Abstract
Background
The clinical assessment of kidney function based on the estimated glomerular filtration rate (GFR) in older patients remains controversial. This study evaluated the concordance and feasibility of using various creatinine-based equations for estimating GFR in elderly Chinese patients with type 2 diabetes mellitus (T2DM).
Methods
A cross-sectional analytical study was conducted in 21,723 older diabetic patients (≥60 years) based on electronic health records (EHR) for Minhang District, Shanghai, China. The concordance of chronic kidney disease (CKD) classification among different creatinine-based equations was assessed based on Kappa values, intraclass correlation coefficient (ICC) statistics, and the eGFR agreement between the equations was tested using Bland–Altman plots. The GFR was estimated using the Cockcroft–Gault (CG), Berlin Initiative Study 1 (BIS1), simplified Modification of Diet in Renal Disease (MDRD), MDRD modified for Chinese populations (mMDRD), chronic kidney disease epidemiology collaboration (CKD-EPI), CKD-EPI in Asians (CKD-EPI-Asia), and Ruijin equations.
Results
Overall, the proportion of CKD stages 3–5 (eGFR <60 mL/min/1.73 m2) was calculated as 28.9%, 39.1%, 11.8%, 8.4%, 14.3%, 11.5%, and 12.7% by the eGFRCG, eGFRBIS1, eGFRMDRD, eGFRmMDRD, eGFRCKD-EPI, eGFRCKD-EPI-Asia, and eGFRRuijin equations, respectively. The concordance of albuminuria and decreased eGFR based on the different equations was poor by both the Kappa (<0.2) and ICC (<0.4) statistics. The CKD-EPI-Asia equation resulted in excellent concordance with the CKD-EPI (ICC =0.931), MDRD (ICC =0.963), mMDRD (ICC =0.892), and Ruijin (ICC =0.956) equations for the classification of CKD stages, whereas the BIS1 equation exhibited good concordance with the CG equation (ICC =0.809). In addition, significant differences were observed for CKD stage 1 among all these equations.
Conclusion
Accurate GFR values are difficult to estimate using creatinine-based equations in older diabetic patients. Kidney function is complex, and the staff need to be aware of the individualized consideration of other risk factors or markers of reduced renal function in clinical practice.
Keywords: estimated glomerular filtration rate, renal function, elderly, type 2 diabetes mellitus, electronic health records
Introduction
The number of older patients with chronic kidney disease (CKD) and/or end-stage renal disease (ESRD) has increased dramatically in China during the past two decades.1 CKD is associated with various comorbid conditions in older people, such as cardiovascular disease and disability, which in turn increase the risk of hospitalization and death. Diabetes mellitus has become one of the main causes of CKD in older patients in China.2 The early identification and appropriate management of CKD in diabetic patients are important measures to slow its progression, for appropriately correcting the dosage of renally eliminated medications, and for avoiding potential drug toxicity. The direct measurement of glomerular filtration rate (GFR) using a substance exclusively filtered by the kidneys, such as inulin or other markers (eg, Tc-99m-diethylene triamine pentaacetic acid, 51Cr-labeled ethylene diamine tetraacetic acid, and 125I-iothalamate), is the most reliable method to assess renal function.3 However, these exogenous markers need to be infused or injected using costly and labor-intensive procedures to measure GFR. These evaluations are impractical in large numbers of older subjects. In clinical practice, serum creatinine (Scr)-based equations for calculating estimated glomerular filtration rate (eGFR) are important tools for identifying geriatric patients with CKD and for allocating appropriate drug dosage in these patients. The most often employed and analyzed equations include the Cockcroft–Gault (CG),4 Modification of Diet in Renal Disease (MDRD),5,6 MDRD modified for Chinese populations (mMDRD),7 and chronic kidney disease epidemiology collaboration (CKD-EPI) equations.8 These equations use information on age, gender, Scr, and race. Weight is also a factor in the CG equation. However, the clinical assessment of kidney function based on eGFR in older patients remains controversial. Older individuals experience age-related loss of muscle mass, and sarcopenia has been reported in 1%–29% of community-dwelling populations,9 which may influence body weight and Scr levels. Malnourished patients are particularly at risk of having decreased eGFR, even when they have normal Scr levels.10 Race is also an important determinant of GFR estimation due to differences in dietary habits and body composition.11 All of these factors can influence the accuracy of the results obtained when applying Scr-based equations. A multiple-race and -ethnicity study suggested that the use of a four-level race CKD-EPI equation (Black, Asian, Native American and Hispanic, White and other) in Asians (CKD-EPI-Asia) may improve the accuracy of results obtained for Asians.12
