Abstract
A worldwide health concern, osteoporosis (OP), increases the risk of bone fracture and results in morbidity. This study examined whether the representative bone absorption marker serum tartrate-resistant acid phosphatase 5b (TRACP-5b) or bone formation marker bone alkaline phosphatase (BAP) could estimate primary OP status and denosumab efficacy in a real-world setting. We retrospectively enrolled 114 female postmenopausal primary OP patients in Japan. Values and percent changes in TRACP-5b, BAP, lumbar 1–4 bone mineral density (L-BMD), and total hip BMD (H-BMD) were assessed before treatment and at 4, 8, and 12 months of therapy to identify the correlations between the percent changes in bone metabolic markers and BMD. We also established two sets of subgroups based on the upper limits of reference values in Japan for serum: TRACP-5b (<420 mU/dL) and (≥420 mU/dL) and BAP (<14.5 µg/L) and (≥14.5 µg/L). Negative correlations were observed for the percent changes of TRACP-5b and H-BMD at 4 months (r=−0.3476) and 8 months (r=−0.3880), for the percent changes of BAP and H-BMD at 8 months (r=−0.3354), and for the percent changes of BAP and L-BMD at 12 months (r=−0.3186). We observed a significant difference between the subgroups for the percent changes of L-BMD at 8 months (p=0.013) and 12 months (p=0.004) in BAP values. These results suggest that TRACP-5b and BAP had negative correlations with BMD, and that BAP represented a useful serum marker to evaluate L-BMD during denosumab therapy for OP.
Keywords: bone mineral density, bone alkaline phosphatase, tartrate-resistant acid phosphatase 5b, denosumab, osteoporosis
Introduction
Osteoporosis (OP) is a worldwide health issue, particularly in aged women, that increases the risk of bone fracture and results in morbidity. Thus, fracture prevention is the primary therapeutic goal in patients with OP.1 To achieve this, data on bone mineral density (BMD) and bone turnover markers (BTMs) are essential in fracture prediction and prevention, and to evaluate treatment efficacy.2
The tartrate-resistant acid phosphatase 5b (TRACP-5b) isozyme is a 35–37 kDa glycoprotein. TRACP-5b is associated with bone remodelling, and the increased levels are often observed during normal bone growth among healthy children. Elevated serum TRACP-5b levels have been detected in diseases characterized by increased bone resorption as well, such as Paget’s disease of the bone, primary hyperparathyroidism, metastatic malignancies involving bone resorption, and OP.3 TRACP-5b is also a specific bone resorptive marker in OP,3,4 while serum bone alkaline phosphatase (BAP) is a representative bone formation marker in OP.5 In addition to N-terminal telopeptide of type-I collagen and procollagen type I N-terminal propeptide, clinical trials and we have suggested that TRACP-5b and BAP are useful bone metaoblic markers for OP, 6,7 however, it is still largely unknown if they are applicable in a real-world setting. Thus, TRACP-5b and BAP were adopted as monitoring markers in this study to evaluate the effects of denosumab on BMD and bone metabolism in Japanese primary OP.
Approved for use in Japan in 2013, denosumab is a fully human monoclonal antibody against receptor activator of nuclear factor-kB ligand (RANKL) that is known to selectively inhibit osteoclastogenesis. There have been numerous reports on the association between BMD and BTMs in postmenopausal women with primary OP, which focused mainly on denosumab.8,9 Cummings et al reported in the FREEDOM study that denosumab reduced bone resorption markers by a median of 86% at 1 month, which was greater than the reduction seen with other antiresorptive drugs, to increase the BMD through RANKL inhibition.8 Miller et al revealed that the drug was associated with greater BMD gains and stronger inhibition of bone remodeling, compared with zoledronate.9 We and others have reported that denosumab increases BMD in OP due to strong bone resorption inhibition, even after bisphosphonate (BP) treatment,10–12 while bone formation is mildly repressed.12 However, there are no reports on the precise correlations between BTMs, especially based on their upper limits of reference values, and BMD during denosumab therapy.
The purpose of this study was to investigate the associations between serum TRACP-5b or BAP with lumbar 1–4 BMD (L-BMD) or bilateral total hip BMD (H-BMD) in Japanese postmenopausal women with primary OP during denosumab treatment for 1 year.
