Supplemental Digital Content is available in the text.
Abstract
Background:
Although the efficacy of collagenase clostridium histolyticum (CCH) injections has been demonstrated by randomized clinical trials, the relative effectiveness of CCH remains uncertain. Our aim was to compare the outcomes of CCH with those of percutaneous needle aponeurotomy (PNA) in daily clinical practice.
Methods:
We analyzed data from patients undergoing PNA or CCH between 2011 and 2014 at 7 practice sites in the Netherlands. We examined the degree of improvement in contracture and adverse effects at 6–12 weeks after surgery or the last injection. Additionally, we invited patients to complete the Michigan Hand Questionnaire before and at 6–12 months follow-up. To minimize the risk of bias, we used propensity score matching.
Results:
Among 130 matched patients (93% Tubiana I or II) undergoing PNA (n = 46) and CCH (n = 84), improvement in contracture was similar: 26 degrees (65% improvement from baseline) for PNA versus 31 degrees (71%) for CCH for affected metacarpophalangeal joints (P = 0.163). This was 16 degrees (50% improvement) versus 17 degrees (42%) for affected proximal interphalangeal joints (P = 0.395), respectively. No serious adverse effects occurred in either of the 2 treatment groups. Of the mild adverse effects, only skin fissures and sensory disturbances were seen in both groups. Through 1-year follow-up, patients reported similar improvements in the overall Michigan Hand Questionnaire score (PNA 5.3 points versus CCH 4.9 points; P = 0.912).
Conclusions:
In patients with mild contractures (Tubiana I or II), CCH was as effective as PNA in reducing contractures. Both treatments were safe and improved hand function to a similar extent in daily practice.
INTRODUCTION
Dupuytren’s disease is an incurable proliferative disorder of the palmar fascia, characterized by the development of palmar nodules and cords.1 Over time, the cords can contract and limit finger extension. Patients report a variable extent of functional impairment and diminished quality of life.2,3
Percutaneous needle aponeurotomy (PNA) and collagenase clostridium histolyticum (CCH) have gained popularity as less-invasive treatment alternatives to limited fasciectomy—the current standard of care.4–7 With PNA, originally popularized by French rheumatologists, surgeons use a hypodermic needle to release cords at multiple levels after which the affected finger is extended to improve contracture. With CCH, which selectively dissolves collagen, a small volume of collagenase solution is injected into the cords. This weakens the treated areas, allowing for subsequent release through forceful manipulation. To date, 2 randomized clinical trials have compared the 2 techniques, both reporting that their efficacy is comparable.8,9 Nevertheless, the extent to which these results can be translated into clinical practice remains incompletely understood because the controlled conditions in such trials may not reflect clinical practice. Patients’ choices, selection, and compliance with treatment regimens in trials can differ substantially from that in actual practice, resulting in a discrepancy in the results achieved in trials versus those in practice.10–12
The aim of this study was to compare the outcomes of PNA and CCH in daily practice using data gathered at multiple practice sites in the Netherlands. We studied the impact of both treatments on the degree of contracture, different domains of hand function, and associated adverse effects.
METHODS
Study Design
This is a study of data compiled from 2 previous comparative studies4,13 performed between 2011 and 2014 at 7 hand surgery practice sites in the Netherlands, including 1 academic institution and a consortium of 6 dedicated hand surgery sites. Briefly, patients were eligible if they were adults with a diagnosis of primary or recurrent Dupuytren’s disease, underwent PNA or CCH, and had contractures affecting the MCP and/or proximal interphalangeal (PIP) joints. Patients were excluded if they underwent treatment for a concomitant hand condition that could confound outcomes assessments (e.g., carpal tunnel release). For this study, we also excluded patients who underwent simultaneous treatment for multiple digits to increase comparability between the groups. Our local institutional review board exempted this study from formal review due to the retrospective nature of this study.
Procedures
Treatments were performed as part of standard clinical practice by the surgeons of the participating practice sites. Hence, treatment selection occurred through shared-decision making.
PNA was performed under local anesthesia. Cords were released using 25 gauge needles at as many levels as possible in the palm and fingers. Patients were instructed to report paresthesias to avoid nerve injury. After release, the treated digit was extended with a progressive force to maximize correction. Patients were encouraged to flex and extend their digits immediately following treatment and to restart normal use of their hands after 24 hours.
