Abstract
Status epilepticus is a common manifestation of nerve agent toxicity and represents a serious medical emergency with high rates of mortality and neurologic injury in those that survive. The aim of the current study was to determine if targeting oxidative stress with the catalytic antioxidant, AEOL10150, would reduce pilocarpine-induced mortality and attenuate neuronal death and neuroinflammation. We found that treatment with AEOL10150 in conjunction with scopolamine and diazepam following pilocarpine-induced SE was able to significantly reduce mortality compared to treatment with just scopolamine and diazepam. Mortality was further reduced when AEOL10150 was used in conjunction with atropine and diazepam which is considered the standard of care for nerve agent exposures. Both treatment paradigms offered significant protection against SE-induced oxidative stress. Additionally, treatment with scopolamine, AEOL10150 and diazepam attenuated SE-induced neuronal loss and neuroinflammation. Taken together, the data suggest that pharmacological targeting of oxidative stress can improve survival and attenuate secondary neurological damage following SE induced by the nerve agent surrogate pilocarpine.
Keywords: Pilocarpine, Nerve agent toxicity, Epilepsy, Glutathione
Introduction
Status epilepticus (SE), a period of seizure activity lasting longer than 5 min, is considered a neurological emergency and is associated with significant morbidity and mortality. In people with epilepsy, the most common precipitant of SE is a change in medication, however SE can occur in people that have experienced brain trauma, metabolic insults or that have been exposed to toxic chemicals including organophosphorus compounds and nerve agents [1, 2]. Prolonged SE can result in neuronal injury, epilepsy and cognitive impairment while mortality associated with SE occurs in approximately 20% of patients [3–5]. Age and the underlying etiology tend to be the best predictors of SE associated mortality however the underlying mechanism(s) responsible for morbidity and mortality are unclear [6].
Current treatment of SE in patients and animals is focused on the prompt delivery of benzodiazepines such as diazepam or midazolam [7]. Additionally, in animal models of SE induced by chemoconvulsants, organophosphate toxicity, or nerve agent exposure, anticholinergics such as scopolamine or atropine are often employed [8]. Despite these various lines of treatment options, SE-associated mortality persists, necessitating the identification of novel therapeutic targets. The CounterACT program is a trans-NIH initiative whose mission is to develop medical countermeasures to prevent and/or treat conditions caused by exposure to nerve agents including SE, SE-related mortality, neuronal injury and cognitive dysfunction [9–11]. Because nerve agent-induced seizures and epileptic seizures share common neuronal mechanisms and phenotypes, countermeasures that prevent mortality and/or treat secondary changes induced by nerve agents, hold promise for the treatment of epilepsy [12]. Additionally, interventions that are identified in epilepsy models hold promise for treating nerve agent exposures particularly those identified in the pilocarpine model as both pilocarpine and nerve agents induce seizures through the cholinergic system. For this reason and its reduced likelihood to be weaponized, pilocarpine is considered a surrogate nerve agent for comparative research [13]. Work from this laboratory has demonstrated that oxidative stress is an important consequence of the nerve agent surrogate, pilocarpine [14]. Oxidative stress occurs when there is an imbalance in the redox environment resulting from excessive production of reactive species [15]. Previous data have demonstrated that prolonged seizures and SE via multiple mechanisms are sufficient to oxidatively damage DNA, proteins and cellular lipids resulting in neuronal damage, gliosis and cognitive dysfunction [16, 17]. Targeting oxidative stress may therefore hold particular promise to improve SE-related neurological consequences and perhaps attenuate SE-related mortality as well.
AEOL10150 is a broad spectrum catalytic antioxidant with the ability to scavenge O2− H2O2, ONOO− and inhibit lipid peroxidation [18–21]. Metalloporphyin catalytic antioxidants have shown efficacy in attenuating oxidative stress in a wide range of animal models of disease [17, 22, 23]. That AEOL10150 inhibits a broad spectrum of reactive oxygen species (ROS) and can cross the blood brain barrier to achieve therapeutic concentrations in brain, make it a useful tool to evaluate our hypothesis that oxidative stress contributes to SE-related mortality and secondary neurological damage [17]. We have previously reported that a brief treatment period with AEOL10150 starting 1 h after pilocarpine-induced SE reduced neurodegeneration measured 24 h after SE and cognitive deficits measured 7 days after SE [17]. The goal of this manuscript was twofold. The first goal was to determine if treatment with AEOL10150 in combination with scopolamine and diazepam or atropine and diazepam could attenuate SE-related mortality and whether scopolamine or atropine, afforded better protection. Atropine is considered part of the standard of care for nerve agent toxicity although scopolamine could serve a similar function in instances when atropine is not available. The second goal was to evaluate indices of oxidative stress after pretreatment or posttreatment with AEOL10150 and neuronal injury following pilocarpine-induced SE. Results from these studies have the potential to identify oxidative stress as a novel therapeutic target to attenuate mortality and secondary injury associated with nerve agent toxicity and epilepsy itself.
