Supplemental Digital Content is available in the text.
Summary:
Plastic and reconstructive surgery relies on the knowledge of angiosomes in the raising of microsurgical flaps. Growing interest in muscle-sparing perforator flaps calls for reliable methods to assess the clinical feasibility of new donor sites in anatomical studies. Several injection techniques are known for the evaluation of vascular territories. Indocyanine green–based fluorescence angiography has found wide application in the clinical assessment of tissue perfusion. In this article, the use of indocyanine green–based fluorescence angiography for the assessment of perforasomes in anatomical studies is described for the first time.
INTRODUCTION
Ongoing striving for new donor sites in plastic and reconstructive surgery has led to the application of various established methods of perforasome visualization in cadaver studies. A perforasome is defined as the arterial vascular territory of a single perforator. The lead oxide-gelatin technique provides high-quality angiograms and is therefore considered the standard method for the study of perforator flaps.1 However, the process is rather time consuming and has its limitations in the visualization of small capillaries. This is the domain of dye studies, which have proven useful in the investigation of a flap’s cutaneous vascular anatomy. In plastic surgery, indocyanine green–based fluorescence angiography (ICG FA) is used mainly to confirm the intraoperative patency of anastomoses2 as well as being an imaging tool in peripheral lymphedema evaluation.3 The following trials demonstrate its feasibility in the visualization of perforasomes in anatomical studies.
MATERIALS AND METHODS
Dissections were carried out in a total of 5 fresh cadavers (2 female) of body donors who willingly donated their bodies to the Institute of Anatomy for academic purposes. The descending genicular artery (DGA) and the superficial circumflex iliac artery (SCIA) were dissected bilaterally and carefully cannulated. The DGA was selectively injected with a mixture of 5 ml ICG solution (ICC-Pulsion, Pulsion Medical System, Germany) and 5 ml methylene blue solution [Methylenblue (C.I. 52015) reinst. Fa. LabChem Röttinger, Germany]. The injection protocol was modified a little for the SCIA by mixing a mere 2.5 ml of indocyanine green with 2.5 ml of methylene blue. Fluorescence angiography was performed using a near-infrared camera (Sony HD Handycam CM05; Sony Corp., Tokyo, Japan). This study was performed according to the local ethics regulation.
DGA
The DGA served as the first injection site. Its saphenous branch supplies the skin of the distal medial thigh by an average 1.8 cutaneous perforators.4 A flap on this vascular basis was first described as the “saphenous flap” by Acland et al.5 in 1981, and its clinical use has been reported by several authors ever since. To assess the feasibility of anatomical perfusion studies by means of ICG FA, the prepared mixture was carefully injected by hand to avoid vascular damage. Visual inspection showed several blue stains without any well-defined borders (Fig. 1). Under visualization with the near-infrared camera, the skin paddle appeared as a homogeneous and well-delimited area (Fig. 2). Furthermore, the ICG FA illustrated substantially larger perforasomes than the methylene blue (Fig. 3).
Fig. 1.
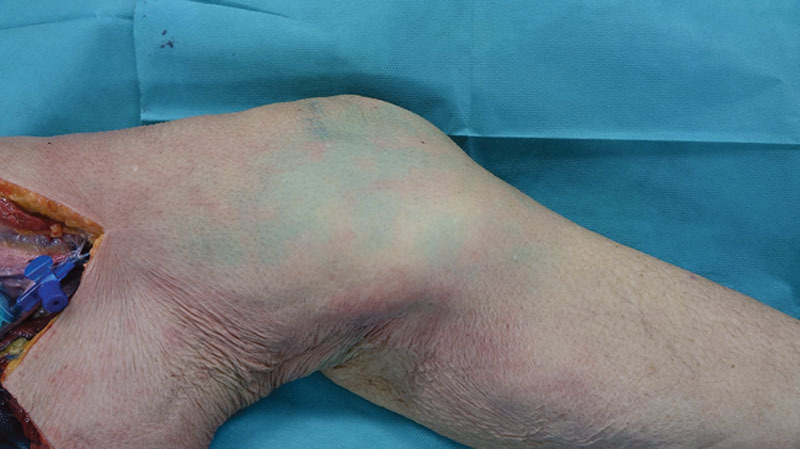
Visual inspection of the medial thigh after injection of 5 ml ICG solution and 5 ml methylene blue solution into the DGA.
Fig. 2.