CCH was administered according to manufacturer instructions, without local anesthesia. Injections were limited to 0.25 mL and 0.20 mL for MCP and PIP joint contractures, respectively. Afterward, compressive dressings were applied. Treated digits were manipulated after 24–72 hours to attempt release of the weakened cords under local anesthesia. Up to 3 injections were offered at 4-week intervals if patients were dissatisfied with the achieved level of correction but were not mandatory. All patients were offered a similar rehabilitation and splinting program as patients undergoing PNA.
Outcome Measures
The primary outcome was early improvement in degree of contracture. The degree of contracture (active extension deficit) was assessed by certified hand therapists using a finger goniometer before treatment and at visits occurring between 6–12 weeks after surgery or the last injection. Any hyperextension was defined as 0° to prevent underestimation of total extension deficit. At the same visits, adverse effects were noted, which were divided based on their severity into 2 categories: serious (nontransient or requiring an intervention) and mild (transient or not requiring an intervention).
Finally, we examined the impact of both treatments on different aspects of hand function using the Michigan Hand outcomes Questionnaire (MHQ).14 Because we assumed that some patients would require at least 6 months for full functional recovery after treatment, we asked patients to complete the MHQ before and between 6 months and 1 year after treatment. The MHQ consists of 37 items evaluating 6 subdomains for each hand separately: overall hand function, ability to perform activities in daily life, work performance, hand appearance, pain, and satisfaction with hand function. The fact that the MHQ includes a subdomain assessing hand appearance increases its scope15 and makes it particularly well-suited for some patients with Dupuytren’s contracture.16,17 Scores range from 0 to 100 (with 100 indicating best hand performance). As this study was directed at assessing improvement in contracture rather than pain reduction, we excluded all pain-related outcomes. We only used the outcomes pertaining to the treated side.
Propensity-Score Matching
In practice, the choice between PNA versus CCH is not random but related to clinical factors, such as the degree of contracture and patient characteristics, as a consequence of differences in patient selection and preference. Therefore, we expected that the PNA and CCH groups would differ with respect to their baseline characteristics. To account for such differences that can otherwise threaten the validity of a comparison due to treatment selection bias, we applied propensity score matching.18,19 This approach has been used previously to examine the comparative effectiveness of treatments for Dupuytren’s contracture, including PNA and CCH.4,13
Propensity scores for the probability of undergoing CCH and PNA were developed using a logistic regression model with the following baseline characteristics as explanatory variables: age,20 gender,21 family history of the disease,22 primary or recurrent disease,23 the baseline degree of contracture24 at the MCP, PIP, and distal interphalangeal joint levels, and which joints were affected.25 These variables were included because they were considered related to (1) either the choice between CCH or PNA or (2) clinical outcomes. After calculating the individual scores, we attempted to match each patient from the PNA group with 2 patients from the CCH group with the closest propensity scores (i.e., who had the most similar characteristics) using a nearest-neighbor algorithm with replacement. We repeated this process until matches had been attempted for all patients from the PNA group. To examine whether propensity score matching improved similarity among the treatment groups, significance testing was performed before and after matching.
Statistical Analysis
Sample size calculations estimated that a total 32 affected MCP joints (16 each group) and 70 proximal interphalangeal contractures (35 each group) would provide 80% power to detect a 10-degree difference in contracture between the 2 treatment groups with the use of 2-sided tests.4
For improvement in contracture (both in percentage and in absolute degrees) and MHQ scores, we used Student t tests to compare the change at follow-up from baseline between treatment groups with corresponding means and 2 standard errors plotted graphically. The proportion of joints with clinical improvement, defined as improvement of ≥ 50% in the degree of contracture, was compared with Chi-square tests. Outcomes were analyzed separately for affected MCP and PIP joints because results at the PIP joint are typically worse. Rates of adverse effects were compared using the Chi-square or Fisher’s exact test if effects occurred in both treatment groups.
Continuous variables were reported as means ± SD and categorical variables with the use of frequencies. P values of < 0.05 were considered to indicate statistical significance.
RESULTS
Study Sample
There were a total of 183 patients of whom 79 underwent PNA and 104 CCH. After excluding 28 patients who were treated with PNA for multiple digits, a total of 155 eligible patients remained: 51 underwent PNA and 104 underwent CCH (Fig. 1).
Fig. 1.
