Summary:
Painful neuropathies can be caused by nerve compression or neuromas. Nerve compressions can arise from scar adhesions causing painful posttraumatic entrapment of nerve branches via fibrosis. The classical treatment methods include neurolysis and nerve transposition. In this case, we present the treatment of recurrent scar entrapment of the saphenous nerve with percutaneous neurolysis and lipofilling in a patient who had previously undergone an open neurolysis procedure. Resolution of the condition without any complications was noted during the 2-month clinical follow-up. Percutaneous neurolysis and lipofilling are shown to be safe and reproducible methods for the treatment of neuropathic compressive scars.
Painful neuropathies can be caused by nerve compression or neuromas. Classically, open approaches have been made to decrease pain through the removal of the neuroma, the neurolysis of perineuronal scar adhesions, and the transposition of neuroma to soft tissue to avoid direct trauma to the nerve.1 However, relapse is frequent, as the healing process causes the formation of new internal adhesions.2 Recent studies postulate that the use of adipose tissue or other substances may create an environment that protects the nerve3 and provides new vascularization.4
We present a case of neuralgia of the saphenous nerve in which the patient was successfully treated with percutaneous neurolysis and fat grafting.
CASE REPORT
A 29-year-old woman was admitted to our center with pain in the lower third of the inner surface of the left inferior limb. The patient was in the armed forces, was a smoker, and was 1.60 m tall and weighed 112 pounds. Her body mass index was 23.8.
The patient reported trauma to the area 6 months earlier, for which she underwent 2 procedures at another center. The first involved drainage of a hematoma and repair of the wound. The patient experienced pain, so a biopsy examination was performed. It showed the presence of fibro-cicatricial tissue and fat necrosis in the damaged area.
At the time she presented to our center, the patient could barely walk due to the intense pain, was on sick leave, and had ceased normal activities. She had an important contouring defect in the inner surface of the leg with a cutaneous scar (Fig. 1). She was positive for Tinel’s sign, with irradiation in the distal part of the limb and anesthesia at the saphenous nerve area.
Fig. 1.
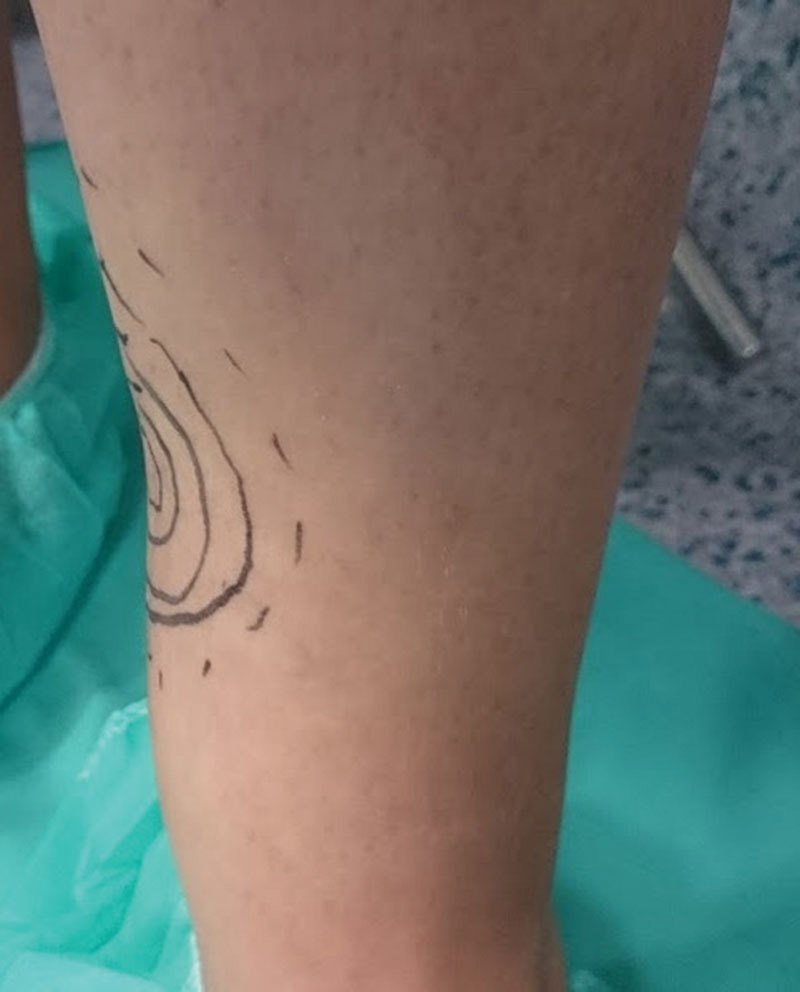
Preoperative photograph.