Materials and Methods
Reagents
All reagents were purchased from Sigma Aldrich unless otherwise noted. Manganese(III) meso-tetrakis (di-N-ethylimidazole) porphyrin or AEOL10150 (also known in the literature MnIIITDE-2-ImP5+) was pharmaceutical grade and obtained from Aeolus Pharmaceuticals.
Animals
Animals were treated in accordance with NIH guidelines and all experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver. Adult, male Sprague–Dawley rats (250–300 g; Harlan Laboratories, Indianapolis, Indiana), were utilized for all experiments. Upon arrival, rats were group housed on a 14/10 light/dark cycle with ad libitum access to food (Harlan rat chow) and filtered water. After 1 week of acclimation, rats were randomly assigned to various treatment groups. Certain experimental rats were injected with scopolamine hydrobromide (1 mg/kg) 30 min prior to pilocarpine (340 mg/kg) or atropine sulfate (2 mg/ kg) 10 min after pilocarpine to limit peripheral cholinergic effects. In certain groups, diazepam (10 mg/kg) was given 90 min after pilocarpine to terminate SE. Rats that served as controls were given either scopolamine or atropine and diazepam, as appropriate per experiment and received saline instead of pilocarpine. All pilocarpine-treated rats were visually monitored during SE and behavioral seizures were scored using a modified Racine scale [24]. Briefly, seizures were scored on the following scale: P1-freezing behavior, P2-head nodding, P3-unilateral forelimb clonus, P4-bilateral forelimb clonus with rearing, and P5-bilateral forelimb clonus with rearing, falling, and/or hind limb clonus.
Catalytic Antioxidant Treatment
Rats were treated with the metalloporphyrin catalytic antioxidant, AEOL10150 (5 mg/kg s.c.) dissolved in saline or saline alone starting 30 min prior to pilocarpine or 90 min after pilocarpine and continuing every 4 h until sacrifice (for most endpoints-24 h). We have previously shown that this compound can pass the blood–brain barrier in therapeutically relevant concentrations when given 1 h after SE and every 4 h thereafter at a dose of 5 mg/kg [17]. This dosing regime was sufficient to attenuate indices of oxidative stress, neuronal loss and cognitive dysfunction induced by pilocarpine administration [17]. Treatment time-points of 30 min prior to and 90 min after pilocarpine were selected to determine a therapeutic window for the protective effects of AEOL10150. Pilot studies determined that treatment of rats with AEOL10150 alone had no effect on indices of oxidative stress in the control group and therefore this group was not included in further analyses.
Measurement of Redox Biomarkers by HPLC
Glutathione (GSH), glutathione disulfide (GSSG), tyrosine and 3-nitrotyrosine (3-NT) assays were performed 24 h after SE with an ESA (Chelmsford, MA) 5600 CoulArray HPLC equipped with eight electrochemical cells following the company instruction (ESA Application Note 70-3993) and previously described in the literature with small modifications [17, 23, 25]. The level of 3-NT was expressed as a ratio of 3-NT to tyrosine. Samples were randomized and blinded before assessment.
Immunohistochemistry
Sections (15 µm) were cut coronally through paraffin-embedded hippocampus and stained with Fluoro-Jade B (FJB; Histo-Chem) as previously described [17, 26]. The staining solution for FJB (0.004% Fluoro-Jade B in 0.1% acetic acid vehicle) was prepared fresh. After a 15 min incubation in the staining solution, the sections were rinsed and dried. The sections were cleared by xylene for at least a minute before coverslipping with DPX. IBA1 staining (Wako, Cat. No. 019-1971) was performed for reactive microgliosis according to the manufacturer’s instructions. Briefly, sections were deparaffinized and rehydrated in sequential steps. Sections were incubated with the primary antibody diluted 1:500 in 1% goat serum PBS-T. After an overnight incubation with the primary antibody, the sections were rinsed and incubated with the secondary antibody, rhodamine red (goat anti-rabbit conjugated Jackson Immuno Research Inc., 1:100 in PBS-T) for 1 h. Sections were washed, cleared in xylene and cover-slipped using DPX mounting medium. A Nikon Eclipse TE2000-U microscope was used to capture images at 10×magnification. Quantification of the average florescence intensity within a given area was performed using Image-J software. Imaging was performed using a Nikon Eclipse TE2000-U microscope. Using Image-J, the FJB or IBA1 positive signal of areas of interest (hippocampal areas CA3 and hilus) was measured. The average of the fluorescent relative density was quantified for each animal and the group average was expressed as percentage of the control.
Statistical Analysis
Effects of group treatment on indices of oxidative stress were compared by one-way analysis of variance (ANOVA). Significant effects (p < 0.05) were probed using Tukey post-tests. Survival analysis was performed using the Kaplan–Meier method. All data are expressed as mean ± SEM unless otherwise noted. All analyses were performed using GraphPad Prism 5 software.