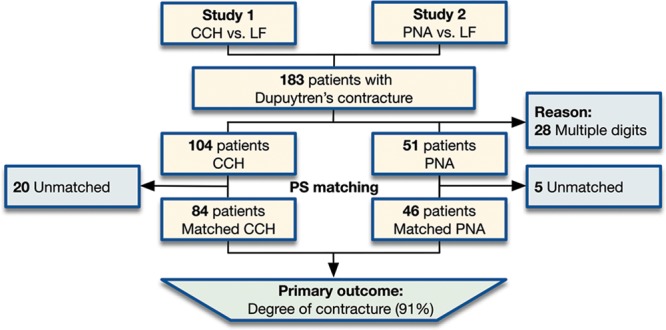
Patient selection flowchart. LF, limited fasciectomy; PS, propensity score.
Differences between the PNA and CCH groups before propensity score matching included that patients undergoing CCH were more often men, were, on average, younger, had less total extension deficit, and proportionally fewer affected MCP joints (Table 1, left). This further highlights the need to account for these differences before comparing the 2 groups since all these factors have been previously found to influence outcomes.21–24 At the same time, all remaining characteristics were not significantly different, showing that the indications for CCH and PNA were not substantially different at the participating sites.
Table 1.
Characteristics of Clostridium Collagenase Histolyticum and PNA Treatment Groups, Before and After Propensity Score–Based Matching*
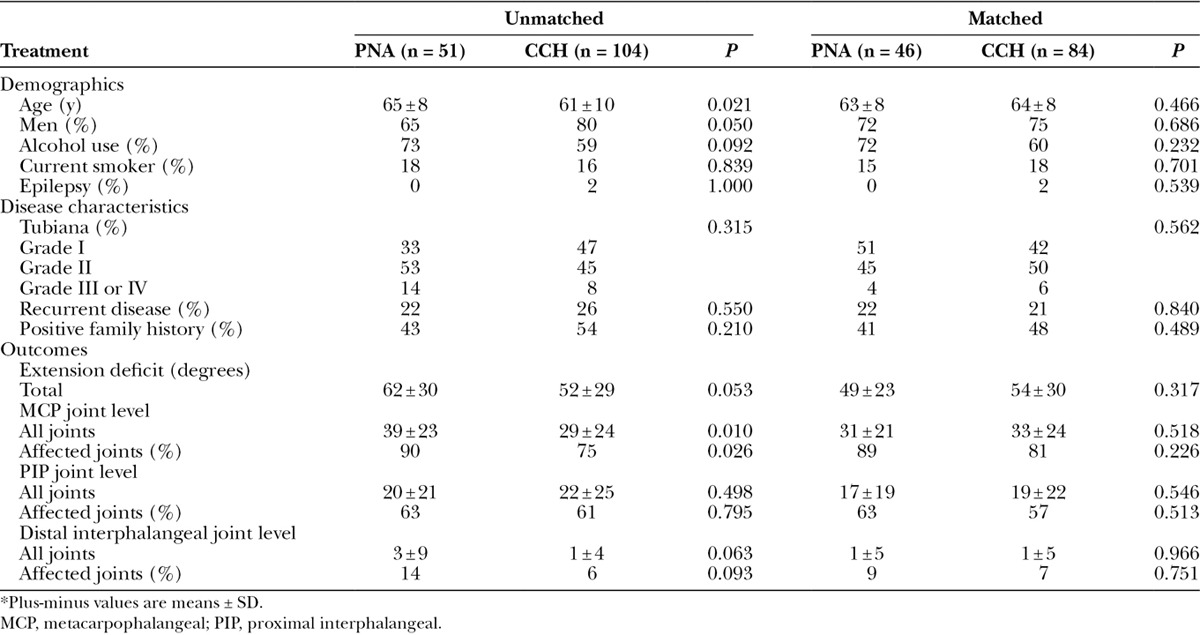
With the use of propensity scores, we were able to match 46 PNA patients to 84 CCH patients who were similar in terms of their baseline degree of contracture and proportions of affected MCP joints (PNA, 34 degrees for 41 joints versus CCH, 41 degrees for 68 joints) and affected PIP joints (PNA, 30 degrees for 26 joints versus CCH, 35 degrees for 46 joints). All other characteristics were also similar between groups (Table 1, right).