Currently, the American Diabetes Association recommends an annual screening for diabetic kidney disease based on an evaluation of the urinary excretion of albumin and GFR, as estimated using equations including Scr, such as the MDRD or the CKD-EPI equation.13 However, some studies suggest that evaluating eGFR based on the CKD-EPI equation provides improved risk prediction for heart failure, ESRD and cardiovascular mortality compared with the value of eGFR obtained using the MDRD equation.14–16 It has also been reported that the MDRD equation significantly underestimates GFR in diabetic patients with microalbuminuria or overt diabetic nephropathy.17 One study, which was conducted in Type 2 diabetic patients in a Korean population, suggested that the value of eGFR obtained using the CKD-EPI equation can more accurately stratify earlier-stage CKD among type 2 diabetic patients with nephropathy than the value of eGFR obtained using the MDRD equation.18 While some studies have suggested that the performances of CKD-EPI and MDRD are comparable,19 others have suggested that the CKD-EPI formula does not exhibit better performance than the simplified MDRD formula for estimating GFR in diabetic patients.20 Using 99mTc-DTPA dynamic renal imaging as the gold standard, some studies in China have suggested that the Ruijin formula is more accurate than MDRD for estimating GFR in Chinese diabetes patients (the rate of achieving 30% accuracy was over 70.0%),21,22 and one study suggested that this method is suitable for older diabetic patients (mean age of 70.3±6.4 years).21 Furthermore, some studies have suggested that the Ruijin formula is more suitable for estimating GFR during the early stage of CKD in Chinese diabetes patients than the CKD-EPI equation.23,24 However, the sample size in each of the previous Chinese studies was not larger than 300 diabetic patients. The Ruijin formula was refitted based on the MDRD by investigating 760 Chinese cases of CKD hospitalized at the Shanghai Jiaotong University-affiliated Ruijin Hospital during 2002–2005.25 The feasibility of using this formula needs verification in a large sample of diabetic patients. Some recent studies have suggested that the use of the Berlin Initiative Study 1 (BIS1) equation based on Scr is more suitable and accurate for estimating GFR in older patients, including diabetic patients; this equation was developed and validated in a population of older adults aged 70 years or more.26 Studies comparing the MDRD, CKD-EPI, BIS1, CKD-EPI-Asia, and Ruijin equations are rare. The present study aimed to evaluate the concordance of estimating renal function using the CG, MDRD, mMDRD, CKD-EPI, BIS1, CKD-EPI-Asia, and Ruijin equations for older patients with type 2 diabetes mellitus (T2DM) based on electronic health record (EHR) data for the Minhang District of Shanghai.
Methods
Subjects
For this study, data collected between October 1, 2012 and September 30, 2013 were extracted from the EHR for all 55,533 patients participating in a diabetes management program in the Minhang district of Shanghai, China, including 13 community care centers. In total, 25,021 elderly patients diagnosed with T2DM (International Classification of Diseases [ICD]-10 codes E10–E14) were eligible for analysis after excluding patients for whom incomplete data were available regarding Scr, urinary albumin to creatinine ratio (ACR), and standard hemoglobin A1c (HbA1c; n=24,491) or who were under the age of 60 years (n=6,021). Details of the data extraction have been described previously.27 After data cleaning and excluding patients with seriously abnormal Scr (<53 µmol/L or >618 µmol/L) levels, a total of 21,723 cases of older diabetic patients remained. Diabetic history and data from physical examinations, including measurements of blood pressure, body height, weight, waist circumference, fasting blood glucose, HbA1c (measured using standard high-performance liquid chromatography), Scr measured by Jaffe’s kinetic method, and the urinary albumin-to-creatinine ratio (ACR, milligram per gram), were extracted. The study adhered to the Declaration of Helsinki and was approved by the ethics committee of the Fifth Hospital of Shanghai, Fudan University, Shanghai, China (EC 2010-024). A consent form was not required because this study was based on the secondary data analysis of a pre-existing, de-identified dataset.