Results
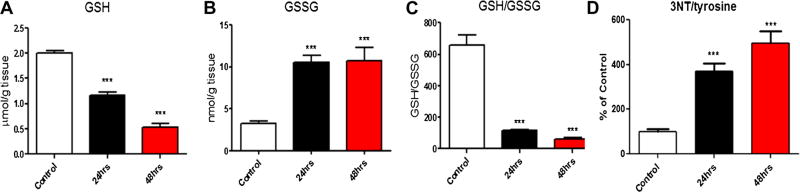
While the direct measurement of ROS is complicated by the innate reactivity and labile nature of the radical, measurement of alterations to the redox status of glutathione (GSH/GSSG) and protein nitration are quantifiable and specific to oxidative/nitrosative stress. To determine whether pilocarpine-induced SE results in oxidative stress, rats pretreated with scopolamine followed 30 min later by pilocarpine and diazepam 90 min after pilocarpine were assessed for glutathione (GSH/GSSG) redox status and protein nitration (3NT/tyr) 24 and 48 h after pilocarpine administration. Relative to control rats that received pretreatment with scopolamine, saline rather than pilocarpine followed by diazepam, the hippocampi of rats pretreated with scopolamine followed by pilocarpine and diazepam had significantly depleted levels of reduced glutathione (GSH) at both 24 h (p < 0.001) and 48 h (p < 0.001; Fig. 1a). Additionally, levels of oxidized glutathione (GSSG) were significantly elevated at both time points (p < 0.001, p < 0.001; Fig. 1b), resulting in a significant depletion of the overall ratio of GSH:GSSG (p < 0.001, p < 0.001; Fig. 1c). The ratio of 3-NT/tyrosine was significantly increased in the hippocampus of the pilocarpine group at both 24 h (p < 0.001) and 48 h (p < 0.001; Fig. 1d).
Fig. 1.
Oxidative stress in rats treated with pilocarpine after 24 and 48 h. a GSH, b GSSG levels, c GSH/GSSG ratio and d 3NT/Tyr ratio were measured in the hippocampi of saline and pilocarpine treated rats at 24 and 48 h. ***p < 0.001 compared to saline injected control
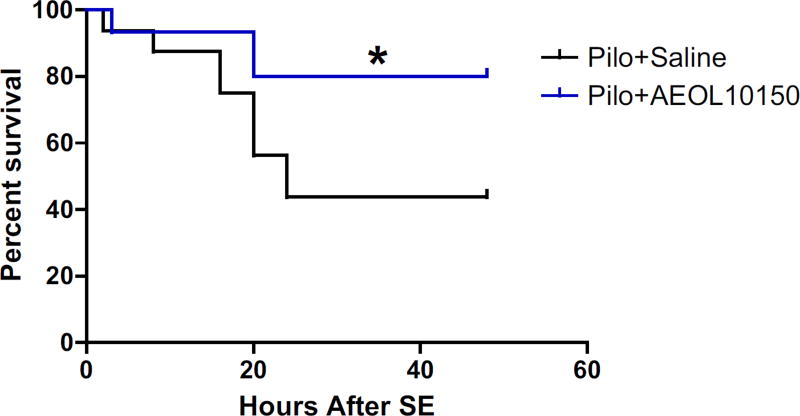
To assess whether oxidative stress contributes to mortality associated with SE, a cohort of rats were treated with scopolamine, pilocarpine, diazepam and either saline or a catalytic antioxidant known to attenuate SE-induced oxidative stress, AEOL10150 [17]. Survival was followed for 48 h and plotted in Fig. 2. Pilocarpine-induced mortality was the highest in the first 24 h after SE with the mortality in the saline group at approximately 56% compared to 20% in the AEOL10150 treated group, representing a significant improvement in survival by AEOL10150 (p < 0.05; Fig. 2).
Fig. 2.
Effect of AEOL10150 on pilocarpine-induced SE survival rates. All rats received scopolamine (1 mg/kg), pilocarpine (340 mg/ kg) and diazepam (10 mg/kg). Treatment with AEOL10150 (5 mg/kg) starting 60 min after SE and continuing every 4 h thereafter significantly improved SE-related mortality up to 48 h after SE. *p < 0.05
We next evaluated SE-related mortality rates of various treatment strategies including pilocarpine alone, with no other standard therapies; scopolamine with pilocarpine and diazepam; atropine with pilocarpine and diazepam; scopolamine, pilocarpine, diazepam and AEOL10150; and atropine, pilocarpine, diazepam and AEOL10150 (Table 1). Pilocarpine alone, without standard therapy resulted in substantial mortality, approximately 57%. Pretreatment with scopolamine and post-treatment with diazepam reduced the mortality rate of pilocarpine treated rats to around 44% and addition of AEOL10150 to scopolamine and diazepam further reduced mortality to 23%. A 31% mortality rate resulted from treatment with pilocarpine followed by atropine and diazepam. When AEOL10150 was given in addition to atropine and diazepam, SE-induced mortality in pilocarpine-treated rats was completely prevented.
Table 1.