Among the matched treatment groups, the average degree of total extension deficit was 52 degrees. This corresponded to 93% of patients being graded as Tubiana I (< 45 degrees) or II (45–90 degrees), which indicated that the majority had mildly affected digits. Follow-up data were available for 91% of the primary outcome (degree of contracture), which was assessed at an average follow-up duration of 8 weeks (range, 6–12 weeks). There were no significant differences in baseline characteristics between those who did and did not have primary outcome data available (see table, Supplementary Digital Content 1, which displays characteristics of the matched treatment groups, divided by those with (respondent) and without data (nonrespondents) available on the primary outcome (degree of contracture), http://links.lww.com/PRSGO/A527). Sixty percentage of PNA patients and 72% of CCH patients completed the MHQ to such an extent that the baseline overall score could be calculated. Of these, 64% in the PNA group and 76% in the CCH group had follow-up data available. We found no significant differences in the baseline characteristics between those with and without MHQ follow-up data.
Change in Angular Correction
For affected MCP joints, the improvement in contracture at follow-up was not significantly different among the matched treatment groups, in percentage correction and in absolute degrees (Fig. 2A, B). The proportion of MCP joints reaching clinical improvement, defined as an improvement of ≥ 50% in the degree of contracture, was also similar between the treatment groups (Fig. 2C).
Fig. 2.
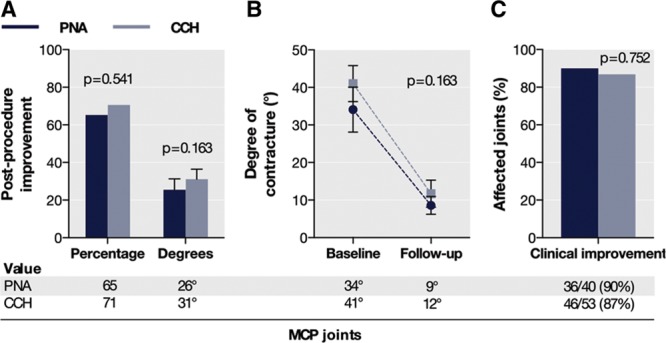
Improvement in contracture for affected MCP joints (A and B) and proportion of joints with clinical improvement (C) among the matched CCH and PNA groups at early follow-up.
For affected PIP joints, improvement in contracture was also not significantly different among the matched treatment groups (Fig. 3A). Consistently, improvement in contracture in degrees was similar (Fig. 3A, B). The proportion of clinically improved PIP joints in the CCH group was not significantly different between groups (Fig. 3C).
Fig. 3.
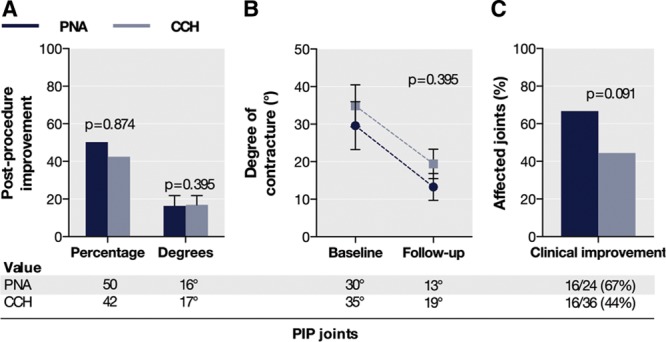
Improvement in contracture for affected PIP joints (A and B) and proportion of joints with clinical improvement (C) among the matched CCH and PNA groups at early follow-up.