Measurements
GFR was estimated using the CG, MDRD, mMDRD, CKD-EPI, CKD-EPI-Asia, Ruijin and BIS1 equations, which are presented below:
The Cockcroft-Gault formula4
MDRD study equation6
mMDRD equation7
CKD-EPI equation8
CKD-EPI-Asia equation12
Ruijin equation25
BIS1 equation26
CKD was defined as either reduced renal function (low eGFR) and/or kidney damage. Kidney damage was estimated as albuminuria >30 mg/g creatinine.29 Albuminuria categories were based on ACR in a spot urine sample: A1, <30 mg/g (normal to mildly increased); A2, 30 to <300 mg/g (moderately increased); and A3, >300 mg/g (severely increased). The stages of CKD were as follows: Stage 1, albuminuria with an eGFR of ≥90 mL/min/1.73 m2; stage 2, albuminuria with an eGFR of 60–89 mL/min/1.73 m2; stage 3a, an eGFR of 45–59 mL/min/1.73 m2; stage 3b, an eGFR of 30–44 mL/min/1.73 m2; stage 4, an eGFR of 15–29 mL/min/1.73 m2; stage 5, an eGFR of <15 mL/min/1.73 m2 or dialysis.30 Stages with eGFR values of <60 mL/min/1.73 m2 (stages 3–5) were considered to indicate reduced renal function.
Statistical analysis
For data processing, the Statistical Product and Service Solutions-IBM SPSS Statistics 19.0 (Armonk, NY, USA) and GraphPad Prism 5 (La Jolla, CA, USA) were used. Qualitative variables are presented as frequencies and percentages, and quantitative variables are presented as the means and standard deviations (mean ± SD). BMI was evaluated in the following 2 ways: 1) as 4 categories (underweight <18.5, normal weight 18.5–23.9, overweight 24–27.9, and obese ≥28 [kg/m2])31 and 2) as a continuous variable. Abdominal obesity was defined as waist circumference ≥90 cm for men and ≥80 cm for women.32 Concordance between the different eGFR estimations was analyzed by calculating intraclass correlation coefficients (ICCs, two-way mixed model) together with the respective 95% confidence interval and Kappa statistic. Kappa values of 0.0–0.2 indicate slight agreement, values of 0.21–0.40 indicate fair agreement, values of 0.41–0.60 indicate moderate agreement, values of 0.61–0.80 indicate substantial agreement, and values of 0.81–1.0 indicate almost perfect or perfect agreement.33,34 Higher ICC values indicate greater inter-rater agreement, with ICC values of <0.4 indicating poor agreement, values of 0.40–0.59 indicating fair agreement, values of 0.60–0.74 indicating good agreement, and values of 0.75–1.0 indicating excellent agreement.34 Bland–Altman plots were used to assess the pairwise agreement between eGFR levels obtained using different equations.35 Two-sided P<0.05 was considered to indicate statistical significance.
Results
Subject characteristics by age group
Table 1 summarizes the demographic and some clinical characteristics of the 21,723 diabetic patients. The mean age of all patients was 70.70±7.35 (range 60–95 years). Almost 55% of the patients were 60–69 years old (11,835, 54.5%), and 2,515 (11.6%) were ≥80 years old. More females (11,146, 51.3%) than males (10,577, 48.7%) were included, especially in the ≥80-year-old age group (females: 1,502, 59.7%; males: 1,013, 40.3%). The proportion of obese (BMI ≥28 kg/m2) and abdominally obese patients decreased with age, while the proportion of underweight (BMI <18.5 kg/m2) patients increased from 1.2% in the 60–69 years age group to 4% in the ≥80 years age group.
Table 1.