Mortality rates in rats treated with various protective compounds 24 h after pilocarpine
| Treatment | Mortality (%) |
|---|---|
| Pilo | 57.1 |
| Scopol + pilo + dzp | 44.4 |
| Pilo + atr + dzp | 31.3 |
| Pilo + scopol + dzp + AEOL10150 | 23.3 |
| Pilo + atr + dzp + AEOL10150 | 0 |
Rats received pilocarpine (pilo; 340 mg/kg) alone or in combination with scopolamine (scopol; 1 mg/kg), or atropine (atr; 2 mg/kg), and diazepam (dzp; 10 mg/kg) and AEOL10150 (5 mg/kg) starting 60 min after SE and continuing every 4 h
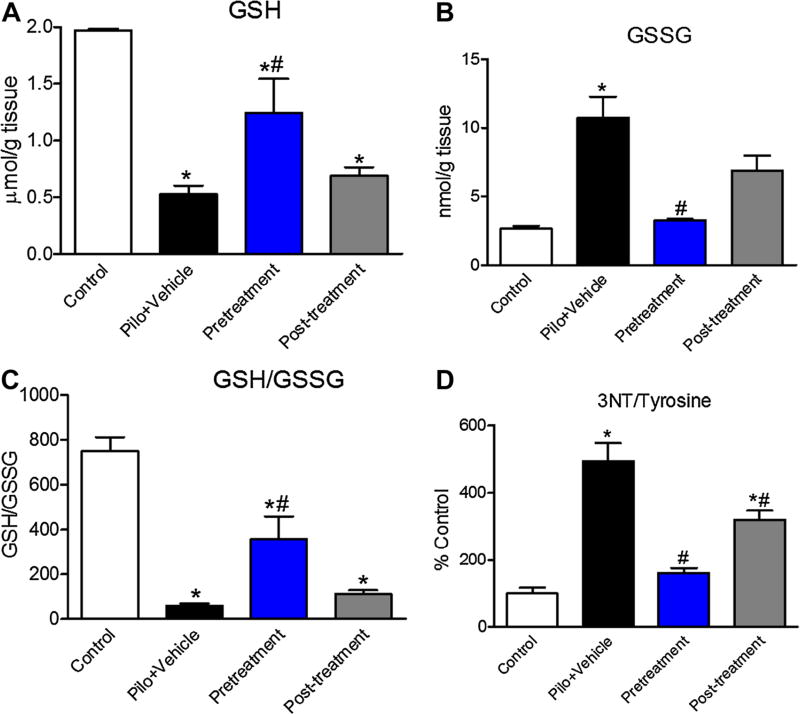
Given that treatment with AEOL10150, scopolamine and diazepam was able to attenuate SE-induced mortality, we next asked if this treatment combination was able to attenuate SE-induced oxidative stress. Additionally we sought to identify a therapeutic window for maximal effects and therefore AEOL10150 was given at two time points: 30 min prior to pilocarpine (pretreatment), and 90 min after pilocarpine (post-treatment) and continuing every 4 h until tissue was collected at 24 h. Previous work from our laboratory has reported the effects of posttreatment of AEOL10150 when started 60 min after pilocarpine in the presence of scopolamine and diazepam on pilocarpineinduced oxidative stress and neuronal injury [17]. Relative to control rats (scopolamine, saline and diazepam), pilocarpine-induced SE in the presence of scopolamine and diazepam resulted in depleted GSH (p < 0.001) which was attenuated by 30 min pretreatment with AEOL10150 (p < 0.01) but not 90 min post-treatment (Fig. 3a). GSSG levels were significantly elevated in those rats experiencing SE relative to controls (p < 0.01) and were attenuated by 30 min pretreatment (p < 0.01) but not 90 min posttreatment with AEOL10150 (Fig. 3b), pretreatment with AEOL10150 thereby significantly improved the ratio of GSH:GSSG in the hippocampus (p < 0.001; Fig. 3c). Similarly, the ratio of 3-NT/tyrosine, which was increased in the hippocampus after pilocarpine-induced SE (p < 0.001), was significantly decreased by both 30 min pretreatment (p < 0.001) and 90 min post-treatment with AEOL10150 (p < 0.05; Fig. 3d).
Fig. 3.