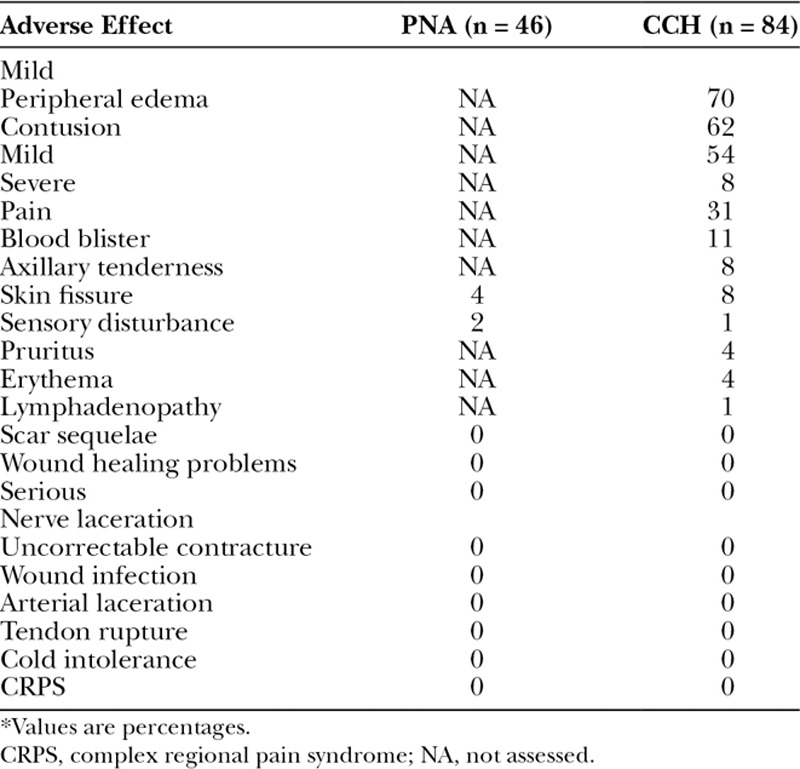
Adverse Effects
No serious adverse effects occurred in either of the matched treatment groups (Table 2). Three of the most common mild adverse effects in the CCH group were peripheral edema, contusion, and transient pain. The only events occurring in both treatment groups were skin fissures and sensory disturbances, which did not significantly differ in their relative incidence between groups (P =0.491 and P = 1.000, respectively).
Table 2.
Adverse Effects in the Matched Treatment Groups, by Severity Grade*
Changes in Hand Function
The overall MHQ score was similar at baseline in the matched PNA and CCH groups. At an average of 11 months follow-up, patients also reported a similar improvement in the overall score (PNA, 5.3 points versus CCH, 4.9 points; P = 0.912).
There were, however, differences between the 2 groups in the extent to which several subdomain scores improved. PNA patients as compared with CCH patients reported, on average, larger improvements in the MHQ subscores of satisfaction (18 points) and hand appearance (8 points), although only the difference in the satisfaction subscore reached significance (Fig. 4). CCH patients, in turn, reported significantly larger improvements in the activities in daily life subscore (4 points). Further exploring these differences, all subdomain scores in absolute terms were similar between-groups at baseline and follow-up with the exception that the satisfaction and appearance subscores were an average of 7 and 9 points, respectively, lower in the PNA group than in the CCH group at baseline (P = 0.204 and P = 0.057, respectively).
Fig. 4.
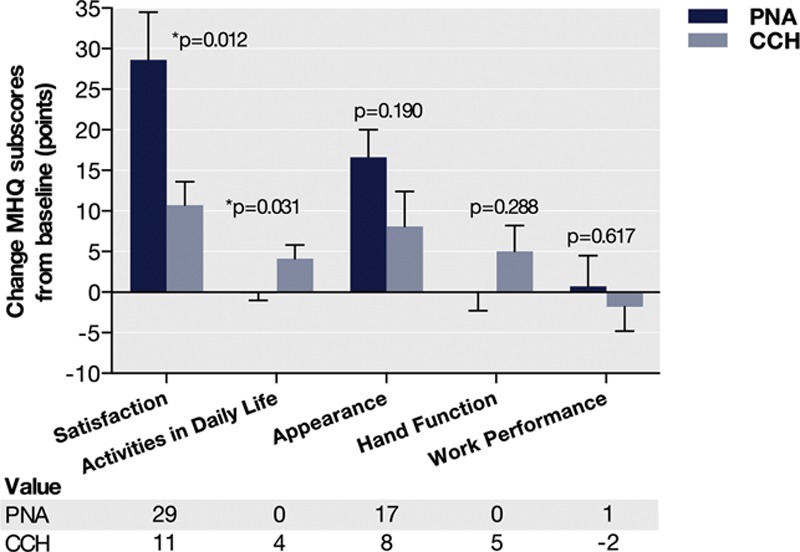
Change in MHQ scores in the matched clostridium collagenase histolyticum and PNA treatment groups.
DISCUSSION
Despite the large number of studies describing the outcomes of various treatments for Dupuytren’s contracture, scarce evidence is available to guide decision-making in the disease.26,27 The aim of this study involving multiple practice sites in the Netherlands was to assess the outcomes of PNA versus CCH in clinical practice. We found that, among patients with mild contractures (93% Tubiana I or II), improvement in contracture with PNA as compared with CCH was similar for affected MCP and PIP joints. No major adverse effects occurred in any of the 2 treatment groups. Over time, the overall MHQ score also improved to a similar extent in both groups.