Magnetic resonance imaging revealed a neurovascular bundle surrounded by scar tissue. Nerve entrapment was suspected, and an electromyography was performed. This revealed involvement of saphenous nerve on the distal scar with severe axonal damage, but with continuity of the nerve. A high-frequency ultrasound examination showed a small volume of subcutaneous tissue over the muscle fascia and a large area of scar reaction (Fig. 2).
Fig. 2.
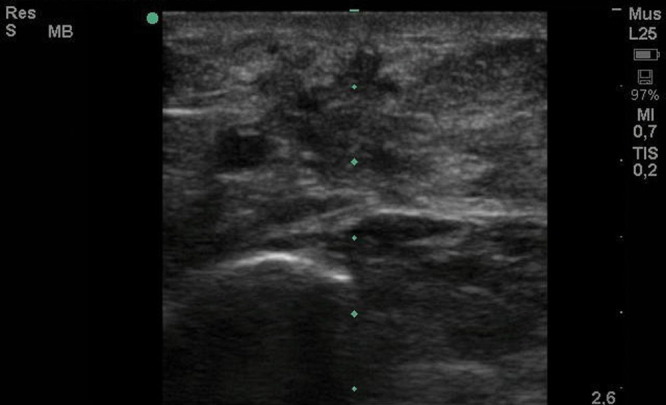
Preoperative ultrasonography.
We decided to perform percutaneous nerve release using a Toledo type cannula, along with fat grafting, to avoid further tissue adhesion and to increase vascularized tissue into the scar area. The need for further open nerve release surgery did not arise because the scar surrounding the nerve was poorly vascularized and prone to new adhesions.
We chose the abdominal area as the donor site, as recommended in the recent literature.5 We used the FAT WASHER system (Groupe SEBBIN, Boissy L’Aillerie, France), a closed system for washing and decanting fat, thus avoiding centrifugation. After infiltration of the donor area with Klein solution, a 3-mm accelerator-type cannula was used to harvest the fat with limited vacuum (0.4 atm). A total of 40 cc of purified fat was transferred into 10 cc syringes and reinjected using 2-hole 2-mm blunt cannulas.
The patient was discharged the following morning with abdominal compression garments. She was requested to keep the limb elevated to avoid edema, and she was administered anticoagulant therapy to prevent deep venous thrombosis. She reported immediate pain relief, which was probably the effect of the neurolysis.
After 2 months of follow-up, the defective contour was corrected (Fig. 3), and the previous pain symptoms were completely alleviated without recurrence. The formation of new vascularized tissue around the neurovascular bundle was confirmed by ultrasound color Doppler examination (Fig. 4). Tinel’s sign did not persist. No complications were observed in the donor or recipient area, and additional lipofilling was not required because the graft was successfully implanted and fat resorption was minimal. The patient was seen back 1 year after surgery with a stable outcome. She remained asymptomatic.
Fig. 3.