Identification of therapeutic window of AEOL10150 when given in conjunction with scopolamine, pilocarpine and diazepam. a GSH, b GSSG, c GSH/GSSG and d 3NT in the hippocampus of rats 24 h after scopolamine, pilocarpine and diazepam with or without AEOL10150 (5 mg/kg) when given 30 min prior to pilocarpine (pretreatment) or 90 min after pilocarpine (post-treatment). *p < 0.05 vs. control, #p < 0.05 vs. pilocarpine treatment
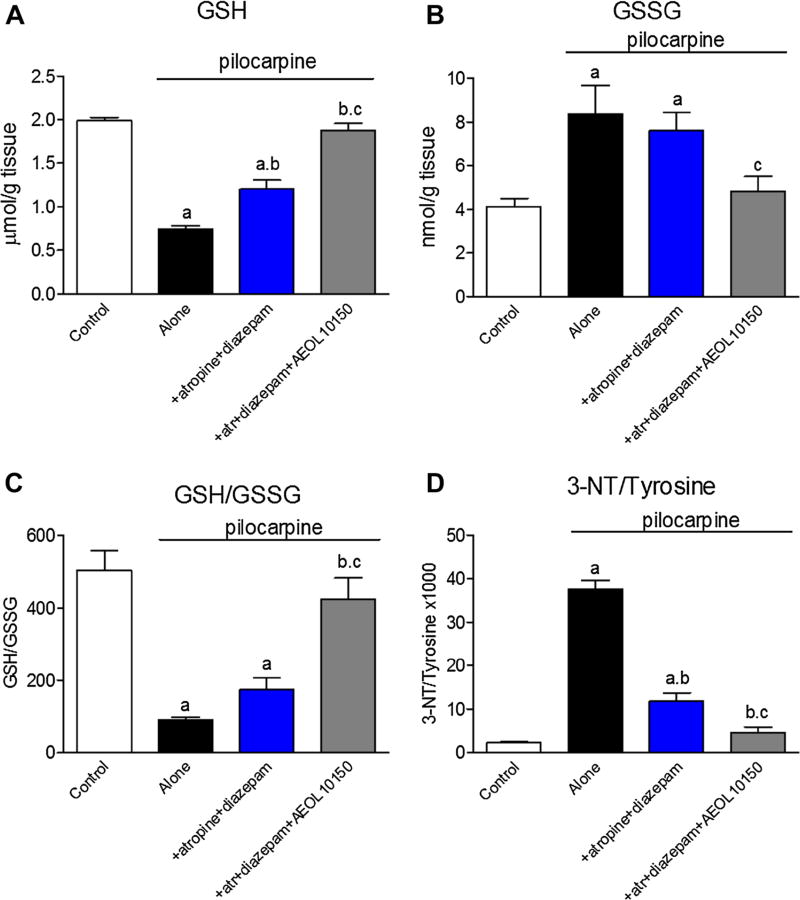
To determine if treatment with atropine in conjunction with diazepam and AEOL10150 offered protection from SE-induced oxidative stress, GSH redox status and protein nitration was assessed. Compared to pilocarpine alone, treatment with atropine and diazepam significantly improved levels of GSH (p < 0.05) which AEOL10150 (5 mg/kg starting 60 min after pilo and every 4 h thereafter) was able to further improve (p < 0.001; Fig. 4a) while neither treatment regiments were able to prevent accumulation of its disulfide, GSSG (Fig. 4b). Despite having no effect on GSSG, treatment with atropine, diazepam and AEOL10150 was able to significantly improve the SE-induced depletion of overall GSH:GSSG relative to atropine and diazepam alone (Fig. 4c). Both atropine and diazepam and atropine, diazepam and AEOL10150 treatment regiments were able to significantly attenuate SE-induced protein nitration (p < 0.001; Fig. 4d).
Fig. 4.
Indices of oxidative stress in rats treated with pilocarpine, atropine, diazepam and AEOL10150. a GSH, b GSSG, c GSH/GSSG ratio and d 3-NT/tyrosine in the hippocampus of rats 24 h after either pilocarpine alone w/o atropine (atr) and diazepam or in the presence of AEOL 10150 (5 mg/kg) starting 60 min after SE and continuing every 4 h. a p < 0.05 versus control, b p < 0.05 versus pilocarpine-treated rats, c p < 0.05 versus pilocarpine + atropine + diazepam treated rats
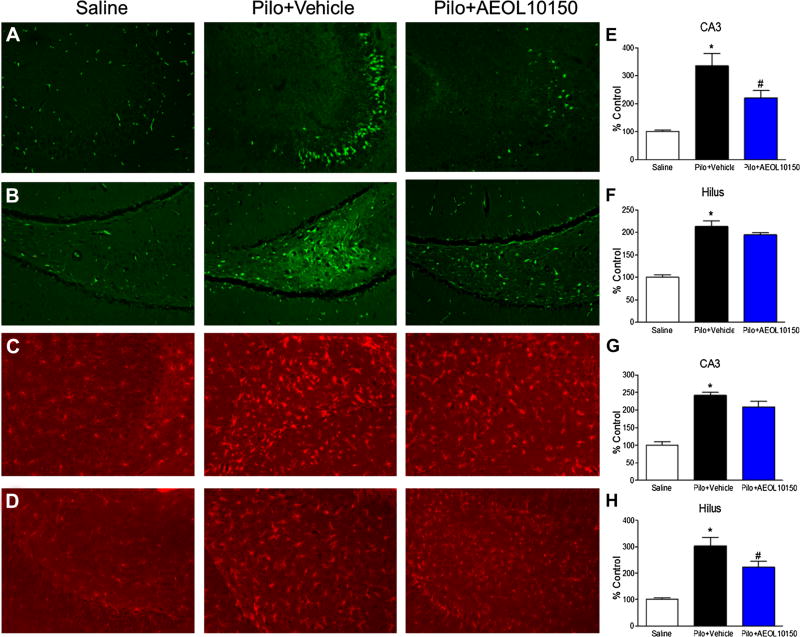
To ascertain whether treatment with AEOL10150 had any effect on neuropathological outcomes, neuronal loss and microgliosis were evaluated via FJB and IBA1 immunofluorescence, respectively. Staining was evaluated in hippocampal areas CA3 and hilus 24 h after scopolamine, pilocarpine, diazepam and AEOL10150 treatment. CA1 was not included in analysis because it did not show any difference in fluorescence intensity when compared to control. Treatment with AEOL10150 starting 90 min after pilocarpine injection significantly attenuated SE-induced neuronal loss in the CA3 (p < 0.05; Fig. 5a) but not hilar region of the hippocampus (p > 0.05; Fig. 5b). Microgliosis was significantly attenuated by AEOL10150 treatment in the hilar region (p < 0.05; Fig. 5c) but not the CA3 region (p > 0.05; Fig. 5d).