As evidenced by both the relative improvement and in absolute degrees, the level of contracture correction achieved in our study at the MCP joint level after CCH was similar to that after PNA. Two previous clinical trials reported similar findings.8,9 In the present study, affected MCP joints improved by 31 and 26 degrees after CCH and PNA, respectively. This agrees well with the similar degree of improvement reported by Scherman et al.8 (46 and 47 degrees for CCH and PNA at 3 months, respectively) and by Strömberg et al.9 (48 and 46 degrees, respectively). The smaller improvement in contracture in absolute terms in our study can be explained by differences in baseline severity of contracture among the study samples as well as differences in assessment methods (passive versus active goniometry). Affected PIP joints in our study improved by 17 and 16 degrees after CCH and PNA, respectively. In comparison, Scherman et al.8 reported in their study that PIP joints also improved to a similar extent (8 and 11 degrees after CCH and PNA, respectively). Again, the difference in absolute improvement can be explained by slight differences in patient inclusion. Collectively, these findings show that the effectiveness of CCH at reducing contractures is similar to that of PNA in actual clinical practice, despite that decision-making processes and compliance in this study probably differed from that in previous clinical trials. We therefore believe that they are an addition to the evidence-base available on CCH and PNA.
We found no serious adverse effects following either treatments, which is consistent with what has previously been reported.9 Skin fissures and sensory disturbances were the only mild adverse effects that occurred after both CCH and PNA but were rare. All other minor effects were unique to the CCH group among which peripheral edema, contusion, and pain in the extremity were the 3 most common. We believe that these findings primarily highlight the different modes of action of the 2 treatments.
During the first year after treatment, we found that the overall MHQ score improved to a similar extent after PNA and CCH. This underscores the effectiveness of both treatments at improving hand function for patients, even for those with mild contractures. Interestingly, the subdomain scores of satisfaction and appearance showed larger improvements after PNA than after CCH at follow-up, whereas the scores in absolute terms were similar. We feel that this is due to the comparatively lower scores in these subdomains in the PNA group at baseline. Considering that both treatment groups were similar with respect to their baseline characteristics, including demographics and disease severity, this suggest less satisfaction and more concern with the appearance of their hands among those opting for PNA. Further research is warranted in this area, which we believe can address a knowledge gap regarding the concerns and needs that influence treatment decision-making among patients with Dupuytren’s disease.28–30
The resources required for CCH and PNA may also be important to consider when deciding between the 2 treatments, particularly considering that associated costs can differ substantially. Although these will vary depending on geographic region, the direct costs of CCH will be higher in most settings due to the low material costs of PNA. In addition, 2 visits are required with CCH, whereas PNA requires only a single visit. CCH may therefore be regarded as the least cost-effective option of the 2, which then ought to be justified by objective advantages (i.e., superior outcomes). To date, we are unaware of any study showing these advantages. Previous economic evaluations have, however, underlined the complexity in comparing the cost-effectiveness of different treatments in Dupuytren’s disease due to the lack and quality of existing literature.27,31 The data presented in the current study allow for refining such economic models that help to identify treatment algorithms for Dupuytren’s contracture that are both cost-effective and broadly applicable.
Our study has several strengths. First, it used prospective data from 7 practice sites that were gathered as part of daily clinical practice, making it a comparative effectiveness study.32 The results from such studies, compared with strictly controlled trials, may be more broadly generalizable because they better reflect the actual decision-making processes, patient compliance, and, ultimately, the outcomes achieved in daily practice.10,32,33 Second, we examined the relative change in MHQ scores rather than a cross-sectional assessment, which enabled a comparison of the impact of CCH and PNA on different aspects of hand function.34 Other strengths include the relatively large sample analyzed, completeness of outcome data (91% primary outcome), and the use of propensity scores to minimize the risk of bias due to observed differences. Despite this study design, a potential limitation of this study is that propensity analyses cannot account for selection bias related to unmeasured characteristics (i.e., genetic constitution). A second limitation is that we could not reliably assess rates of recurrence, which may be as relevant to patients in treatment decision-making as early outcomes, because of the limited time-horizon of our study.30 Third, only a subset of patients completed the MHQ. Although this might have influenced our results, the possibility for attrition bias seems small because there were no differences in the characteristics between those who did and did not complete the MHQ at follow-up. Finally, the rare incidence of adverse effects in both treatment groups precludes strong inferences to be made about the comparative risk profile of both treatments.
