Supplemental Digital Content is available in the text.
Abstract
Enhanced recovery after surgery is a multidisciplinary perioperative clinical pathway that uses evidence-based interventions to improve the patient experience as well as increase satisfaction, reduce costs, mitigate the surgical stress response, accelerate functional recovery, and decrease perioperative complications. One of the most important elements of enhanced recovery pathways is multimodal pain management. Herein, aspects relating to multimodal analgesia following breast surgical procedures are discussed with the understanding that treatment decisions should be individualized and guided by sound clinical judgment. A review of liposomal bupivacaine, a prolonged-release formulation of bupivacaine, in the management of postoperative pain following breast surgical procedures is presented, and technical guidance regarding optimal administration of liposomal bupivacaine is provided.
INTRODUCTION
Enhanced recovery after surgery (ERAS) care pathways are aimed at improving the patient experience with recovery while reducing health care costs and complications.1,2 ERAS guidelines address key reasons for prolonged length of stay (LOS): (1) continued need for intravenous hydration; (2) need for parenteral analgesia; (3) decreased mobility; and (4) intolerance of enteral nutrition.3–5 A multidisciplinary approach is required to manage all aspects of patient treatment to improve postsurgical patient recovery and decrease perioperative morbidity.5 ERAS guidelines may decrease morbidity by reducing physiologic alterations caused by surgery and postsurgical care. Use of ERAS pathways may also improve quality of care and patient satisfaction.3 Several recent studies have demonstrated the benefits of ERAS and other “fast-track surgery” guidelines in breast surgery in terms of improved outcomes in LOS, along with decreased postsurgical opioid usage, health care costs, and readmission rates.3,6–8
Use of multimodal analgesia for postsurgical pain management is a critical component of ERAS (Fig. 1).5,9–11 Multiple societies and governmental organizations have developed or endorsed evidence-based guidelines supporting the use of multimodal analgesia to manage postsurgical pain.11–14 Multimodal regimens for postsurgical analgesia vary based on procedure but may include a combination of oral or intravenous nonopioids administered perioperatively, such as gabapentinoids, acetaminophen, anti-inflammatory agents including dexamethasone,15,16 α2-adrenergic receptor agonists including clonidine,17,18 dexmedetomidine,17 muscle relaxants including diazepam,19 methocarbamol,20 and tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs).21,22 Intraoperatively, administration of local anesthetics via regional anesthesia techniques or local infiltration into a surgical site is considered effective, with low risk of complications, and is a common component of multimodal analgesic regimens.22,23
Fig. 1.
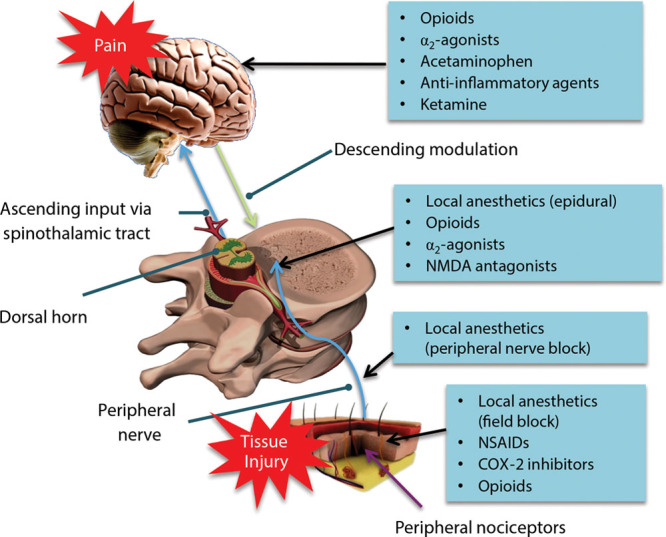
Multimodal analgesia addresses multiple sources of pain.5,9–11 COX-2, cyclooxygenase 2; NMDA, N-methyl-D-aspartate. Adapted with permission from Henrik Kehlet and Jørgen B. Dahl, The value of “multimodal” or “balanced analgesia” in postoperative pain treatment, Anesthesia & Analgesia, volume 77, issue 5, pages 1048–1056, ©1993 by the International Anesthesia Research Society, and from Dave Klemm, Washington, DC, ©2001.
The Breast Reconstruction Advisory Group, a panel of 9 experts in anesthesiology and breast reconstructive surgery, convened on September 18, 2015, to develop a consensus approach to best practices for providing analgesia for breast surgical procedures while reducing opioid use and opioid-related adverse events. This article will review input received from the advisory group regarding optimal multimodal analgesic regimens that may be utilized with or without ERAS for breast surgical procedures. Technical aspects relating to regional and local anesthetic administration during common breast surgical procedures, both oncologic and reconstructive, will be emphasized. The clinical pathway proposed in this article was contributed by multiple individuals employing a multidisciplinary approach. Like any proposed protocol or pathway, they should serve as starting points only and should be tailored to individual patients and their clinical presentations, as well as to clinicians and their practices, experience, and comfort levels. Although the recommendations put forth in this article represent a consensus of the majority of contributing authors, they do not necessarily represent the practice guideline of every author.
Pain Associated with Surgical Treatment for Breast Cancer
Acute postsurgical pain is commonly associated with both mastectomies and reconstructions.24 Inadequate pain management in the acute setting may increase risk of chronic pain development.11 In a study of 28 women undergoing breast cancer surgery,25 patients who reported persistent postsurgical pain at 3 months postoperatively (29%) had greater pain on postoperative day (POD) 5 compared with those who did not report persistent postsurgical pain. Acute postsurgical pain was also a predictor of persistent postsurgical pain in a larger study of 537 women followed up to 1 year after breast cancer surgery.26 Severe pain after mastectomy has been associated with development of postmastectomy pain syndrome and occurs in 20–52% of patients, adding to an already psychologically devastating experience and negatively impacting quality of life.27
Postsurgical Pain Management following Breast Reconstruction
Postsurgical pain after breast surgery contributes to delayed patient mobilization and prolonged LOS.6 Although traditional pain management for patients undergoing breast reconstruction has relied heavily on opioids,27 the American Society of Anesthesiologists Task Force recommends moving away from sole reliance on opioids to routine use of multimodal analgesic strategies including NSAIDs, cyclooxygenase-2 inhibitors, acetaminophen, and regional blockade with local anesthetics.11
The authors propose an opioid-sparing multimodal analgesic clinical pathway (Fig. 2) for 4 common breast procedures—lumpectomy, bilateral mastectomy with and without tissue expanders, and deep inferior epigastric perforator (DIEP) flap reconstruction—which has provided superior analgesia for their patients and can be utilized in ERAS.