Fig. 5.
Neuronal loss and microgliosis in hippocampus of rats treated with scopolamine, pilocarpine, diazepam and with or without AEOL10150 (5 mg/kg) starting 90 min after SE and continuing every 4 h. Representative images of FJB staining in a CA3 and b Hilus. Representative images of IBA1 staining in c CA3 and d Hilus. e Quantification of FJB staining indicative of neuronal loss in the f CA3 region and hilar region of the hippocampus. g Quantification of IBA1 staining indicative of microgliosis in the CA3 and h hilus of the hippocampus of rats 24 h after SE. *p < 0.05 vs. control, #p < 0.05 vs. pilocarpine treatment
Discussion
Using a catalytic antioxidant, we have shown that pharmacologically targeting oxidative stress can reduce mortality associated with SE produced by pilocarpine, a nerve agent surrogate. The greatest reduction in pilocarpine-associated mortality was observed when AEOL10150 was used in conjunction with atropine and diazepam. This was also the treatment paradigm that resulted in the greatest attenuation of oxidative stress. Treatment with AEOL10150 resulted in modest improvements in neuronal loss and microgliosis in the hippocampus. Taken together, the data suggest a novel target to reduce mortality and improve neurological outcomes associated with SE.
Increasing evidence suggests that SE is associated with oxidative stress in the brain [17, 24, 27, 28]. Indeed, the data presented here suggest that SE results in oxidative stress as indicated by significant alterations in the glutathione redox status and increased protein nitration at both 24 and 48 h following pilocarpine administration. Organophosphates such as diisopropyl fluorophosphates (DFP) and malathion are also considered surrogate nerve agents due to their effects on the cholinergic system resulting in SE. Studies have shown that SE induced by these compounds is also sufficient to increase markers of oxidative stress suggesting that SE and not the inciting compound is responsible for increased oxidative stress in the brain [29, 30]. Although most people who have experienced SE do not die during the event, the highest mortality is typically within 30 days of the event [31]. Previous research has shown that glutathione redox status alterations and mitochondrial oxidative stress persists when measured up to 3 months after SE [32, 33]. The current study only evaluated oxidative stress and mortality up to 2 days after SE and oxidative stress was present at both 24 and 48 h while mortality was greatest in the first 24 h after SE. Treatment with AEOL10150 was able to significantly reduce mortality suggesting that oxidative stress may play an underlying role in acute SE-associated death.
Treatment with AEOL10150 as well as scopolamine or atropine and diazepam was able to provide the most protection against SE-induced mortality. Both scopolamine and atropine act to reduce SE-associated damage and likely mortality by blocking muscarinic cholinergic receptors within the nervous system and reducing SE duration and intensity [8]. Treatment with diazepam improves mortality in much the same way, by activating GABAergic receptors and reducing SE duration and intensity [9]. Duration of SE is a significant predictor of morbidity and mortality therefore quick treatment with atropine or scopolamine and diazepam would be expected and indeed has been shown to improve mortality and secondary consequences compared to no treatment at all [8, 34]. The greater ability of atropine to protect against mortality relative to scopolamine when used in conjunction with diazepam may be related to atropine’s greater ability to pass the blood brain barrier than scopolamine hydrobromide [35]. Indeed, treatment with atropine and diazepam protected the glutathione redox ratio in the hippocampus approximately three-fold better than did scopolamine and diazepam. Addition of AEOL10150 to treatment with atropine and diazepam was able to improve the glutathione redox status four-fold relative to treatment with scopolamine, diazepam and AEOL10150. AEOL10150 itself, does not have any direct effects on the intensity or duration of SE [17]. Therefore improvement in the glutathione redox status may provide a possible rationale as to why mortality was completely prevented upon treatment with atropine, diazepam and AEOL10150.