Demographic and clinical characteristics of study population by age groups
| Characteristics | Total | 60–69 years | 70–79 years | ≥80 years | P-value |
|---|---|---|---|---|---|
| Number | 21,723 | 11,835 (54.5%) | 7,373 (33.9%) | 2,515 (11.6%) | – |
| Age (years) | 70.70±7.35 | 64.99±2.98 | 75.34±2.90 | 83.95±2.86 | <0.001 |
| Female | 11,146 (51.3%) | 5,803 (49.0%) | 3,841 (52.1%) | 1,502 (59.7%) | <0.001 |
| Duration of T2DM (years) | 8.95±6.25 | 8.05±5.52 | 9.84±6.73 | 10.60±7.25 | <0.001 |
| Fasting glucose (mmol/L) | 8.11±2.80 | 8.17±2.77 | 8.11±2.81 | 7.85±2.89 | <0.001 |
| Waist circumference (cm) | 84.82±7.79 | 85.07±7.74 | 84.60±7.70 | 84.36±8.22 | <0.001 |
| Abdominal obesity | 11,353 (52.3%) | 6,175 (54.4%) | 3,784 (33.3%) | 1,394 (12.3%) | 0.002 |
| BMI (kg/m2) | 24.20±2.98 | 24.45±2.97 | 23.99±2.95 | 23.66±3.02 | <0.001 |
| <18.5 | 409 (1.9%) | 139 (1.2%) | 170 (2.3%) | 100 (4.0%) | <0.001 |
| 18.5–23.9 | 10,435 (48.0%) | 5,389 (45.5%) | 3,725 (50.5%) | 1,321 (52.5%) | – |
| 24–27.9 | 8,664 (39.9%) | 4,958 (41.9%) | 2,813 (38.2%) | 893 (35.5%) | – |
| ≥28 | 2,215 (10.2%) | 1,349 (11.4%) | 665 (9.0%) | 201 (8.0%) | – |
| Serum creatinine (mmol/L) | 77.98±25.55 | 75.08±22.99 | 80.07±26.65 | 85.48±31.04 | <0.001 |
| HbA1c (%) | 7.29±1.72 | 7.30±1.69 | 7.30±1.74 | 7.22±1.76 | 0.077 |
| HbA1c (%, ≤7.0%) | 11,663 (53.7%) | 6,312 (53.3%) | 3,931 (53.3%) | 1,420 (56.5%) | 0.012 |
| Hypertension (yes) | 13,112 (60.4%) | 6,361 (53.7%) | 4,953 (67.2%) | 1,798 (71.5%) | <0.001 |
| Albuminuria (ACR ≥30 mg/g) | 7,637 (35.2%) | 3,742 (31.6%) | 2,874 (39.0%) | 1,021 (40.6%) | <0.001 |
| eGFRCG (mL/min/1.73 m2) | 70.20±18.18 | 78.67±16.05 | 63.18±14.45 | 50.92±13.26 | <0.001 |
| eGFRBIS1 (mL/min/1.73 m2) | 70.11±15.48 | 77.35±13.53 | 63.87±12.49 | 54.35±11.93 | <0.001 |
| eGFRmMDRD (mL/min/1.73 m2) | 91.61±23.04 | 96.50±21.88 | 87.78±22.54 | 79.82±23.38 | <0.001 |
| eGFRMDRD (mL/min/1.73 m2) | 83.86±20.73 | 88.46±19.79 | 80.29±20.09 | 72.69±20.63 | <0.001 |
| eGFRCKD-EPI (mL/min/1.73 m2) | 78.23±16.41 | 83.79±14.40 | 73.81±15.49 | 64.97±16.38 | <0.001 |
| eGFRCKD-EPI-Asia (mL/min/1.73 m2) | 82.36±17.35 | 88.23±15.25 | 77.70±16.37 | 68.35±17.29 | <0.001 |
| eGFRRuijin (mL/min/1.73 m2) | 76.91±15.29 | 80.97±14.25 | 73.67±14.68 | 67.32±15.28 | <0.001 |
Notes: Data are means ± SD or n (%). All percentages are column percentage.