Fig. 2.
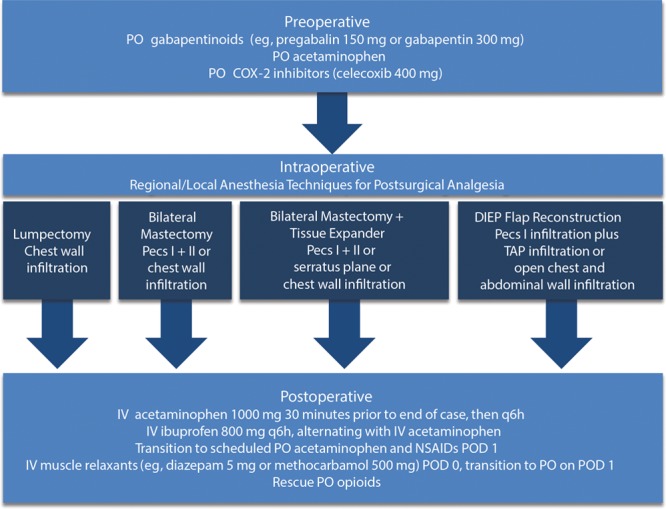
Proposed multimodal regimen for controlling postsurgical pain following common breast surgical procedures. COX-2, cyclooxygenase 2; IV, intravenous; Pecs I + II, pectoral nerve block types I and II; PO, oral; POD, postoperative day.
Analgesic Options for the Pre- and Postoperative Periods
The authors’ recommendations for analgesic options in the pre- and postoperative periods are presented in Table 1.
Table 1.
Analgesic Options for the Pre- and Postoperative Periods
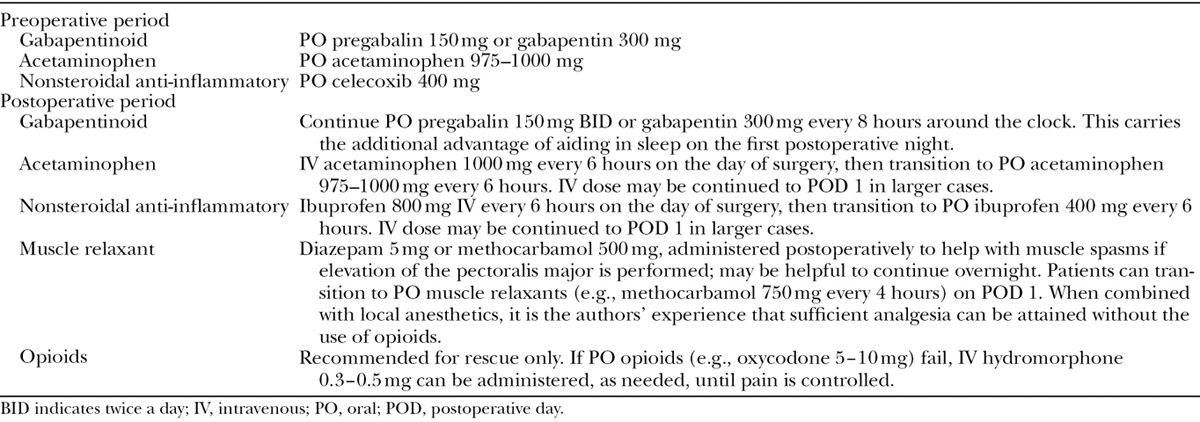
Analgesic Options for the Intraoperative Period
Both regional and local anesthesia techniques are commonly used intraoperatively to provide analgesia. Thoracic epidural and paravertebral block have been shown to improve pain control and recovery, compared with general anesthesia alone.24,28 There is a depth of literature that reviews both the utility and drawbacks of epidural and paravertebral blocks, so the primary objective of this report is to discuss other local anesthetic techniques including Pecs I and II infiltration blocks,28,29 serratus block,30 local infiltration,23 field block infiltration,31,32 and transversus abdominis plane (TAP) infiltration.22,33 The relatively short duration of action of traditional local anesthetics is a limitation to their use and has led to different approaches to prolong the duration of postsurgical analgesia (e.g., continuous wound infusion). However, for the intraoperative local anesthetic techniques described herein, it is the authors’ opinion that maximal use of longer-acting local agents is key. Although technique and timing of administration may vary depending on the procedure,5 surgical-site infiltration or field blocks can be effective in relieving postsurgical pain.31,34 Local infiltration into the chest wall with anesthetics such as bupivacaine HCl 0.25% is suitable for lumpectomies, but for more invasive procedures, field block infiltration or fascial plane infiltration may be more appropriate. In the fascial plane between the pectoralis major and minor muscles lie the lateral and medial pectoral nerves; in the plane between the pectoralis minor and serratus anterior muscles lie spinal nerves T2–T4 and the long thoracic nerve. Ultrasound guidance can be utilized to identify these fascial planes and local anesthetics can be deposited to provide analgesia to these anatomic regions, with some spread to T6, using novel techniques referred to as Pecs I and II field infiltration28,29 (Figs. 3–4; see video, Supplementary Digital Content 1, which demonstrates Pecs I and II infiltration technique. Pecs I and II, pectoral nerve block types I and II. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A514). In Pecs I infiltration, local anesthetic is injected between the pectoralis major and minor muscles.28,35 Pecs II infiltration is more technically challenging and involves injection of local anesthetic between the pectoralis muscles and between the serratus anterior muscle and pectoralis minor muscle28,35 (see video, Supplementary Digital Content 2, which demonstrates Pecs II and serratus plane infiltration (sometimes referred to as Pecs III) technique. Pecs I and II, pectoral nerve block types I and II. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A513). However, Pecs I does not reliably block pain at the serratus muscle, whereas Pecs II infiltration anesthetizes the entire breast and axilla.28
Fig. 3.
