 15267: Cardiac-Specific Insulin-Like Growth Factor-1 Receptor (IGF-1R) Expression Targets Maladaptive Hexosamine Biosynthesis and O-Linked GlcNAc Modification of Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA2a) in Diabetic Myocardium. Circulation. 2016;134(Suppl 1):A15267–A15267. [Google Scholar]
- 55.Ramirez-Correa G, et al. Removal of abnormal myofilament O-GlcNAcylation restores Ca2+ sensitivity in diabetic cardiac muscle. Diabetes. 2015;64(10):3573–87. doi: 10.2337/db14-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gawlowski T, et al. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-β-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem. 2012;287(35):30024–34. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dassanayaka S, et al. High glucose induces mitochondrial dysfunction independently of protein O-GlcNAcylation. Biochem J. 2015;467(1):115–26. doi: 10.1042/BJ20141018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banerjee PS, Ma J, Hart GW. Diabetes-associated dysregulation of O-GlcNAcylation in rat cardiac mitochondria. Proc Natl Acad Sci U S A. 2015;112(19):6050–6055. doi: 10.1073/pnas.1424017112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma J, et al. O-GlcNAcomic Profiling Identifies Widespread O-GlcNAcylation in Oxidative Phosphorylation System Regulating Cardiac Mitochondrial Function. Journal of Biological Chemistry. 2015 doi: 10.1074/jbc.M115.691741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma J, et al. Comparative Proteomics Reveals Dysregulated Mitochondrial O-GlcNAcylation in Diabetic Hearts. Journal of Proteome Research. 2016 doi: 10.1021/acs.jproteome.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, et al. O-GlcNAcase deficiency suppresses skeletal myogenesis and insulin sensitivity in mice through the modulation of mitochondrial homeostasis. Diabetologia. 2016;59(6):1287–1296. doi: 10.1007/s00125-016-3919-2. [DOI] [PubMed] [Google Scholar]
- 62.Federici M, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106(4):466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- 63.Beleznai T, Bagi Z. Activation of hexosamine pathway impairs nitric oxide (NO)-dependent arteriolar dilations by increased protein O-GlcNAcylation. Vascular Pharmacology. 2012;56(3–4):115–121. doi: 10.1016/j.vph.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makino A, et al. O-GlcNAcase overexpression reverses coronary endothelial cell dysfunction in type 1 diabetic mice. American Journal of Physiology - Cell Physiology. 2015;309(9):C593–C599. doi: 10.1152/ajpcell.00069.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lima VV, et al. High-fat diet increases O-GlcNAc levels in cerebral arteries: a link to vascular dysfunction associated with hyperlipidaemia/obesity? Clin Sci (Lond) 2016;130(11):871–80. doi: 10.1042/CS20150777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heath JM, et al. Activation of AKT by O-linked N-acetylglucosamine induces vascular calcification in diabetes mellitus. Circ Res. 2014;114(7):1094–102. doi: 10.1161/CIRCRESAHA.114.302968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lima VV, et al. Impaired Vasodilator Activity in Deoxycorticosterone Acetate-Salt Hypertension Is Associated With Increased Protein O-GlcNAcylation. Hypertension. 2009;53(2):166–174. doi: 10.1161/HYPERTENSIONAHA.108.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lima VV, et al. O-GlcNAcylation: a novel pathway contributing to the effects of endothelin in the vasculature. Am J Physiol Regul Integr Comp Physiol. 2011;300(2):R236–50. doi: 10.1152/ajpregu.00230.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xing D, et al. Increased protein O-GlcNAc modification inhibits inflammatory and neointimal responses to acute endoluminal arterial injury. American Journal of Physiology - Heart and Circulatory Physiology. 2008;295(1):H335–H342. doi: 10.1152/ajpheart.01259.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xing D, et al. O-GlcNAc Modification of NFκB p65 Inhibits TNF-α-Induced Inflammatory Mediator Expression in Rat Aortic Smooth Muscle Cells. PLOS ONE. 2011;6(8):e24021. doi: 10.1371/journal.pone.0024021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hilgers RHP, et al. Acute O-GlcNAcylation prevents inflammation-induced vascular dysfunction. American Journal of Physiology - Heart and Circulatory Physiology. 2012;303(5):H513–H522. doi: 10.1152/ajpheart.01175.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Zhao V, et al. Spliced X-Box Binding Protein 1 Couples the Unfolded Protein Response to Hexosamine Biosynthetic Pathway. Cell. 2014;156(6):1179–1192. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]