Abbreviations: ACR, albumin to creatinine ratio; BIS1, Berlin Initiative Study 1 equation; BMI, body mass index; CG, Cockcroft–Gault equation; CKD-EPI, chronic kidney disease epidemiology collaboration; CKD-EPI-Asia, CKD-EPI equation in Asians; Chinese-Ruijin, Ruijin equation; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; MDRD, modification of diet in renal disease equation; mMDRD, MDRD modified for Chinese equation; T2DM, type 2 diabetes mellitus.
Mean eGFR differed according to the formula used and decreased with aging. The lowest levels were observed for GFR values that were estimated using the BIS1 equation (70.11±15.48), similar values were obtained using the CG equation (70.20±18.18), and the highest value for eGFR was obtained using the mMDRD equation (91.61±23.04). The CKD-EPI-Asia (68.35±17.29), CKD-EPI (64.97±16.38), and Ruijin (67.32±15.28) equations provided very similar estimations of eGFR for the ≥80-year-old group (Figure 1).
Figure 1.
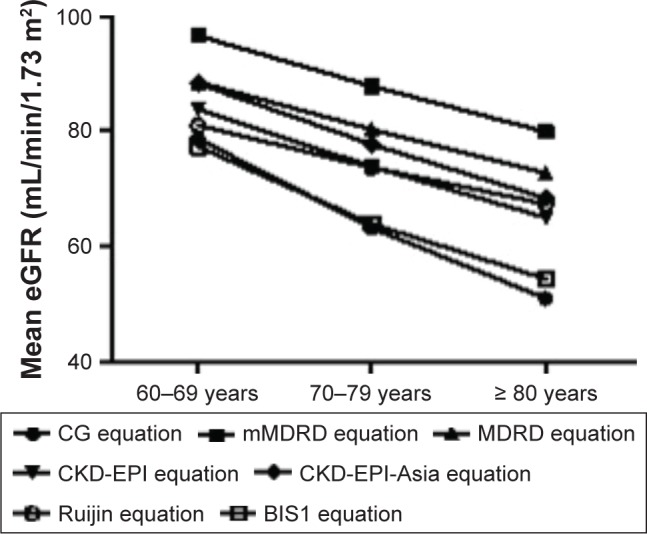
Estimated GFR of various equations by age groups.
Abbreviations: BIS1, Berlin initiative study 1 equation; CG, Cockcroft–Gault; CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease; mMDRD, MDRD modified for Chinese populations; Ruijin, Chinese Ruijin equation.
Subject characteristics according to the presence of reduced eGFR
Table 2 compares the differences in the demographics and clinical characteristics regarding decreases in renal function between the various GFR estimation equations. In the case of GFR values of <60 mL/min/1.73 m2, one can observe decreases in renal function of 28.9%, 39.1%, 11.8%, 8.4%, 14.3%, 11.5%, and 12.7% when estimated using the eGFRCG, eGFRBIS1, eGFRMDRD, eGFRmMDRD, eGFRCKD-EPI, eGFRCKD-EPI-Asia, and eGFRRuijin equations, respectively. Although different methods for calculating eGFR were used in this study, patients with reduced renal function were older and mostly female (except for values calculated using the eGFRBIS1 equation), their conditions had been diagnosed for longer times, and the patients showed higher proportions of hypertension and albuminuria. There were significantly lower values of waist circumference and BMI for patients with reduced renal function compared with those with GFR ≥60 mL/min/1.73 m2 according to the CG formula (P<0.001).
Table 2.