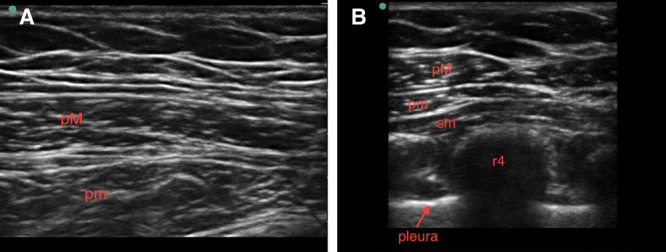
Ultrasound images of Pecs I (A) and Pecs II (B). Pecs I and II, pectoral nerve block types I and II; pM, pec major; pm, pec minor; r4, rib 4; sm, serratus muscle. Images courtesy of Jacob Hutchins, MD.
Fig. 4.
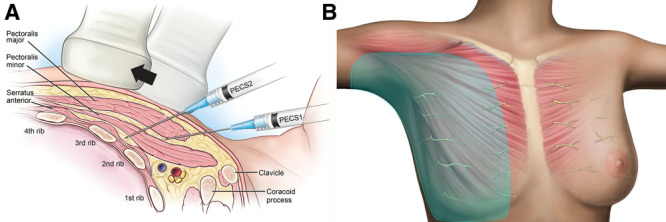
Overview of Pecs I and II infiltration (A) internal sagittal view and (B) external anterior view.28,29 Pecs I and II, pectoral nerve block types I and II. Images courtesy of Pacira Pharmaceuticals, Inc.
Video Graphic 1.
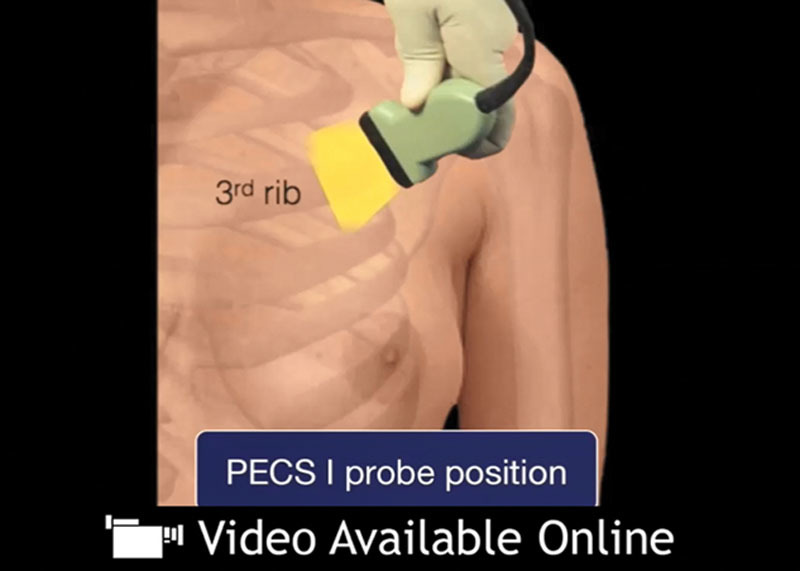
See video, Supplementary Digital Content 1, which demonstrates Pecs I and II infiltration technique. Pecs I and II, pectoral nerve block types I and II. Video courtesy of Jeffrey C. Gadsden, MD. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A514.
Video Graphic 2.
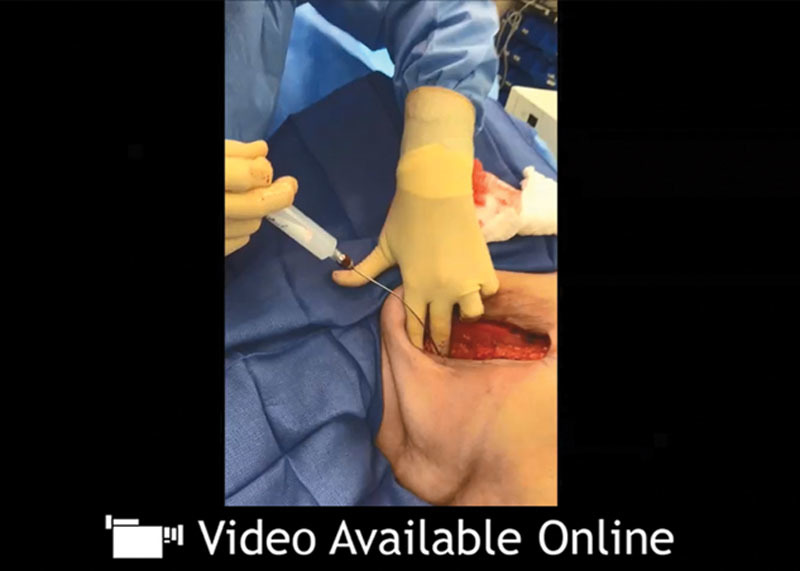
See video, Supplementary Digital Content 2, which demonstrates Pecs II and serratus plane infiltration (sometimes referred to as Pecs III) technique. Pecs I and II, pectoral nerve block types I and II. Video courtesy of Mark Brzezienski, MD, Chattanooga, TN. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A513.