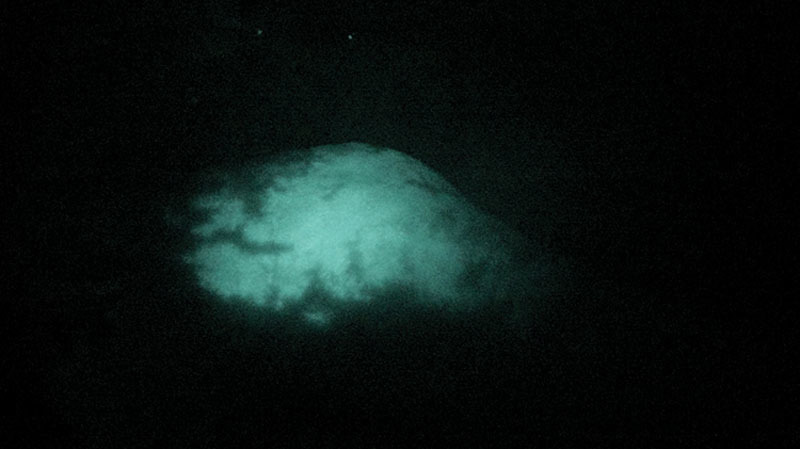
The medial thigh under visualization with the near-infrared camera after injection of 5 ml ICG solution and 5 ml methylene blue solution into the DGA.
Fig. 3.
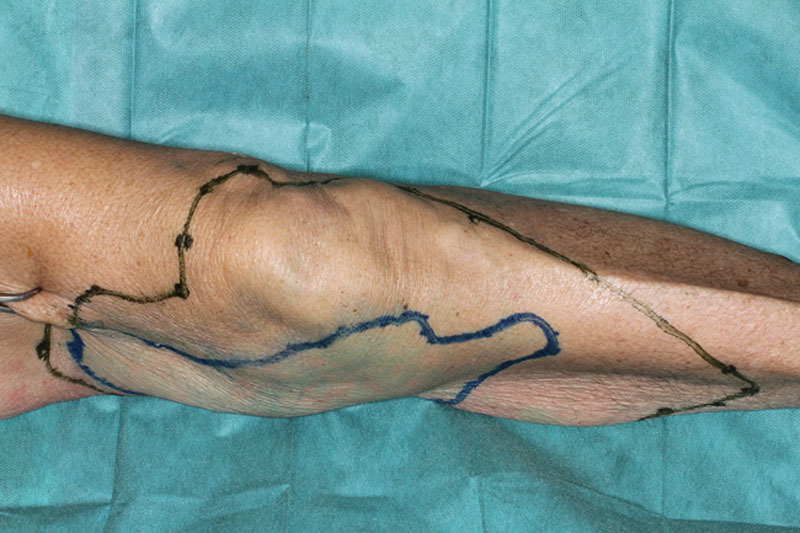
Comparison between the staining properties of methylene blue and ICG marked on the medial thigh.
SCIA
A second trial was conducted to assess the feasibility of ICG FA in a different body region. The groin flap is supplied by the SCIA, once serving as a workhorse in reconstructive microsurgery. Its clinical use as a free flap was first reported by Daniel and Taylor6 and soon gained popularity because of some of the flap’s favorable features, such as the hidden donor site scar and the availability of large cutaneous tissue. However, the groin flap has several limitations, the most drastic being its short pedicle and variable arterial anatomy.7 Moreover, its bulkiness in obese patients, as well as the risk for postoperative seroma or even iatrogenic lymphedema, led to a decline in popularity. A modified version currently experiences a revival in the form of the SCIA perforator flap since its publication by Hong et al.8 Recent studies show a continuous interest in this donor site.9 The SCIA was therefore chosen to test the feasibility of ICG FA for a second time. The results confirmed the findings made in the first trial. This time, visual examination showed minimal to no blue staining whatsoever, whereas visualization with the near-infrared camera revealed well-defined perforasomes (see video, Supplemental Digital Content 1, which displays the groin region under visualization with the near-infrared camera after injection of 2.5 ml ICG solution and 2.5 ml methylene blue solution into the SCIA, http://links.lww.com/PRSGO/A543).
Video Graphic 1.