In conclusion, we found that, among patients with mildly affected digits, CCH and PNA were similarly effective at improving contractures. Even among these patients, we found a significant and similar improvement in overall hand function, which reinforces the usefulness of both treatments as first-line treatments. Our findings also underscore the safety of both techniques in daily practice. Until longer term studies are conducted that are urgently needed to better understand the durability of the outcomes of both treatments, we believe that these findings may help patients with Dupuytren’s disease, payers and providers decide between these 2 minimally invasive treatment options.
ACKNOWLEDGMENTS
The authors thank all patients, hand therapists, surgeons, and Nick Hart in particular, for their contribution in the gathering of the data.
Supplementary Material
Footnotes
Disclosure: Dr. Feitz has been a consultant to Pfizer and Sobi, 2 manufacturers of injectable collagenase in Europe. Prof. Hovius and Dr. Selles have been consultants to Pfizer. All authors agreed not to personally receive any form of financial compensation for their advisory services. Support was received from Pfizer in the form that the injections used in the present study were provided free of charge. The present study was conducted without any form of involvement from either pharmaceutical company. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Lanting R, van den Heuvel ER, Werker PM. Clusters in short-term disease course in participants with primary Dupuytren disease. J Hand Surg Am. 2016;41:354–361.; quiz 361. [DOI] [PubMed] [Google Scholar]
- 2.Engstrand C, Krevers B, Nylander G, et al. Hand function and quality of life before and after fasciectomy for Dupuytren contracture. J Hand Surg Am. 2014;39:1333–1343.e2.. [DOI] [PubMed] [Google Scholar]
- 3.Thoma A, Kaur MN, Ignacy TA, et al. Health-related quality of life in patients undergoing palmar fasciectomy for Dupuytren’s disease. Plast Reconstr Surg. 2014;133:1411–1419.. [DOI] [PubMed] [Google Scholar]
- 4.Zhou C, Hovius SE, Slijper HP, et al. Collagenase clostridium histolyticum versus limited fasciectomy for Dupuytren’s contracture: outcomes from a multicenter propensity score matched study. Plast Reconstr Surg. 2015;136:87–97.. [DOI] [PubMed] [Google Scholar]
- 5.Hurst LC, Badalamente MA, Hentz VR, et al. ; CORD I Study Group. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968–979.. [DOI] [PubMed] [Google Scholar]
- 6.Riester SM, van WAJ, Rizzo M, et al. Pathogenesis and treatment of Dupuytren disease. JBJS Reviews. 2014;2:75–76.. [DOI] [PubMed] [Google Scholar]
- 7.Denkler KA, Vaughn CJ, Dolan EL, et al. Evidence-based medicine: options for Dupuytren’s contracture: incise, excise, and dissolve. Plast Reconstr Surg. 2017;139:240e–255e.. [DOI] [PubMed] [Google Scholar]
- 8.Scherman P, Jenmalm P, Dahlin LB. One-year results of needle fasciotomy and collagenase injection in treatment of Dupuytren’s contracture: a two-centre prospective randomized clinical trial. J Hand Surg. 2016;41:577–582.. [DOI] [PubMed] [Google Scholar]
- 9.Strömberg J, Ibsen-Sörensen A, Fridén J. Comparison of treatment outcome after collagenase and needle fasciotomy for Dupuytren contracture: a randomized, single-blinded, clinical trial with a 1-year follow-up. J Hand Surg Am. 2016;41:873–880.. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93.. [DOI] [PubMed] [Google Scholar]
- 11.Peimer CA, Skodny P, Mackowiak JI. Collagenase clostridium histolyticum for Dupuytren contracture: patterns of use and effectiveness in clinical practice. J Hand Surg Am. 2013;38:2370–2376.. [DOI] [PubMed] [Google Scholar]
- 12.Farrokhyar F, Karanicolas PJ, Thoma A, et al. Randomized controlled trials of surgical interventions. Ann Surg. 2010;251:409–416.. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Selles RW, Slijper HP, et al. Comparative effectiveness of percutaneous needle aponeurotomy and limited fasciectomy for Dupuytren’s contracture: a multicenter observational study. Plast Reconstr Surg. 2016;138:837–846.. [DOI] [PubMed] [Google Scholar]