Demographic and clinical characteristics of subjects by reduced renal function according to different GFR estimated equations
| Variables | Reduced renal function (eGFR <60 mL/min/1.73 m2)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFR by adjusted CG
|
eGFR by BIS1
|
eGFR by mMDRD
|
eGFR by MDRD
|
eGFR by CKD-EPI
|
eGFR by EPI-Asian
|
eGFR by Ruijin
|
||||||||
| No N=15,445 (71.1) |
Yes N=6,278 (28.9) |
No N=13,228 (60.9) |
Yes N=8,495 (39.1) |
No N=19,907 (91.6) |
Yes N=1,816 (8.4) |
No N=19,165 (88.2) |
Yes N=2,558 (11.8) |
No N=18,613 (85.7) |
Yes N=3,110 (14.3) |
No N=19,220 (88.5) |
Yes N=2,503 (11.5) |
No N=18,954 (87.3) |
Yes N=2,769 (12.7) |
|
| Age (years) | 68.35±6.09 | 76.48±6.99*** | 67.96±6.08 | 74.97±7.12*** | 70.28±7.17 | 75.25±7.72*** | 70.11±7.10 | 75.09±7.68*** | 69.84±6.96 | 75.85±7.51*** | 70.00±7.04 | 76.10±7.46*** | 70.01±7.06 | 75.43±7.54*** |
| Female | 7,616 (49.3%) | 3,530 (56.2%)*** | 7,850 (59.3%) | 3,296 (38.8%)*** | 10,126 (50.9%) | 1,020 (56.2%)*** | 9,484 (49.5%) | 1,662 (65.0%)*** | 9,218 (49.5%) | 1,928 (62.0%)*** | 9,573 (49.8%) | 1,573 (62.8%)*** | 9,536 (50.3%) | 1,610 (58.1%)*** |
| Duration of T2DM (years) | 8.35±5.79 | 10.42±7.06*** | 8.37±5.79 | 9.85±6.81*** | 8.75±6.10 | 11.19±7.34*** | 8.69±6.05 | 10.91±7.32*** | 8.66±6.02 | 10.68±7.26*** | 8.68±6.05 | 11.06±7.33*** | 8.68±6.04 | 10.80±7.29 |
| BMI (kg/m2) | 24.59±2.95 | 23.25±2.85*** | 24.30±3.01 | 24.04±2.93*** | 24.22±2.98 | 24.03±3.00* | 24.21±2.97 | 24.12±3.05* | 24.22±2.97 | 24.10±3.02* | 24.22±2.97 | 24.08±3.04* | 24.22±2.97 | 24.10±3.03 |
| Waist circumference (cm) | 85.34±7.75 | 83.55±7.73*** | 84.53±7.66 | 85.28±7.97*** | 84.82±7.76 | 84.90±8.10 | 84.58±7.75 | 84.66±8.12 | 84.83±7.75 | 84.76±8.00 | 84.84±7.75 | 84.74±8.08 | 84.81±7.74 | 84.95±8.11 |
| Abdominal obese (yes) | 8,252 (72.7%) | 3,101 (27.3%)*** | 7,288 (55.1%) | 4,065 (47.9%)*** | 10,346 (91.1%) | 1,007 (8.9%)** | 9,839 (86.7%) | 1,514 (13.3%)*** | 9,545 (84.1%) | 1,808 (15.9%)*** | 9,891 (87.1%) | 1,462 (12.9%)*** | 9,779 (86.1%) | 1,574 (13.9%)*** |
| Fasting glucose (mmol/L) | 8.15±2.77 | 8.02±2.86** | 8.15±2.82 | 8.04±2.77** | 8.11±2.78 | 8.13±2.96 | 8.12±2.78 | 8.06±2.92 | 8.12±2.79 | 8.03±2.87 | 8.12±2.79 | 8.02±2.91 | 8.12±2.79 | 8.03±2.88 |
| HbA1c (%) | 7.31±1.70 | 7.23±1.75** | 7.32±1.72 | 7.24±1.71** | 7.28±1.72 | 7.34±1.71 | 7.29±1.72 | 7.29±1.68 | 7.29±1.72 | 7.29±1.68 | 7.29±1.72 | 7.29±1.67 | 7.29±1.72 | 7.29±1.68 |
| HbA1c ≤7.0 | 8,208 (53.1%) | 3,455 (55.0%)* | 7,075 (53.5%) | 4,588 (54.0%) | 10,722 (53.9%) | 941 (51.8%) | 10,307 (53.8%) | 1,356 (53.0%) | 10,002 (53.7%) | 1,661 (53.4%) | 10,337 (53.8%) | 1,326 (53.0%) | 10,188 (53.8%) | 1,475 (53.3%) |