For Pecs I and II, direct injection during the open surgical procedure is also possible. These techniques provide good analgesia during and after breast surgery and, unlike thoracic paravertebral and epidural blocks, are not associated with sympathetic block.19 Pecs I and II infiltration were evaluated in a randomized trial19 in which 60 patients received Pecs I and II plus general anesthesia and 60 patients received general anesthesia alone before modified radical mastectomy surgery. Lower pain scores, lower morphine consumption through 12 hours postsurgery, and fewer opioid-related complications were observed in the Pecs I and II group.19 Another prospective study compared ultrasound-guided pectoral nerve block versus single ipsilateral paravertebral block in 60 patients undergoing modified radical mastectomy; levobupivacaine 0.25% was used in both techniques.36 Pectoral nerve block was associated with reduced morphine consumption within the first 24 hours after surgery and lower pain scores within the first 12 hours after surgery versus paravertebral block.36
Serratus plane infiltration (sometimes referred to as Pecs III)37 is an ultrasound-guided technique that can produce numbness and paresthesia from T2 to T9.30 A local anesthetic is deposited in the fascial plane between the latissimus dorsi and serratus anterior muscles (identified at the level between the fourth and fifth ribs); a superficial rather than deep injection to the serratus muscle optimizes the duration of action and dermatomal spread of local anesthetic (Supplementary Digital Content 2). This technique provides analgesia to nearly an entire hemithorax on the side on which the local anesthetic is administered.30 Depending on the coverage needed, the techniques of Pecs I, Pecs II, and serratus plane infiltration may be suitable for mastectomies with or without tissue expanders or implants.
For reconstruction using DIEP flaps, TAP infiltration is recommended. In TAP infiltration, local anesthetic is deposited, usually under ultrasound guidance, in the fascial plane between the internal oblique and transversus muscles,31 providing analgesia to the abdominal wall (Figs. 5–6; see video, Supplementary Digital Content 3, which shows an overview of classic TAP infiltration technique. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A516; see video, Supplementary Digital Content 4, which demonstrates an overview of subcostal TAP infiltration technique. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A515).
Fig. 5.
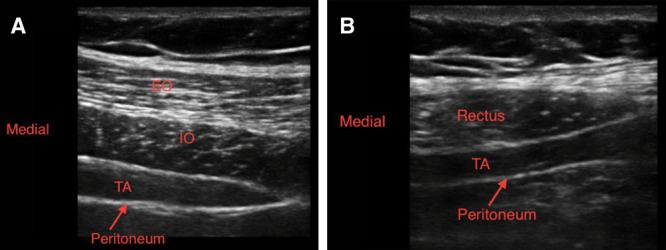
Ultrasound images of TAP infiltration: classic TAP (A) and subcostal TAP (B). EO, external oblique; IO, internal oblique; TA, transversus abdominis. Images courtesy of Jacob Hutchins, MD.
Fig. 6.
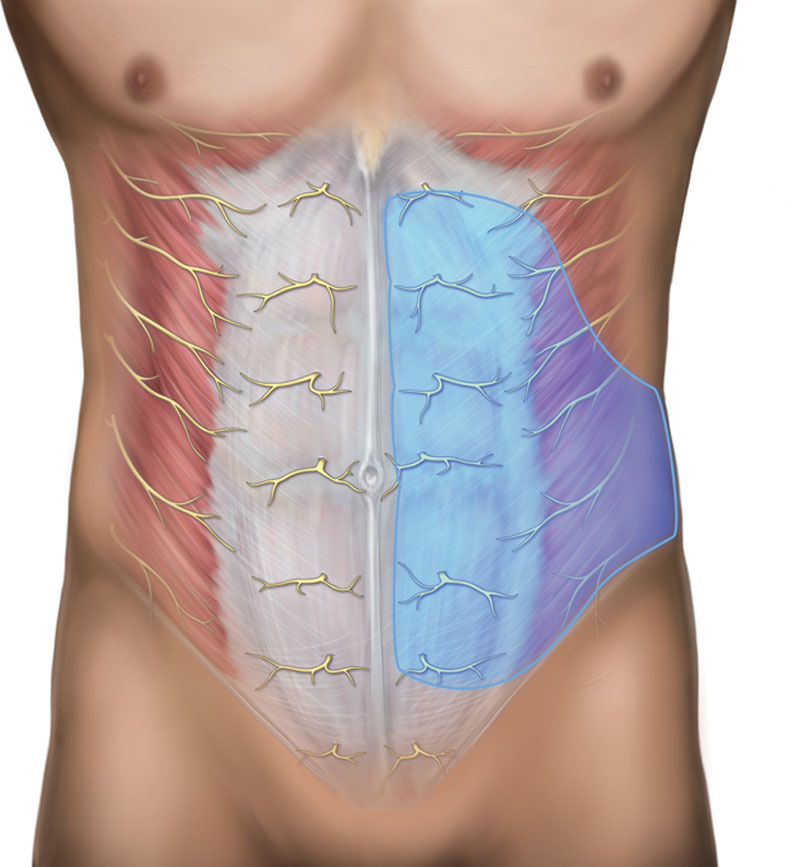
Overview of TAP infiltration. Image courtesy of Pacira Pharmaceuticals, Inc.
Video Graphic 3.
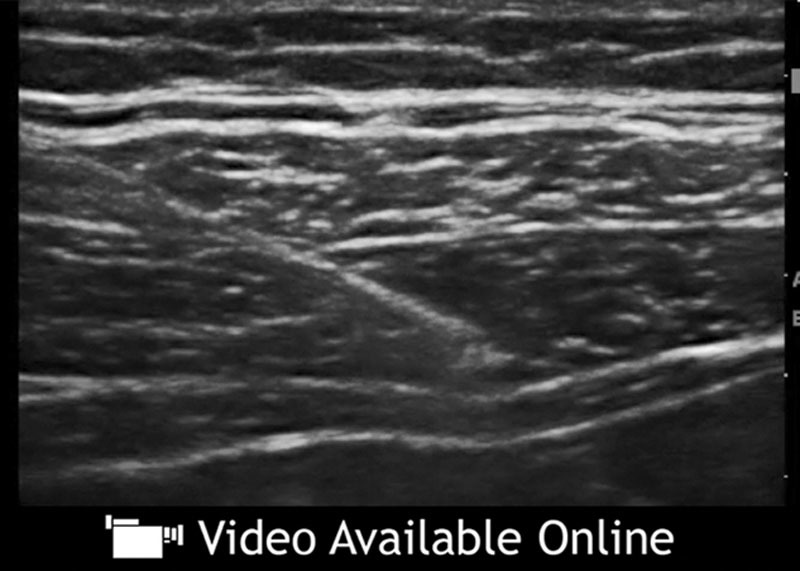
See video, Supplementary Digital Content 3, which shows an overview of classic TAP infiltration technique. Video courtesy of Jacob Hutchins, MD. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A516.