- 14.Shauver MJ, Chung KC. The Michigan hand outcomes questionnaire after 15 years of field trial. Plast Reconstr Surg. 2013;131:779e–787e.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badalamente M, Coffelt L, Elfar J, et al. ; American Society for Surgery of the Hand Clinical Trials and Outcomes Committee. Measurement scales in clinical research of the upper extremity, part 2: outcome measures in studies of the hand/wrist and shoulder/elbow. J Hand Surg Am. 2013;38:407–412.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ball C, Pratt AL, Nanchahal J. Optimal functional outcome measures for assessing treatment for Dupuytren’s disease: a systematic review and recommendations for future practice. BMC Musculoskelet Disord. 2013;14:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou C, Hovius SER, Slijper HP, et al. Predictors of patient satisfaction with hand function after fasciectomy for Dupuytren’s contracture. Plastic Reconstr Surg 2016;138:649–655.. [DOI] [PubMed] [Google Scholar]
- 18.Freemantle N, Marston L, Walters K, et al. Making inferences on treatment effects from real world data: propensity scores, confounding by indication, and other perils for the unwary in observational research. BMJ. 2013;347:f6409. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469–477.. [DOI] [PubMed] [Google Scholar]
- 21.Anwar MU, Al Ghazal SK, Boome RS. Results of surgical treatment of Dupuytren’s disease in women: a review of 109 consecutive patients. J Hand Surg Am. 2007;32:1423–1428.. [DOI] [PubMed] [Google Scholar]
- 22.Abe Y, Rokkaku T, Ofuchi S, et al. An objective method to evaluate the risk of recurrence and extension of Dupuytren’s disease. J Hand Surg Br. 2004;29:427–430.. [DOI] [PubMed] [Google Scholar]
- 23.Coert JH, Nérin JP, Meek MF. Results of partial fasciectomy for Dupuytren disease in 261 consecutive patients. Ann Plast Surg. 2006;57:13–17.. [DOI] [PubMed] [Google Scholar]
- 24.Badalamente MA, Hurst LC, Benhaim P, et al. Efficacy and safety of collagenase clostridium histolyticum in the treatment of proximal interphalangeal joints in Dupuytren contracture: combined analysis of 4 phase 3 clinical trials. J Hand Surg Am. 2015;40:975–983.. [DOI] [PubMed] [Google Scholar]
- 25.Foucher G, Medina J, Navarro R. Percutaneous needle aponeurotomy: complications and results. J Hand Surg Br. 2003;28:427–431.. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues JN, Becker GW, Ball C, et al. Surgery for Dupuytren’s contracture of the fingers. Cochrane Database Syst Review. 2015;12:CD010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen NC, Shauver MJ, Chung KC. Cost-effectiveness of open partial fasciectomy, needle aponeurotomy, and collagenase injection for Dupuytren contracture. J Hand Surg Am. 2011;36:1826–1834.e32.. [DOI] [PubMed] [Google Scholar]
- 28.Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345:e6572. [DOI] [PubMed] [Google Scholar]
- 29.Say RE, Thomson R. The importance of patient preferences in treatment decisions—challenges for doctors. BMJ. 2003;327:542–545.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kan HJ, de Bekker-Grob EW, van Marion ES, et al. Patients’ preferences for treatment for Dupuytren’s disease: a discrete choice experiment. Plast Reconstr Surg. 2016;137:165–173.. [DOI] [PubMed] [Google Scholar]
- 31.Baltzer H, Binhammer PA. Cost-effectiveness in the management of Dupuytren’s contracture. A Canadian cost-utility analysis of current and future management strategies. Bone Joint J. 2013;95-B:1094–1100.. [DOI] [PubMed] [Google Scholar]
- 32.Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations. N Engl J Med. 2009;361:325–328.. [DOI] [PubMed] [Google Scholar]
- 33.Mushlin AI, Ghomrawi H. Health care reform and the need for comparative-effectiveness research. N Engl J Med. 2010;362:e6. [DOI] [PubMed] [Google Scholar]
- 34.Leversedge FJ. Hardly depressing and far from painful: commentary on an article by Daniel A. London, BA, et al.: “the impact of depression and pain catastrophization on initial presentation and treatment outcomes for atraumatic hand conditions”. J Bone Joint Surg Am. 2014;96:e88. [DOI] [PubMed] [Google Scholar]