Materials and methods
The inclusion criteria for this retrospective study were postmenopausal patients with primary OP and low L-BMD and/or H-BMD of <−3.0 of standard deviation (SD). In total, 114 patients were recruited at our institutions between October 2013 and September 2015. Sixteen patients were excluded due to insufficient data, leaving 98 patients for further analyses. Among them, 36 patients had taken BPs (alendronate: 12, risedronate: 5, minodronate: 12, ibandronate: 7) and 43 had received teriparatide (PTH) prior to denosumab therapy. BP and PTH treatment were ceased just prior to denosumab administration. Subcutaneous denosumab injections of 60 mg were given every 6 months. Sixty-six in 98 patients also received vitamin D supplementation (native vitamin D and calcium: 40, alfacalcidol: 10, eldecalcitol: 13, calcitriol: 3). Male patients were excluded to avoid sex-related bias. Other exclusion criteria in this study were obvious complications that might affect OP, such as chronic renal failure (estimated glomerular filtration rate <40 mL/min/1.73 m2), bone metabolic disorder, liver dysfunction, and diabetes mellitus, as well as fracture within 1 year prior to the study. Patient characteristics prior to denosumab treatment were expressed as mean ± SD (Table 1). No hypocalcemia, fractures, or other serious adverse events occurred during observation.
Table 1.
Characteristics of patients prior to the study
| Age (years) | 73.9±9.1 |
| Sex (F:M) | 98:0 |
| BMI (kg/m2) | 21.5±4.0 |
| Serum BAP (µg/L) | 16.2±7.2 |
| Serum TRACP-5b (mU/dL) | 496.7±251.4 |
| BP pretreatment, n | 36 |
| PTH combination, n | 43 |
| Vitamin D combination, n | 66 |
| 1. Native vitamin D and calcium, n | 40 |
| 2. Alfacalcidol, n | 10 |
| 3. Eldecalcitol, n | 13 |
| 4. Calcitriol, n | 3 |
| L-BMD (g/cm2) | 0.799±0.15 |
| H-BMD (g/cm2) | 0.635±0.11 |
Notes: Results are expressed as mean ± standard deviation. p<0.05 was considered statistically significant.
Abbreviations: BAP, bone alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; BP, bisphosphonate; H-BMD, total hip BMD; L-BMD, lumbar 1–4 BMD; PTH, teriparatide; TRACP-5b, tartrate-resistant acid phosphatase 5b.
We measured serum BAP (µg/L) by a chemiluminescent enzyme immunoassay and antibody radioimmunoassay, and TRACP-5b (mU/dL) with an enzyme-linked immunosorbent assay at the first visit, 4, 8, and 12 months of treatment. L-BMD and H-BMD were quantified using a dual-energy X-ray absorption fan-beam bone densitometer (Lunar Prodigy; GE Healthcare, Waukesha, WI, USA) at the same time points. The coefficients of variation of the L-BMD and H-BMD measurements were 1.0% and 0.6%, respectively.13 All TRACP-5b and BAP measurements were performed by SRL Inc. (Tokyo, Japan). Results in Table 1–3 were expressed as mean ± SD, and results in Figure 1 were expressed as mean ± standard error (SE). Correlations between the percent changes of TRACP-5b and L-BMD or H-BMD, as well as between the percent changes of BAP and L-BMD or H-BMD were determined using Spearman correlation methods.
Table 2.
Patient characteristics prior to the study after classification of TRACP-5b values, and L-BMD and H-BMD values at 0, 4, 8, and 12 months of denosumab treatment
| Characteristic | TRACP-5b (<420 mU/dL) (n=47) |
TRACP-5b (≥420 mU/dL) (n=51) |
p-value |
|---|---|---|---|
| Age (years) | 73.2±8.5 | 74.1±8.8 | 0.59 |
| BMI (kg/m2) | 22.0±3.7 | 21.5±4.2 | 0.52 |
| Serum BAP (µg/L) | 11.8±4.6 | 21.3±8.4 | <0.001 |
| Serum TRACP-5b (mU/dL) | 283.9±99.3 | 683.3±197.5 | <0.001 |
| BP pretreatment, n | 32 | 7 | |
| Duration of BP use, years | 2.9±2.5 | 9.9±8.3 | 0.20 |
| PTH combination, n | 19 | 25 | |
| L-BMD (g/cm2) at 0 months | 0.796±0.11 | 0.783±0.17 | 0.64 |
| H-BMD (g/cm2) at 0 months | 0.664±0.10 | 0.640±0.10 | 0.24 |
| L-BMD (g/cm2) at 4 months | 0.825±0.10 | 0.830±0.10 | 0.83 |
| H-BMD (g/cm2) at 4 months | 0.666±0.07 | 0.656±0.10 | 0.63 |
| L-BMD (g/cm2) at 8 months | 0.832±0.09 | 0.839±0.10 | 0.75 |
| H-BMD (g/cm2) at 8 months | 0.674±0.10 | 0.665±0.10 | 0.71 |
| L-BMD (g/cm2) at 12 months | 0.850±0.11 | 0.854±0.11 | 0.88 |
| H-BMD (g/cm2) at 12 months | 0.680±0.10 | 0.668±0.10 | 0.59 |
Notes: Results are expressed as mean ± standard deviation. p<0.05 was considered statistically significant.