Oxidative stress can cause free-radical induced damage to proteins, lipids and DNA as well as inflammation and neurotoxicity. This is particularly the case when levels of GSH are altered, as it functions as one of the primary antioxidants responsible for free-radical detoxification. One important consequence of altered GSH levels is activation of cell death cascades [36]. Indeed, we demonstrate that alterations in GSH redox status are sufficient to contribute to SE-induced neuronal loss. Additionally nitration of tyrosine is considered an indicator of cell damage and inflammation and this marker was significantly attenuated with AEOL10150 treatment [37]. After nerve agent exposure, oximes and anticholinergics can mitigate direct toxicity but seizure activity and its consequences such as neuronal damage and neuroinflammation are major sequalae. Therefore, individuals who survive the initial exposure are at risk for secondary damage and cognitive dysfunction. We have previously shown that treatment with AEOL10150 is neuroprotective and provide further evidence here that delaying treatment with AEOL10150 up to 90 min following pilocarpine administration provides some protection against neuronal loss and microglial activation in areas of the hippocampus, which is a brain region known to be highly epileptogenic and a known target of SE-induced neuronal injury [17]. Initiating treatment at an earlier time-point may provide more protection to all areas of the hippocampus which may have functional effects in improving learning and memory. Based on this data, it appears that AEOL10150 can significantly attenuate mortality as well as secondary SE mediated sequalae such as oxidative stress, neuroinflammation and neuronal injury.
Acknowledgments
This work was funded by Grants NIHRO1NS039587 (M.P.), NIHRO1NS086423 (M.P.) UO1NS083422 (M.P.), F31NS086405 (J.N.P.) and R21NS072099 (M.P.).
Footnotes
Compliance with Ethical Standards
Conflict of interest Drs. Day and Patel are consultants for Aeolus Pharmaceuticals which develops catalytic antioxidants for human diseases including the compound used in this work. Dr. Day holds equity in Aeolus Pharmaceuticals.
References
- 1.Trinka E, Höfler J, Zerbs A. Causes of status epilepticus. Epilepsia. 2012;53:127–138. doi: 10.1111/j.1528-1167.2012.03622.x. [DOI] [PubMed] [Google Scholar]
- 2.de Araujo Furtado M, Rossetti F, Chanda S, Yourick D. Exposure to nerve agents: from status epilepticus to neuroinflammation, brain damage, neurogenesis and epilepsy. Neurotoxicology. 2012;33(6):1476–1490. doi: 10.1016/j.neuro.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Hauser WA. Status epilepticus: epidemiologic considerations. Neurology. 1990;40(5 Suppl 2):9–13. [PubMed] [Google Scholar]
- 4.DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12(4):316–325. [PubMed] [Google Scholar]
- 5.Logroscino G, Hesdorffer DC, Cascino G, Annegers JF, Hauser WA. Short-term mortality after a first episode of status epilepticus. Epilepsia. 1997;38(12):1344–1349. doi: 10.1111/j.1528-1157.1997.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 6.Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994;35(1):27–34. doi: 10.1111/j.1528-1157.1994.tb02908.x. [DOI] [PubMed] [Google Scholar]
- 7.Manno EM. Status epilepticus: current treatment strategies. Neurohospitalist. 2011;1(1):23–31. doi: 10.1177/1941875210383176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shih T-M, Rowland TC, McDonough JH. Anticonvulsants for nerve agent-induced seizures: the influence of the therapeutic dose of atropine. J Pharmacol Exp Ther. 2006;320(1):154. doi: 10.1124/jpet.106.111252. [DOI] [PubMed] [Google Scholar]
- 9.Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188(2):69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 10.Shih T, McDonough JH, Jr, Koplovitz I. Anticonvulsants for soman-induced seizure activity. J Biomed Sci. 1999;6(2):86–96. doi: 10.1007/BF02256439. [DOI] [PubMed] [Google Scholar]
- 11.Jett DA, Yeung DT. The counteract research network: basic mechanisms and practical applications. Proc Am Thorac Soc. 2010;7(4):254–256. doi: 10.1513/pats.201001-003SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jett DA. Finding new cures for neurological disorders: a possible fringe benefit of biodefense research? Sci Transl Med. 2010;2(23):23ps12. doi: 10.1126/scitranslmed.3000752. [DOI] [PubMed] [Google Scholar]
- 13.Tang FR, Loke WK, Ling EA. Comparison of status epilepticus models induced by pilocarpine and nerve agents: a systematic review of the underlying aetiology and adopted therapeutic approaches. Curr Med Chem. 2011;18(6):886–899. doi: 10.2174/092986711794927720. [DOI] [PubMed] [Google Scholar]