Video Graphic 4.
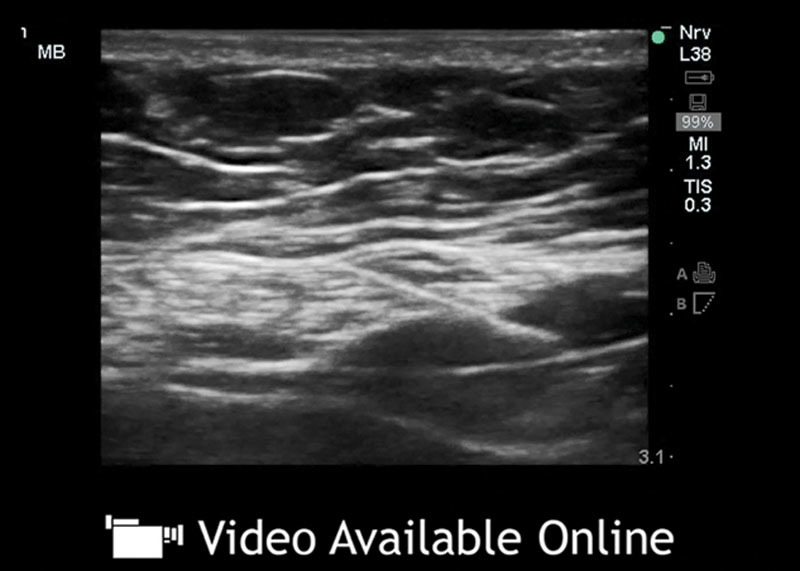
See video, Supplementary Digital Content 4, which demonstrates an overview of subcostal TAP infiltration technique. Video courtesy of Jacob Hutchins, MD. This video is available in the “Related Videos” section of the Full-Text article on PRSGlobalOpen.com or available at http://links.lww.com/PRSGO/A515.
TAP infiltration can be performed preoperatively with minimal sedation. Intravenous midazolam 1–2 mg, with or without intravenous ketamine 10 mg, can be administered for sedation and/or anxiolysis. Alternatively, TAP infiltration can be performed intraoperatively under direct vision, before abdominal closure during DIEP flap surgery. With direct visualization (e.g., during field block or infiltration of the breast or pectoralis), the entire operative field is wide open and directly accessible. Although administering TAP infiltration before beginning a case may be beneficial during surgery, the surgeon can perform the block 45–60 minutes before final closure to ensure adequate analgesia in the postanesthesia care unit. Combining this technique with the use of bupivacaine HCl may provide similar early postoperative pain relief. Intravenous acetaminophen can also be initiated approximately 30 minutes before the end of the case but no earlier than 4 hours after an oral dose of preoperative acetaminophen to ensure onset of analgesia.
In the past, longer-acting local anesthetics, such as bupivacaine HCl or ropivacaine, were commonly used in field block infiltrations.38,39 More recently, a novel, prolonged-release local anesthetic, liposomal bupivacaine, marketed in the United States by Pacira Pharmaceuticals, Inc., Parsippany, N.J., as Exparel (bupivacaine liposome injectable suspension), has become available.40 Liposomal bupivacaine is indicated for surgical-site infiltration and field block infiltrations where the anesthetic is placed in the fascial plane between muscles.32,40 This includes TAP block for DIEP and muscle-sparing transverse rectus abdominis myocutaneous flap procedures, to produce postsurgical analgesia, and is consistent with Pecs I and II infiltration.19 Liposomal bupivacaine is an encapsulated formulation of bupivacaine released over time by multivesicular liposomes.40,41 Liposomes are deposited throughout the surgical site upon administration; as they are metabolized through normal biologic processes, bupivacaine is continually released over a period of several days.42
Clinical Evidence Supporting the Use of Liposomal Bupivacaine in Breast Reconstruction
Liposomal bupivacaine has been used in a wide range of surgical sites, and improved analgesia with reduced postsurgical opioid usage has been demonstrated in clinical trials.43 Liposomal bupivacaine has been evaluated for use in breast surgical procedures, including oncologic, reconstructive, and cosmetic procedures.3,27,44–48
A retrospective chart review27 evaluated postsurgical analgesia and LOS for 90 patients undergoing mastectomy and immediate implant-based breast reconstruction; all were transitioned to oral opioids.27 Patients receiving field block with liposomal bupivacaine had a numerically shorter mean LOS compared with patients receiving intravenous/oral opioids (1.5 versus 2.0 days; P = 0.062), a higher rate of hospital discharge within 1 day after surgery (P = 0.016),27 and significantly lower pain scores at 4, 8, 12, 16, and 24 hours (P < 0.01).27 It should be noted that intercostal field block infiltration and regional (e.g., intercostal) nerve block are different techniques27,33 and that liposomal bupivacaine is indicated for infiltration into the surgical site but not for regional nerve block.40
Another retrospective review of patients undergoing mastectomy with immediate tissue expander reconstruction44 compared 53 patients who received intraoperative local infiltration with liposomal bupivacaine with 44 patients who were administered preoperative ultrasound-guided paravertebral block. Liposomal bupivacaine was administered into the base of the mastectomy skin flaps, the pectoralis major muscle and serratus fascia, periaxillary tissues, and drain sites following the mastectomy and before tissue expander placement. In the liposomal bupivacaine group, opioid use in the recovery room was significantly lower versus the paravertebral block group [mean (SD) = 9.4 (16.4) versus 24.8 (23.9) oral morphine equivalents, respectively; P < 0.001]. LOS in the recovery unit was comparable between groups. Pain scores on the day of surgery were lower in the liposomal bupivacaine group than in the paravertebral block group [mean (SD) = 3.2 (1.8) versus 4.2 (1.5), respectively; P = 0.008]. Time to first postsurgical opioid dose was significantly longer for patients in the liposomal bupivacaine group versus patients in the paravertebral block cohort [mean (SD) = 210 (212) versus 125 (171) minutes, respectively; P = 0.04). Further, those who received liposomal bupivacaine were less likely to require opioids during the recovery period (44% versus 74%, respectively; P = 0.006).44
In another study of 100 women undergoing abdominally based microsurgical free-flap breast reconstruction,3 an ERAS pathway that included intraoperative use of liposomal bupivacaine was compared with traditional care after surgery. In the ERAS cohort, mean hospital LOS was significantly decreased compared with the traditional care after surgery cohort (3.9 versus 5.5 days, respectively; P < 0.001). For the first 3 PODs, total inpatient opioid usage (oral morphine equivalents) was 71% less for the ERAS group versus the traditional care after surgery group (167.3 versus 574.3 mg, respectively; P < 0.001), with similar pain scores for both groups at most time points.3