| Scr (mmol/L) | 69.42±11.13 | 99.05±36.48*** | 66.33±8.53 | 96.11±31.88*** | 73.03±13.28 | 132.20±51.67*** | 72.30±12.90 | 120.52±47.42*** | 71.65±12.33 | 115.85±44.47*** | 72.35±12.91 | 121.23±47.69*** | 71.86±12.31 | 119.84±45.57*** |
| Hypertension (yes) | 8,883 (57.5%) | 4,229 (67.4%)*** | 7,517 (56.8%) | 5,595 (65.9%)*** | 11,817 (59.4%) | 1,295 (71.3%)*** | 11,313 (59.0%) | 1,799 (70.3%)*** | 10,927 (58.7%) | 2,185 (70.3%)*** | 11,323 (58.9%) | 1,789 (71.5%)*** | 11,156 (58.9%) | 1,956 (70.6%)*** |
| Albuminuria (yes) | 4,840 (31.3%) | 2,797 (44.6%)*** | 4,128 (31.2%) | 3,509 (41.3%)*** | 6,594 (33.1%) | 1,043 (57.4%)*** | 6,256 (32.6%) | 1,381 (54.0%)*** | 6,017 (32.3%) | 1,620 (52.1%)*** | 6,272 (32.6%) | 1,365 (54.5%)*** | 6,161 (32.5%) | 1,476 (53.3%)*** |
Notes: Subjects with missing data were excluded. Data are means ± SD or n (%).
Statistically significant difference of reduced renal function compared to those with eGFR ≥60 mL/min/1.73 m2 (P<0.05).
Statistically significant difference of reduced renal function compared to those with eGFR ≥60 mL/min/1.73 m2 (P<0.01).
Statistically significant difference of reduced renal function compared to those with with eGFR ≥60 mL/min/1.73 m2 (P<0.001).
Abbreviations: BIS1, Berlin initiative study 1 equation; BMI, body mass index; CG, Cockcroft–Gault equation; CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; EPI-Asian, CKD-EPI equation in Asians; HbA1c, hemoglobin A1c; MDRD, modification of diet in renal disease equation; mMDRD, MDRD modified for Chinese equation; Scr, serum creatinine; Ruijin, Chinese Ruijin equation; T2DM, type 2 diabetes mellitus.
Status of albuminuria and decreased eGFR according to the different equations
Albuminuria status and low eGFR (<60 mL/min/1.73 m2) as calculated by the different equations are shown in Table 3. The total rate of albuminuria based on urine ACR >30 mg/g was 35.2% (7,637), 31.0% (6,717) with moderately increased (ACR 30–300 mg/g) and 4.2% (920) with severely increased (ACR >300 mg/g) albuminuria. Most of the patients had albuminuria with eGFR ≥60 mL/min/1.73 m2 according to the various equations, ranging from 20.4% to 27.5%. It can be seen that the calculated proportions of patients with both albuminuria and reduced renal function were quite similar among the MDRD (6.4%), CKD-EPI (7.5%), CKD-EPI-Asia (6.3%), and Ruijin equations (6.8%) but were higher for the BIS1 (16.2%) and adjusted CG (12.9%) equations and lower for the mMDRD equation (4.8%). The concordance of albuminuria and decreased eGFR (<60 mL/min/1.73 m2) based on the different equations was poor according to both the Kappa (<0.2) and ICC (<0.4) statistics. The CKD-EPI (ICC =0.243, Kappa =0.123) and Ruijin equations (ICC =0.240, Kappa =0.119) had a similar concordance.
Table 3.