Abbreviations: BAP, bone alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; BP, bisphosphonate; H-BMD, total hip BMD; L-BMD, lumbar 1–4 BMD; PTH, teriparatide; TRACP-5b, tartrate-resistant acid phosphatase 5b.
Table 3.
Patient characteristics prior to the study after classification of BAP values, and L-BMD and H-BMD values at 0, 4, 8, and 12 months of denosumab treatment
| Characteristic | BAP (<14.5 µg/L) (n=45) |
BAP (≥14.5 µg/L) (n=53) |
p-value |
|---|---|---|---|
| Age (years) | 73.1±8.0 | 74.1±9.9 | 0.60 |
| BMI (kg/m2) | 21.3±2.7 | 22.0±4.7 | 0.37 |
| Serum BAP (µg/L) | 10.3±2.8 | 22.2±7.4 | <0.001 |
| Serum TRACP-5b (mU/dL) | 336.3±150.3 | 624.7±252.4 | <0.001 |
| BP pretreatment, n | 28 | 11 | |
| Duration of BP use, years | 3.6±3.2 | 4.7±7.1 | 0.72 |
| PTH combination, n | 15 | 29 | |
| L-BMD (g/cm2) at 0 month | 0.778±0.12 | 0.796±0.17 | 0.51 |
| H-BMD (g/cm2) at 0 month | 0.661±0.10 | 0.630±0.11 | 0.17 |
| L-BMD (g/cm2) at 4 months | 0.794±0.10 | 0.817±0.10 | 0.33 |
| H-BMD (g/cm2) at 4 months | 0.678±0.10 | 0.640±0.12 | 0.13 |
| L-BMD (g/cm2) at 8 months | 0.796±0.11 | 0.830±0.10 | 0.14 |
| H-BMD (g/cm2) at 8 months | 0.681±0.10 | 0.640±0.09 | 0.09 |
| L-BMD (g/cm2) at 12 months | 0.816±0.11 | 0.839±0.15 | 0.48 |
| H-BMD (g/cm2) at 12 months | 0.688±0.09 | 0.641±0.12 | 0.11 |
Notes: Results are expressed as mean ± standard deviation. p<0.05 was considered statistically significant.
Abbreviations: BAP, bone alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; BP, bisphosphonate; H-BMD, total hip BMD; L-BMD, lumbar 1–4 BMD; PTH, teriparatide; TRACP-5b, tartrate-resistant acid phosphatase 5b.
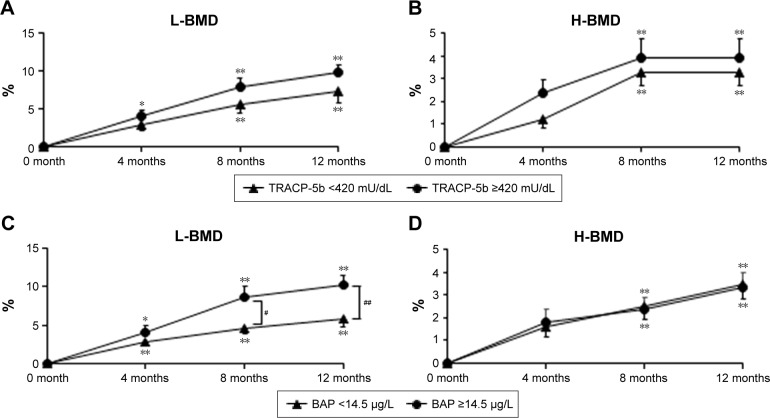
Figure 1.
Comparisons of percent changes in L-BMD and H-BMD between TRACP-5b (<420 mU/dL) and TRACP-5b (≥420 mU/dL) groups just prior to and during denosumab administration (A, B). Comparisons of percent changes in L-BMD and H-BMD in BAP (<14.5 µg/L)and BAP (≥14.5 µg/L) groups just prior to and during denosumab administration (C, D). *p<0.05; **p<0.01; #p<0.05; ##p<0.01. Results are expressed as mean ± standard error.