Use of liposomal bupivacaine in cosmetic breast procedures has also been studied. In a phase 3, randomized, multicenter, double-blind study, 136 patients undergoing bilateral submuscular breast augmentation under general anesthesia were administered either the 266-mg maximum dose of liposomal bupivacaine40 (n = 66) or 100 mg of bupivacaine HCl/epinephrine 1:200,000 (n = 70) into each implant pocket.48 Pain intensity with activity was significantly lower at 8 hours and 12 hours after study drug administration in the liposomal bupivacaine group versus the bupivacaine HCl/epinephrine group [4.9 (0.41) versus 6.7 (0.40) at 8 hours; P = 0.0016 and 5.6 (0.40) versus 6.9 (0.37) at 12 hours; P = 0.0143). Mean total postsurgical rescue opioid consumption was lower in the liposomal bupivacaine group through 72 hours.48
A phase 4, multicenter, prospective, observational study34 evaluated the effect of a single intraoperative administration of liposomal bupivacaine 266 mg into the surgical site on postsurgical pain, opioid use, and opioid-related adverse events in 49 subjects undergoing breast surgery and/or abdominoplasty. Low pain intensity scores and reduced opioid consumption were observed compared with investigators’ previous experiences with patients not receiving liposomal bupivacaine. Mean numeric rating scale pain scores were ≤ 4.3 from discharge through POD 3. Median daily oral opioid consumption was approximately 1 tablet postsurgically on the day of surgery and approximately 2 tablets by POD 3. Subjects’ satisfaction with postsurgical analgesia was high, with a low rate of opioid-related adverse events.34
In a telephone survey of 75 women who had received liposomal bupivacaine at the time of either a cosmetic breast/abdominal procedure (n = 23) or breast reconstruction (n = 52), mean pain scores using a 1–10 verbal scale were 2.6 (0–9) on POD 1 and 3.6 (0–8) on POD 3. All patients preferred liposomal bupivacaine over their perceptions of an elastomeric pump device, and 97% reported they would want to receive liposomal bupivacaine should they need surgery again.38
In a recently published case report37 describing ultrasound-guided lateral and medial pectoral nerve block using liposomal bupivacaine before surgical incision for submuscular breast augmentation, the authors reported complete relaxation of the pectoralis major muscle, facilitating surgical dissection and markedly diminishing postsurgical pain and muscle spasms. The patient required no opioids from discharge through POD 10.37
Technical Considerations for Administration of Liposomal Bupivacaine
The recommended dose of liposomal bupivacaine is based on various factors including volume required to cover the site, allowed maximum dose of 266 mg (20 mL), and patient risk factors.40 Liposomal bupivacaine is intended for single-dose administration only; a single 20-mL vial may be expanded with ≤ 280 mL of normal sterile saline or lactated Ringer’s solution to ensure sufficient coverage of larger surgical sites. Administration technique can greatly affect the efficacy of analgesia in wound infiltration; liposomal bupivacaine should be injected slowly into soft tissue using a 25-gauge or larger bore needle. Also, liposomal bupivacaine does not spread as extensively as bupivacaine HCl, so the “moving needle” technique is crucial to ensure adequate spread of the anesthetic. For larger areas (e.g., during lower abdominal incision for DIEP flap reconstruction or abdominoplasty), it may be easier to use a blunt tip infiltration cannula rather than a 25-gauge needle. Accurate infiltration of all relevant tissue layers of the surgical site is recommended for efficacy, with frequent aspirations to check for blood, to reduce the chance of an inadvertent intravascular injection.22,40
There are precautions to consider when using liposomal bupivacaine in conjunction with other local anesthetics. Nonbupivacaine local anesthetics, such as lidocaine, can be administered before administration of liposomal bupivacaine, into the same site, but a separation of ≥ 20 minutes is required. Rapid release of bupivacaine from liposomes may occur when lidocaine or similar nonbupivacaine local anesthetics are locally coadministered.40 This rapid release of bupivacaine from liposomes could potentially affect properties of liposomal bupivacaine and alter its efficacy and safety.40 Other formulations of bupivacaine HCl may be admixed with liposomal bupivacaine in the same syringe, as long as the ratio of milligram dose of bupivacaine HCl:liposomal bupivacaine does not exceed 1:2. For example, there are 266 mg of bupivacaine in a 20-mL vial of liposomal bupivacaine, which is equivalent to 300 mg bupivacaine HCl40; therefore, up to 150 mg of bupivacaine HCl can be coadministered with a 20-mL vial of liposomal bupivacaine.
CONCLUSIONS
Postoperative pain management is a key component of ERAS pathways for breast surgical procedures. Perioperative multimodal analgesia and opioid-sparing analgesic regimens utilizing a combination of oral and intravenous nonopioids, as well as regional and local infiltration techniques, are optimal for providing pain relief following surgery. However, the proposed analgesic options should supplement, not replace, clinical judgment when making treatment decisions. Evidence supports the use of liposomal bupivacaine infiltration to produce postsurgical analgesia for a broad range of surgical procedures utilizing a wide range of injection techniques. Studies evaluating liposomal bupivacaine in breast surgical procedures have shown reduced postoperative opioid usage coupled with a low burden of opioid-related adverse events, reduced hospital LOS, lower postsurgical pain scores, and high patient satisfaction. Liposomal bupivacaine can be used in a variety of local and field infiltration techniques, including Pecs I and II, serratus plane infiltration, TAP infiltration for DIEP flaps, chest wall infiltration, and incisional or drain site infiltration. Meticulous administration to all tissue layers is essential for optimal results.