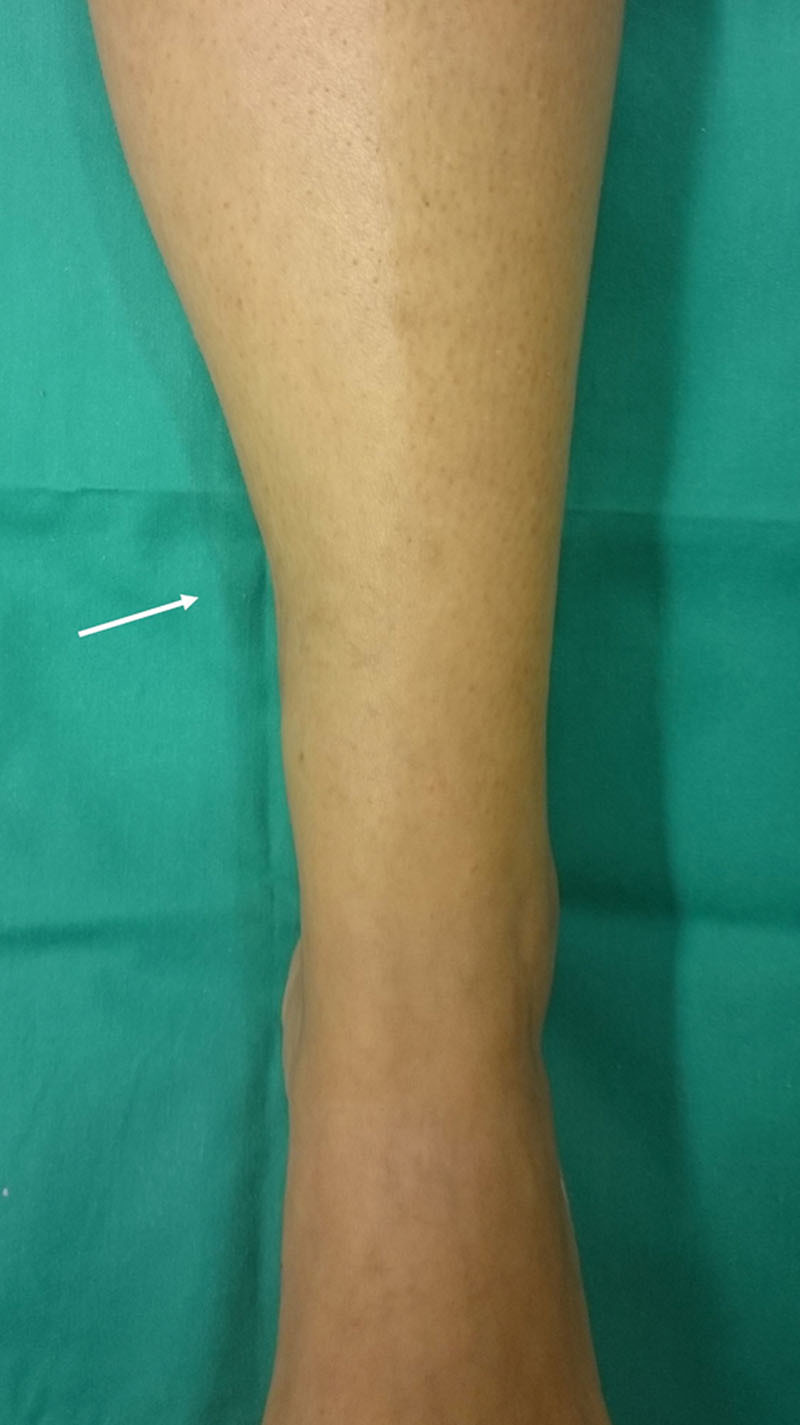
Postoperative photograph at 2 months. The white arrow indicates the location of the defect.
Fig. 4.
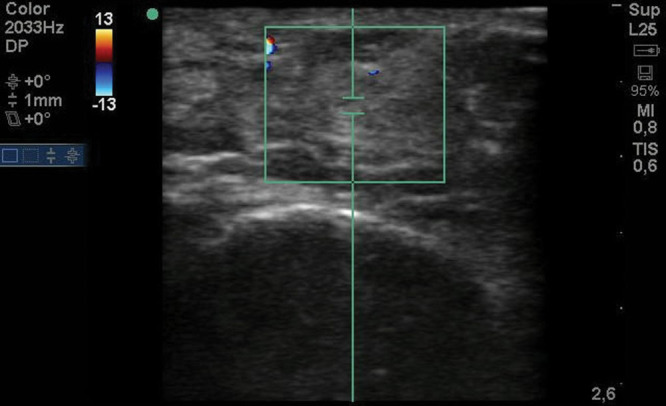
Doppler ultrasonography at 2 months postoperative.