Abbreviations: BAP, bone alkaline phosphatase; BMD, bone mineral density; H-BMD, total hip BMD; L-BMD, lumbar 1–4 BMD; TRACP-5b, tartrate-resistant acid phosphatase 5b.
BTM cut-off values were selected based on the Guidelines for the Use of Bone Metabolic Markers in the Diagnosis and Treatment of Osteoporosis (2012 edition).5 Based on the upper limits of reference values in Japan for serum TRACP-5b (420 mU/dL) and BAP (14.5 µg/L), we established the following 4 groups with respect to each marker: TRACP-5b (<420 mU/dL), TRACP-5b (≥420 mU/dL), BAP (<14.5 µg/L), and BAP (≥14.5 µg/L). The percentage changes for each group at each time point were determined using Bonferroni correction for multiple comparisons, compared to those before treatment. Comparisons of markers between the groups at each time point were performed by Welch’s t-test.
This study was approved by the institutional ethics committee of Shinshu University School of Medicine and Showa-Inan General Hospital, and informed consent was obtained from all patients. All methods were carried out in accordance with the approved guidelines.
Results
The patients’ characteristics prior to the start of the study are summarized in Table 1.
The percent changes of serum TRACP-5b and BAP, L-BMD and H-BMD at 4 months were −21.7±39.1, −26.1±3.0, 4.0±0.7, and 1.8±0.7, respectively. The percent changes of those markers were −68.6±2.2, −40.3±3.0, 6.9±0.9, and 3.0±0.7, respectively, at 8 months, and −49.5±4.2, −33.9±5.8, 8.9±1.0, and 3.4±0.5, respectively, at 12 months.
We observed significant negative correlations between the percent changes of TRACP-5b and H-BMD at 4 months (r=−0.3476, p<0.01) and 8 months (r=−0.3880, p<0.01). There were negative correlations between the percent changes of BAP and H-BMD at 8 months (r=−0.3354, p<0.05), and between the percent changes of BAP and L-BMD at 12 months (r=−0.3186, p<0.05). Since the percent changes of the BTMs and BMD showed a negative correlation, we next analyzed the groups based on the upper limits of reference values of TRACP-5b and BAP in Japan (Tables 2 and 3).
The TRACP-5b (<420 mU/dL) group consisted of 47 patients and the TRACP-5b (≥420 mU/dL) group had 51 patients (Table 2). There were no significant differences in patient characteristics except for serum BAP and TRACP-5b values prior to the study. However, pre-treatment BAP values were significantly lower in the TRACP-5b (<420 mU/dL) group than those in the TRACP-5b (≥420 mU/dL) group (p<0.001). With respect to the percent changes in L-BMD from baseline, significant differences were noted at 4, 8, and 12 months (4.0%, 7.8%, and 9.7%, respectively) in the TRACP-5b (≥420 mU/dL) group (Figure 1). There were significant differences at 8 and 12 months (5.6% and 7.4%, respectively) in the TRACP-5b (<420 mU/dL) group, compared with the pre-treatment levels of L-BMD (Figure 1). Compared with the pre-treatment levels of H-BMD, there were significant differences at 8 and 12 months in the TRACP-5b (≥420 mU/dL) and (<420 mU/dL) groups (3.9% and 3.9%, and 3.3% and 3.3%, respectively), as shown in Figure 1.
Similar studies were performed on 45 BAP (<14.5 µg/L) and 53 BAP (≥14.5 µg/L) group patients (Table 3). There were no significant differences in patient characteristics except for serum BAP and TRACP-5b values prior to the study. The BAP (<14.5 µg/L) group showed significantly lower TRACP-5b values than those in the BAP (≥14.5 µg/L) group (p<0.001). There was a significant difference between the BAP groups for percent changes in L-BMD at 8 months (p=0.013) and 12 months (p=0.004). Within each group, significant differences were observed at 4, 8, and 12 months for the percent changes in L-BMD (BAP [≥14.5 µg/L]: 4.0%, 8.6%, and 10.3%, respectively; BAP [<14.5 µg/L]: 2.9%, 4.5%, and 5.7%, respectively). In both BAP (≥14.5 µg/L) and BAP (<14.5 µg/L) groups, we noted significant differences for percent changes in H-BMD at 8 and 12 months (2.4% and 3.3%, and 2.5% and 3.5%, respectively), compared with the pre-treatment levels.