ACKNOWLEDGMENTS
Under author direction, editorial and medical writing assistance was provided by Michael D. Morren, RPh, MBA, of Peloton Advantage, LLC, supported by Pacira Pharmaceuticals, Inc., the manufacturer of liposomal bupivacaine. The authors were fully responsible for the content, editorial decisions, and opinions expressed in the current article. All authors were involved in the critical revision and review of the article text and figures, as well as approval of the final draft for submission. The authors did not receive an honorarium related to the development of this article.
Supplementary Material
Footnotes
Disclosure: Funding was not provided to the authors for the development of this article. Under author direction, editorial and medical writing assistance was provided by Michael D. Morren, RPh, MBA, of Peloton Advantage, LLC, supported by Pacira Pharmaceuticals, Inc., the manufacturer of liposomal bupivacaine. Dr. Afonso has received research funding from Pacira Pharmaceuticals, Inc. Dr. Newman is a paid consultant and speaker for Novadaq Technologies Inc. and Pacira Pharmaceuticals, Inc. Dr. Seeley is a consultant for Pacira Pharmaceuticals, Inc. and speaker for Edwards Lifesciences. Dr. Hutchins is a member of the speakers bureau for Halyard Health and is a consultant for and has received research funding from Pacira Pharmaceuticals, Inc. Dr. Smith is a paid consultant and speaker for Allergan and Pacira Pharmaceuticals, Inc. and a consultant for VitaThreads, LLC. Dr. Mena is an expert consultant for Pacira Pharmaceuticals, Inc. and Edwards Lifesciences. Dr. Selber has received a one-time consulting fee from Pacira Pharmaceuticals, Inc. for participation in a focus group. Dr. Saint-Cyr is a consultant for Pacira Pharmaceuticals, Inc. Dr. Gadsden is a consultant for B. Braun Medical Inc. The Article Processing Charge was paid for by Peloton Advantage, LLC.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Miller TE, Gan TJ, Thacker JKM. Enhanced recovery pathways for major abdominal surgery. Anesthesiology News. 2014. 1–8..December (Special Report): [Google Scholar]
- 2.Miller TE, Thacker JK, White WD, et al. ; Enhanced Recovery Study Group. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118:1052–1061.. [DOI] [PubMed] [Google Scholar]
- 3.Batdorf NJ, Lemaine V, Lovely JK, et al. Enhanced recovery after surgery in microvascular breast reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:395–402.. [DOI] [PubMed] [Google Scholar]
- 4.White PF, Kehlet H, Neal JM, et al. ; Fast-Track Surgery Study Group. The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg. 2007;104:1380–1396.. [DOI] [PubMed] [Google Scholar]
- 5.Joshi GP, Schug SA, Kehlet H. Procedure-specific pain management and outcome strategies. Best Pract Res Clin Anaesthesiol. 2014;28:191–201.. [DOI] [PubMed] [Google Scholar]
- 6.Arsalani-Zadeh R, ElFadl D, Yassin N, et al. Evidence-based review of enhancing postoperative recovery after breast surgery. Br J Surg. 2011;98:181–196.. [DOI] [PubMed] [Google Scholar]
- 7.Bonde C, Khorasani H, Eriksen K, et al. Introducing the fast track surgery principles can reduce length of stay after autologous breast reconstruction using free flaps: a case control study. J Plast Surg Hand Surg. 2015;49:367–371.. [DOI] [PubMed] [Google Scholar]
- 8.Hainsworth AJ, Lobo CR, Williams P, et al. ‘23 h Model’ for breast surgery: an early experience. Breast. 2013;22:898–901.. [DOI] [PubMed] [Google Scholar]
- 9.Gottschalk A, Smith DS. New concepts in acute pain therapy: preemptive analgesia. Am Fam Physician. 2001;63:1979–1984.. [PubMed] [Google Scholar]
- 10.Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–1056.. [DOI] [PubMed] [Google Scholar]
- 11.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273.. [DOI] [PubMed] [Google Scholar]
- 12.Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–157.. [DOI] [PubMed] [Google Scholar]
- 13.Paice JA, Gordon DB, Contreras J, Jarzyna D. Safe use of opioids in hospitals. Sentinel Event Alert. 2012;49:1–5.. [PubMed] [Google Scholar]
- 14.Gordon DB, Dahl JL, Miaskowski C, et al. American Pain Society recommendations for improving the quality of acute and cancer pain management: American Pain Society Quality of Care Task Force. Arch Intern Med. 2005;165:1574–1580.. [DOI] [PubMed] [Google Scholar]
- 15.Gärtner R, Kroman N, Callesen T, et al. Multimodal prevention of pain, nausea and vomiting after breast cancer surgery. Minerva Anestesiol. 2010;76:805–813.. [PubMed] [Google Scholar]
- 16.Gómez-Hernández J, Orozco-Alatorre AL, Domínguez-Contreras M, et al. Preoperative dexamethasone reduces postoperative pain, nausea and vomiting following mastectomy for breast cancer. BMC Cancer. 2010;10:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baptista JF, Gomez RS, Paulo DN, et al. Epidural anesthesia with ropivacaine with or without clonidine and postoperative pain in hemorrhoidectomies. Acta Cir Bras. 2014;29:201–208.. [DOI] [PubMed] [Google Scholar]
- 18.Burlacu CL, Frizelle HP, Moriarty DC, et al. Fentanyl and clonidine as adjunctive analgesics with levobupivacaine in paravertebral analgesia for breast surgery. Anaesthesia. 2006;61:932–937.. [DOI] [PubMed] [Google Scholar]
- 19.Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth Pain Med. 2015;40:68–74.. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy CM, Pusic AL, Hidalgo DA. Efficacy of pocket irrigation with bupivacaine and ketorolac in breast augmentation: a randomized controlled trial. Ann Plast Surg. 2009;62:15–17.. [DOI] [PubMed] [Google Scholar]