- 14.Waldbaum S, Patel M. Mitochondrial dysfunction and oxidative stress: a contributing link to acquired epilepsy? J Bioenerg Biomembr. 2010;42(6):449–455. doi: 10.1007/s10863-010-9320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemp M, Go Y-M, Jones DP. Non-equilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Rad Biol Med. 2008;44(6):921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Rad Biol Med. 2004;37(12):1951–1962. doi: 10.1016/j.freeradbiomed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Pearson JN, Rowley S, Liang L-P, White AM, Day BJ, Patel M. Reactive oxygen species mediate cognitive deficits in experimental temporal lobe epilepsy. Neurobiol Dis. 2015;82:289–297. doi: 10.1016/j.nbd.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day BJ, Batinic-Haberle I, Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Rad Biol Med. 1999;26(5–6):730–736. doi: 10.1016/s0891-5849(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 19.Day BJ, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced lung injuryin vivo. Toxicol Appl Pharmacol. 1996;140(1):94–100. doi: 10.1006/taap.1996.0201. [DOI] [PubMed] [Google Scholar]
- 20.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347(2):256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 21.Day BJ. Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov Today. 2004;9(13):557–566. doi: 10.1016/S1359-6446(04)03139-3. [DOI] [PubMed] [Google Scholar]
- 22.Liang L-P, Waldbaum S, Rowley S, Huang T-T, Day BJ, Patel M. Mitochondrial oxidative stress and epilepsy in sod2 deficient mice: attenuation by a lipophilic metalloporphyrin. Neurobiol Dis. 2012;45(3):1068–1076. doi: 10.1016/j.nbd.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang L-P, Huang J, Fulton R, Day BJ, Patel M. An orally active catalytic metalloporphyrin protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in vivo. J Neurosci. 2007;27(16):4326. doi: 10.1523/JNEUROSCI.0019-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang LP, Ho YS, Patel M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience. 2000;101(3):563–570. doi: 10.1016/s0306-4522(00)00397-3. [DOI] [PubMed] [Google Scholar]
- 25.Lakritz J, Plopper CG, Buckpitt AR. Validated high-performance liquid chromatography-electrochemical method for determination of glutathione and glutathione disulfide in small tissue samples. Anal Biochem. 1997;247(1):63–68. doi: 10.1006/abio.1997.2032. [DOI] [PubMed] [Google Scholar]
- 26.Schmued LC, Hopkins KJ. Fluoro-jade b: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874(2):123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 27.Bruce AJ, Baudry M. Oxygen free radicals in rat limbic structures after kainate-induced seizures. Free Rad Biol Med. 1995;18(6):993–1002. doi: 10.1016/0891-5849(94)00218-9. [DOI] [PubMed] [Google Scholar]
- 28.Frantseva MV, Perez Velazquez JL, Tsoraklidis G, Mendonca AJ, Adamchik Y, Mills LR, Carlen PL, Burnham MW. Oxidative stress is involved in seizure-induced neurodegeneration in the kindling model of epilepsy. Neuroscience. 2000;97(3):431–435. doi: 10.1016/s0306-4522(00)00041-5. [DOI] [PubMed] [Google Scholar]
- 29.Zaja-Milatovic S, Gupta RC, Aschner M, Milatovic D. Protection of DFP-induced oxidative damage and neurodegeneration by antioxidants and nmda receptor antagonist. Toxicol Appl Pharmacol. 2009;240(2):124–131. doi: 10.1016/j.taap.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortunato JJ, Feier G, Vitali AM, Petronilho FC, Dal-Pizzol F, Quevedo J. Malathion-induced oxidative stress in rat brain regions. Neurochemical Res. 2006;31(5):671–678. doi: 10.1007/s11064-006-9065-3. [DOI] [PubMed] [Google Scholar]
- 31.Logroscino G, Hesdorffer DC, Cascino GD, Annegers JF, Bagiella E, Hauser WA. Long-term mortality after a first episode of status epilepticus. Neurology. 2002;58(4):537–541. doi: 10.1212/wnl.58.4.537. [DOI] [PubMed] [Google Scholar]
- 32.Jarrett SG, Liang L-P, Hellier JL, Staley KJ, Patel M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol Dis. 2008;30(1):130–138. doi: 10.1016/j.nbd.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waldbaum S, Liang L-P, Patel M. Persistent impairment of mitochondrial and tissue redox status during lithium-pilocarpine-induced epileptogenesis. J Neurochem. 2010;115(5):1172–1182. doi: 10.1111/j.1471-4159.2010.07013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandt C, Töllner K, Klee R, Bröer S, Löscher W. Effective termination of status epilepticus by rational polypharmacy in the lithium–pilocarpine model in rats: window of opportunity to prevent epilepsy and prediction of epilepsy by biomarkers. Neurobiol Dis. 2015;75:78–90. doi: 10.1016/j.nbd.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 35.Tullberg S. Scopolamine. In: Enna J, Bylund DB, editors. Xpharm: the comprehensive pharmacology reference. Elsevier; New York: 2007. pp. 1–6. [Google Scholar]
- 36.Jones DP, Brown LAS, Sternberg P. Variability in glutathione-dependent detoxication in vivo and its relevance to detoxication of chemical mixtures. Toxicology. 1995;105(2–3):267–274. doi: 10.1016/0300-483x(95)03221-z. [DOI] [PubMed] [Google Scholar]
- 37.Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356(1):1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]