Discussion
There have been no reports on specific correlations between BTMs and BMD during denosumab therapy in postmenopausal women with OP. We herein examined the correlations between the percent changes in BTMs and BMD during denosumab therapy for 1 year.
Our results uncovered several remarkable negative correlations. One was between the percent changes of TRACP-5b and H-BMD at 4 and 8 months, −21.7%±39.1% and 1.8%±0.7%, respectively, at 4 months, and −68.6%±2.2% and 3.0%±0.7%, respectively, at 8 months. These findings suggest that denosumab increased H-BMD through the reduction of TRACP-5b during 4–8 months of denosumab treatment. Other negative correlations were found between the percent changes in BAP and H-BMD at 8 months (−40.3%±3.0% and 3.0%±0.7%, respectively), and in BAP and L-BMD at 12 months (−33.9%±5.8% and 8.9%±1.0%, respectively), suggesting that denosumab increased H-BMD and L-BMD through the reduction of BAP during 8–12 months of therapy. We have reported that denosumab potentially might be a stronger bone resorption agent than BPs in postmenopausal OP women and in OP with rheumatoid arthritis, according to alterations in BTM.12,14,15 We also found that the agent had strong bone absorptive effects, but caused mild inhibition of bone formation in primary OP.15 Collectively, these findings suggest an inversely proportional relationship between percent changes of BTMs and BMD with denosumab.
We next searched for relationships between BTMs, particularly based on the upper limit of reference values, and BMD during denosumab therapy. Both the percent changes of L-BMD and H-BMD were significantly increased at 12 months. A significant difference was seen between the BAP groups for the percent changes of L-BMD at 8 and 12 months. Catalano et al recently reported associations of BAP with L-BMD changes using a bone resorption marker, collagen type 1 cross-linked C-telopeptide (CTX), in aromatase inhibitor-treated female breast cancer patients receiving denosumab.16 In their study, the percent changes of each CTX or BAP were negatively correlated with the percent changes of L-BMD,16 which are consistant with current findings. On the other hand, Bauer et al described that greater reduction in BAP with alendronate therapy was associated with fewer hip, non-spine, and vertebral fractures.17 Although fracture prevention could not be judged due to the absence of fracture cases in our cohort, BAP might also be a marker to evaluate fracture risk during denosumab treatment.
In other studies, denosumab therapy significantly increased L-BMD and H-BMD in postmenopausal Japanese OP patients for 12 months. Ebina et al reported that the percent change in L-BMD at 1 year was 6.4% and in H-BMD was 3.3%,18 while Suzuki et al found the percent change in L-BMD at 1 year was 7.6% and in H-BMD was 2.8%.19 In this study, the percent changes in L-BMD and H-BMD at 1 year were 8.9% and 3.4%, respectively. These discrepancies were potentially and partly due to differences in the number of PTH-treated cases prior to denosumab therapy (16.7% in Suzuki’s study, 31.7% in Ebina’s study, and 43.9% in our own).18,19 Despite the fact that the BMD increase in this investigation was partly affected by PTH therapy, since we have previously reported that denosumab upregulates BMD in postmenopausal Japanese OP patients,19 denosumab was considered to clearly increase BMD in those series.
The limitations of this study were a small sample size and inclusion of BP and PTH pre-treatment prior to denosumab therapy, which might have affected the results.
In conclusion, this investigation showed that the increased effects of L-BMD could be observed when the BAP values were high (≧14.5 ug/L) prior to denosumab therapy. Thus, BAP represents a useful marker to evaluate L-BMD during denosumab therapy in OP.
Acknowledgments
We thank Mr. Yuji Takanashi for collecting patient data at Showa-Inan General Hospital and Shinshu University School of Medicine. We also thank Mr Trevor Ralph for editing the manuscript.