- 21.Buvanendran A. Multimodal analgesia for perioperative pain management [presentation]. Presented at: Annual Meeting of the International Anesthesia Research Society; May 21–24, 2011; Vancouver, BC, Canada. [Google Scholar]
- 22.Joshi GP. Putting it all together: recommendations for improving pain management in plastic surgical procedures. Plast Reconstr Surg. 2014;134:94S–100S.. [DOI] [PubMed] [Google Scholar]
- 23.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22:588–593.. [DOI] [PubMed] [Google Scholar]
- 24.Amaya F, Hosokawa T, Okamoto A, et al. Can acute pain treatment reduce postsurgical comorbidity after breast cancer surgery? A literature review. Biomed Res Int. 2015;2015:641508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey OT, Burke SM, Hafeez P, et al. Severity of acute pain after breast surgery is associated with the likelihood of subsequently developing persistent pain. Clin J Pain. 2010;26:556–560.. [DOI] [PubMed] [Google Scholar]
- 26.Andersen KG, Duriaud HM, Jensen HE, et al. Predictive factors for the development of persistent pain after breast cancer surgery. Pain. 2015;156:2413–2422.. [DOI] [PubMed] [Google Scholar]
- 27.Butz DR, Shenaq DS, Rundell VLM, et al. Postoperative pain and length of stay lowered by use of Exparel in immediate, implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2015;3:e391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012;59:470–475.. [DOI] [PubMed] [Google Scholar]
- 29.Blanco R. The ‘pecs block’: a novel technique for providing analgesia after breast surgery. Anaesthesia. 2011;66:847–848.. [DOI] [PubMed] [Google Scholar]
- 30.Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 2013;68:1107–1113.. [DOI] [PubMed] [Google Scholar]
- 31.Joshi GP, Rawal N, Kehlet H, et al. ; PROSPECT collaboration. Evidence-based management of postoperative pain in adults undergoing open inguinal hernia surgery. Br J Surg. 2012;99:168–185.. [DOI] [PubMed] [Google Scholar]
- 32.Woodcock J. FDA warning withdrawal letter for Exparel. Available at http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/enforcementactivitiesbyfda/warninglettersandnoticeofviolationletterstopharmaceuticalcompanies/ucm477250.pdf. Accessed January 6, 2016.
- 33.Gadsden J, Ayad S, Gonzales JJ, et al. Evolution of transversus abdominis plane infiltration techniques for postsurgical analgesia following abdominal surgeries. Local Reg Anesth. 2015;8:113–117.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta A. Wound infiltration with local anaesthetics in ambulatory surgery. Curr Opin Anaesthesiol. 2010;23:708–713.. [DOI] [PubMed] [Google Scholar]
- 35.Murata H, Ichinomiya T, Hara T. Pecs block for anesthesia in breast surgery of the elderly. J Anesth. 2015;29:644. [DOI] [PubMed] [Google Scholar]
- 36.Wahba SS, Kamal SM. Thoracic paravertebral block versus pectoral nerve block for analgesia after breast surgery. Egypt J Anaesth 2014;30:129–115.. [Google Scholar]
- 37.Blanco R. A reply. Anaesthesia. 2015;70:509. [DOI] [PubMed] [Google Scholar]
- 38.Buitelaar D, Huitink J, Oldenburg H, et al. Field block: an additional technique of potential value for breast surgery under general anaesthesia. Eur J Anaesthesiol. 2008;25:253–255.. [DOI] [PubMed] [Google Scholar]
- 39.Gill P, Kiani S, Victoria BA, et al. Pre-emptive analgesia with local anaesthetic for herniorrhaphy. Anaesthesia. 2001;56:414–417.. [DOI] [PubMed] [Google Scholar]
- 40.Exparel [prescribing information]. 2016Parsippany, NJ: Pacira Pharmaceuticals, Inc.. [Google Scholar]
- 41.Pacira Pharmaceuticals, Inc. Pacira Pharmaceuticals announces favorable resolution with U.S. Food and Drug Administration, which reaffirms the broad indication for EXPAREL [press release]. Available at http://investor.pacira.com/phoenix.zhtml?c=220759&p=RssLanding&cat=news&id=2122491. Accessed March 29, 2016.
- 42.Lambrechts M, O’Brien MJ, Savoie FH, et al. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013;7:885–890.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dasta J, Ramamoorthy S, Patou G, et al. Bupivacaine liposome injectable suspension compared with bupivacaine HCl for the reduction of opioid burden in the postsurgical setting. Curr Med Res Opin. 2012;28:1609–1615.. [DOI] [PubMed] [Google Scholar]
- 44.Abdelsattar JM, Boughey JC, Fahy AS, et al. Comparative study of liposomal bupivacaine versus paravertebral block for pain control following mastectomy with immediate tissue expander reconstruction. Ann Surg Oncol. 2016;23:465–470.. [DOI] [PubMed] [Google Scholar]
- 45.Eberle N, Newman M. Patient perception of postoperative pain after administration of liposomal bupivacaine in plastic surgery. Ann Plast Surg. 2015;74:S198–S200.. [DOI] [PubMed] [Google Scholar]
- 46.Leiman D, Barlow M, Carpin K, et al. Medial and lateral pectoral nerve block with liposomal bupivacaine for the management of postsurgical pain after submuscular breast augmentation. Plast Reconstr Surg Glob Open. 2014;2:e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edwards MC, Sorokin E, Brzezienski M, et al. Impact of liposome bupivacaine on the adequacy of pain management and patient experiences following aesthetic surgery: results from an observational study. Plast Surg (Oakv). 2015;23:15–20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smoot JD, Bergese SD, Onel E, et al. The efficacy and safety of DepoFoam bupivacaine in patients undergoing bilateral, cosmetic, submuscular augmentation mammaplasty: a randomized, double-blind, active-control study. Aesthet Surg J. 2012;32:69–76.. [DOI] [PubMed] [Google Scholar]