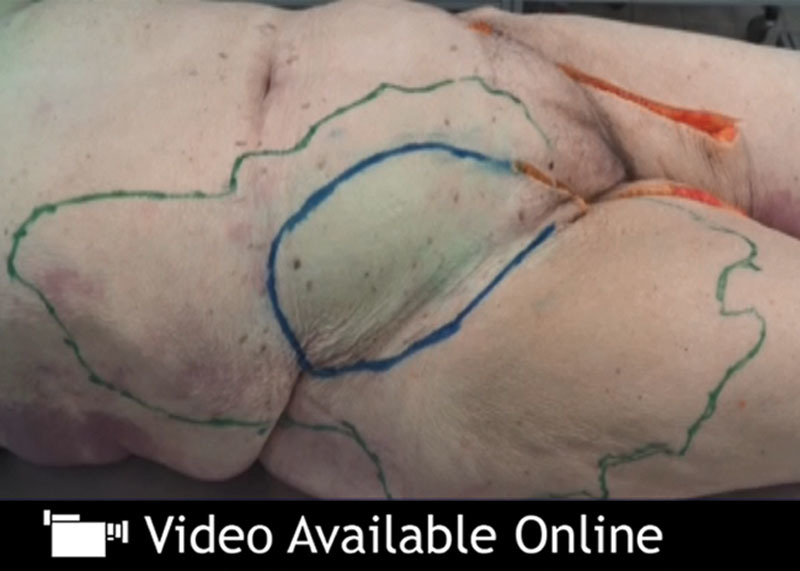
See video, Supplemental Digital Content 1, which displays the groin region under visualization with the near-infrared camera after injection of 2.5 ml ICG solution and 2.5 ml methylene blue solution into the SCIA, http://links.lww.com/PRSGO/A543.
DISCUSSION
Dye studies are well established in the assessment of vascular territories. To our knowledge, there is no report of using ICG FA in anatomical studies. In comparison with the conventional dye methylene blue, ICG stains a substantially larger area when injecting equal volumes. The findings seem to confirm the fact that postmortem studies with methylene blue generally rather under- than overestimate the maximum vascular territory.10 The FA produces a clear image of the potential skin paddle with well-defined borders. Paired with its excellent staining properties, which require only a low volume to completely outline perforasomes, ICG is an outstanding dye in cadaver studies. Basically any perforator flap might benefit from this technique. In contrast to methylene blue or some radiopaque materials like lead oxide, ICG is nontoxic, requiring no safety precautions during the application or a special waste disposal facility.
Due to the high costs of a near-infrared camera, purchasing such a device for the sole purpose of carrying out anatomical perfusion studies would probably exceed the budget of most departments. Nevertheless, if a camera is already available for clinical use, it might as well serve in cadaver studies. Further investigations will be carried out to evaluate the exceptional staining properties of ICG in the assessment of angiosomes.
CONCLUSIONS
ICG FA is a useful tool in perfusion studies for the visualization of perforasomes. It excels in outlining the borders of vascular territories in comparison with conventional dyes, opening new ways in the field of cadaver studies.
Supplementary Material
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Bergeron L, Tang M, Morris SF. A review of vascular injection techniques for the study of perforator flaps. Plast Reconstr Surg. 2006;117:2050–2057.. [DOI] [PubMed] [Google Scholar]
- 2.Iida T, Yoshimatsu H, Yamamoto T, et al. A pilot study demonstrating the feasibility of supermicrosurgical end-to-side anastomosis onto large recipient vessels in head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:1662–1668.. [DOI] [PubMed] [Google Scholar]
- 3.Narushima M, Yamamoto T, Ogata F, et al. Indocyanine green lymphography findings in limb lymphedema. J Reconstr Microsurg. 2016;32:72–79.. [DOI] [PubMed] [Google Scholar]
- 4.García-Pumarino R, Franco JM. Anatomical variability of descending genicular artery. Ann Plast Surg. 2014;73:607–611.. [DOI] [PubMed] [Google Scholar]
- 5.Acland RD, Schusterman M, Godina M, et al. The saphenous neurovascular free flap. Plast Reconstr Surg. 1981;67:763–774.. [DOI] [PubMed] [Google Scholar]
- 6.Daniel RK, Taylor GI. Distant transfer of an island flap by microvascular anastomoses. A clinical technique. Plast Reconstr Surg. 1973;52:111–117.. [DOI] [PubMed] [Google Scholar]
- 7.Chuang DC, Jeng SF, Chen HT, et al. Experience of 73 free groin flaps. Br J Plast Surg. 1992;45:81–85.. [DOI] [PubMed] [Google Scholar]
- 8.Hong JP, Sun SH, Ben-Nakhi M. Modified superficial circumflex iliac artery perforator flap and supermicrosurgery technique for lower extremity reconstruction: a new approach for moderate-sized defects. Ann Plast Surg. 2013;71:380–383.. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimatsu H, Yamamoto T, Hayashi A, et al. Proximal-to-distally elevated superficial circumflex iliac artery perforator flap enabling hybrid reconstruction. Plast Reconstr Surg. 2016;138:910–922.. [DOI] [PubMed] [Google Scholar]
- 10.Acland RD. Outlining a free flap exactly. Plast Reconstr Surg. 1977;59:113. [PubMed] [Google Scholar]