Footnotes
Author contributions
YN directed this study. TS collected and analyzed patient data. YN and TS wrote the main manuscript. HK gave suggestions on the study. YN, TS, and HK revised the manuscript. All authors reviewed and approved the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Christiansen C. Skeletal osteoporosis. J Bone Miner Res. 1993;8(Suppl 2):S475–S480. doi: 10.1002/jbmr.5650081308. [DOI] [PubMed] [Google Scholar]
- 2.Orimo H, Nakamura T, Hosoi T, Iki M, et al. Japanese 2011 guidelines for prevention and treatment of osteoporosis–executive summary. Arch Osteoporos. 2012;7:3–20. doi: 10.1007/s11657-012-0109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halleen J, Hentunen TA, Hellmann J, Väänänen HK. Tartrate-resistant acid phosphatase from human bone: purification and development of an immunoassay. J Bone Min Res. 1996;11(10):1444–1452. doi: 10.1002/jbmr.5650111011. [DOI] [PubMed] [Google Scholar]
- 4.Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Väänänen HK. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000;15(7):1337–1345. doi: 10.1359/jbmr.2000.15.7.1337. [DOI] [PubMed] [Google Scholar]
- 5.Nishizawa Y, Ohta H, Miura M, et al. Guidelines for the use of bone metabolic markers in the diagnosis and treatment of osteoporosis (2012 edition) J Bone Miner Metab. 2013;31(1):1–15. doi: 10.1007/s00774-012-0392-y. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura T, Matsumoto T, Sugimoto T, et al. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT) J Clin Endocrinol Metab. 2014;99(7):2599–2607. doi: 10.1210/jc.2013-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura Y, Suzuki T, Kamimura M, Ikegami S, Uchiyama S, Kato H. Vitamin D and calcium are required at the time of denosumab administration during osteoporosis treatment. Bone Res. 2017;5:16055. doi: 10.1038/boneres.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings SR, San Martin J, McClung MR, et al. FREEDOM Trial Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 9.Miller PD, Pannacciulli N, Brown JP, et al. Denosumab or Zoledronic acid in postmenopausal women with osteoporosis previously treated with oral bisphosphonates. J Clin Endocrinol Metab. 2016;101(8):3163–3170. doi: 10.1210/jc.2016-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Recknor C, Czerwinski E, Bone HG, et al. Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol. 2013;121(6):1291–1299. doi: 10.1097/AOG.0b013e318291718c. [DOI] [PubMed] [Google Scholar]
- 11.Roux C, Hofbauer LC, Ho PR, et al. Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone. 2014;58:48–54. doi: 10.1016/j.bone.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Kamimura M, Nakamura Y, Ikegami S, Uchiyama S, Kato H, Taguchi A. Significant improvement of bone mineral density and bone turnover markers by denosumab therapy in bisphosphonate-unresponsive patients. Osteoporos Int. 2017;28(2):559–566. doi: 10.1007/s00198-016-3764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura Y, Suzuki T, Kamimura M, Ikegami S, Uchiyama S, Kato H. Alfacalcidol increases the therapeutic efficacy of ibandronate on bone mineral density in Japanese women with primary osteoporosis. Tohoku J Exp Med. 2017;241(4):319–326. doi: 10.1620/tjem.241.319. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Suzuki T, Yoshida T, Yamazaki H, Kato H. Vitamin D and calcium are required during denosumab treatment in osteoporosis with rheumatoid arthritis. Nutrients. 2017;9(5) doi: 10.3390/nu9050428. pii: E428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura Y, Kamimura M, Ikegami S, et al. Changes in serum vitamin D and PTH values using denosumab with or without bisphosphonate pre-treatment in osteoporotic patients: a short-term study. BMC Endocr Disord. 2015;15:81. doi: 10.1186/s12902-015-0077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalano A, Gaudio A, Morabito N, et al. Quantitative ultrasound and DXA measurements in aromatase inhibitor-treated breast cancer women receiving denosumab. J Endocrinol Invest. 2017;40(8):851–857. doi: 10.1007/s40618-016-0606-6. [DOI] [PubMed] [Google Scholar]
- 17.Bauer DC, Black DM, Garnero P, et al. Fracture Intervention Trial Study Group Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004;19:1250–1258. doi: 10.1359/JBMR.040512. [DOI] [PubMed] [Google Scholar]
- 18.Ebina K, Kashii M, Hirao M, et al. Comparison of the effects of denosumab between a native vitamin D combination and an active vitamin D combination in patients with postmenopausal osteoporosis. J Bone Miner Metab. 2016 Nov 9; doi: 10.1007/s00774-016-0792-5. Epub. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Nakamura Y, Kamimura M, Ikegami S, Uchiyama S, Kato H. Compliance and discontinuation of denosumab treatment in postmenopausal Japanese women with primary osteoporosis or rheumatoid arthritis and osteoporosis. Osteoporos Sarcopenia. 2017;3(2):108–111. doi: 10.1016/j.afos.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]