DISCUSSION
To the best of our knowledge, there is no report on the treatment of saphenous nerve scar entrapment with neurolysis and fat grafting, so the positive outcomes observed here are rather interesting and indicate the potential usefulness of this technique in similar cases.
To understand the underlying mechanism, we performed ultrasound examination before and 2 months after fat grafting. The preoperative examination showed a fibrous bed with decreased vascularization. The postoperative examination revealed the reappearance of normal fat tissue on the neurovascular bundle and an increase in the density of vascularization. The increase in vascularization promotes the replacement of scar tissue with healthy tissue.
In agreement with this, fat grafting has been reported to increase vascularization in the grafted site, probably thanks to the endothelial differentiation of the adipose-derived stem cells, as well as to reduce nerve compression and facilitate the movement of the nerve away from scar tissue.6 In addition, an anti-inflammatory effect is also noted,7 which could be associated with reduction in the scar tissue after implantation of the fat graft. Further, fat tissue may act as a mechanical barrier that prevents the growth of aberrant neuronal tissue from the nerve damage, which could decrease the formation of neuromas.8
The methods of treatment and injection of adipose tissue are important,9 as they affect graft survival. We used a novel device for washing and decanting the adipose tissue and avoided the centrifugation of the cells, so the fraction of living cells was increased and the graft was cleaned more efficiently.10 Further, fat grafting was performed in the whole area to release the adhesions and prevent future adhesions and create a cushioning effect. As the adhesions are released, the technique allowed for the correction of the secondary defect of the aesthetic contour that our patient presented with.
CONCLUSIONS
Based on the findings in this case, fat grafting appears to be an advantageous treatment option for scar neuralgias and painful neuromas and overcomes some limitations of the open approach. However, since this is the first such reported case, more case series are required before the technique can be recommended with confidence.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by Groupe SEBBIN SAS.
REFERENCES
- 1.Mackinnon SE. Evaluation and treatment of the painful neuroma. Tech Hand Up Extrem Surg. 1997;1:195–212.. [DOI] [PubMed] [Google Scholar]
- 2.Vaienti L, Merle M, Battiston B, et al. Perineural fat grafting in the treatment of painful end-neuromas of the upper limb: a pilot study. J Hand Surg Eur Vol. 2013;38:36–42.. [DOI] [PubMed] [Google Scholar]
- 3.Agenor A, Dvoracek L, Leu A, et al. Hyaluronic acid/carboxymethyl cellulose directly applied to transected nerve decreases axonal outgrowth. J Biomed Mater Res B Appl Biomater. 2017;105:568–574.. [DOI] [PubMed] [Google Scholar]
- 4.Thanik VD, Chang CC, Lerman OZ, et al. A murine model for studying diffusely injected human fat. Plast Reconstr Surg. 2009;124:74–81.. [DOI] [PubMed] [Google Scholar]
- 5.Geissler PJ, Davis K, Roostaeian J, et al. Improving fat transfer viability: the role of aging, body mass index, and harvest site. Plast Reconstr Surg. 2014;134:227–232.. [DOI] [PubMed] [Google Scholar]
- 6.Carlstedt T. An overture to basic science aspects of nerve injuries. J Hand Surg Eur Vol. 2011;36:726–729.. [DOI] [PubMed] [Google Scholar]
- 7.González MA, Gonzalez-Rey E, Rico L, et al. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989.. [DOI] [PubMed] [Google Scholar]
- 8.Lutz BS, Ma SF, Chuang DC, et al. Interposition of a pedicle fat flap significantly improves specificity of reinnervation and motor recovery after repair of transected nerves in adjacency in rats. Plast Reconstr Surg. 2001;107:116–123.. [DOI] [PubMed] [Google Scholar]
- 9.Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118:108S–120S.. [DOI] [PubMed] [Google Scholar]
- 10.Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63:1375–1381.. [DOI] [PubMed] [Google Scholar]