Concordance between albuminuria and declined renal function
| Reduced renal function
|
eGFR by adjusted CG (n, %)
|
eGFR by BIS1
|
eGFR by mMDRD
|
eGFR by MDRD
|
eGFR by CKD-EPI
|
eGFR by EPI -Asian
|
eGFR by Ruijin
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Albuminuria | No 15,445 (71.1%) |
Yes 6,278 (28.9%) |
No 13,228 (60.9) |
Yes 8,495 (39.1) |
No 19,907 (91.6%) | Yes 1,816 (8.4%) | No 19,165 (88.2%) | Yes 2,558 (11.8%) | No 18,613 (85.7%) |
Yes 3,110 (14.3%) |
No 19,221 (88.5%) |
Yes 2,502 (11.5%) |
No 18,954 (87.3%) | Yes 2,769 (12.7%) |
| Normal, N (%) | 10,605 (48.8%) |
3,481 (16.0%) |
9,100 (41.9%) |
4,986 (23.0%) |
13,313 (61.3%) |
773 (3.6%) | 12,909 (59.4%) |
1,177 (5.4%) | 12,596 (58.0%) |
1,490 (6.9%) |
12,948 (59.6%) |
1,138 (5.2%) |
12,793 (58.9%) |
1,293 (6.0%) |
| Microalbuminuria, N (%) | 4,423 (20.4%) |
2,294 (10.6%) |
3,783 (17.4%) |
2,934 (13.5%) |
5,969 (27.5%) |
748 (3.4%) | 5,675 (26.1%) |
1,042 (4.8%) | 5,459 (25.1%) |
1,258 (5.8%) |
5,678 (26.1%) |
1,039 (4.8%) |
5,587 (25.7%) |
1,130 (5.2%) |
| Macroalbuminuria, N (%) | 417 (1.9%) |
503 (2.3%) |
345 (1.6%) |
575 (2.6%) |
625 (2.9%) |
295 (1.4%) |
581 (2.7%) |
339 (1.6%) |
558 (2.6%) |
362 (1.7%) |
594 (2.7%) |
326 (1.5%) |
574 (2.6%) |
346 (1.6%) |
| Micro + macroalmuminuria, N (%) | 4,840 (22.3%) |
2,797 (12.9%) |
4,128 (19.0%) |
3,509 (16.2%) |
6,594 (30.4%) |
1,043 (4.8%) |
6,256 (28.8%) |
1,381 (6.4%) |
6,017 (27.7%) |
1,620 (7.5%) |
6,272 (28.8%) |
1,365 (6.3%) |
6,161 (28.3%) |
1,476 (6.8%) |
| Kappa (95% CI) |
0.124 (0.111–0.137) |
0.103 (0.089–0.116) |
0.099 (0.089–0.108) |
0.115 (0.103–0.127) |
0.123 (0.111–0.135) |
0.116 (0.104–0.128) |
0.119 (0.107–0.131) |
|||||||
| ICC (95% CI) |
0.223 (0.202–0.243) |
0.187 (0.165–0.208) |
0.218 (0.197–0.238) |
0.236 (0.215–0.256) |
0.243 (0.223–0.263) |
0.238 (0.218–0.258) |
0.240 (0.220–0.260) |
|||||||
Note: Microalbuminuria: ACR 30 to <300 mg/g, macroalbuminuria: ACR ≥300 mg/g, reduced renal function: eGFR <60 mL/min/1.73 m2.
Abbreviations: BIS1, Berlin initiative study 1 equation; 95% CI, 95% confidence interval; ACR, albumin to creatinine ratio; CG, Cockcroft–Gault equation; CKD-EPI, chronic kidney disease epidemiology collaboration; CKD-EPI-Asia, CKD-EPI equation in Asians; eGFR, estimated glomerular filtration rate; ICC, intraclass correlation coefficient; MDRD, modification of diet in renal disease equation; mMDRD, MDRD modified for Chinese equation; Ruijin, Chinese Ruijin equation.
Comparison of the proportions of diabetic kidney disease stages calculated using the different equations
Table 4 shows the calculated proportions of the various stages of CKD. The total proportion of CKD stages 1–5 was 51.2%, 58.7%, 40.6%, 38.7%, 42.0%, 40.4%, and 41.1% according to the eGFRCG, eGFRBIS1, eGFRMDRD, eGFRmMDRD, eGFRCKD-EPI, eGFRCKD-EPI-Asia, and eGFRRuijin equations, respectively. Significant differences in the proportion of stage 1 CKD were observed according to the various equations. The proportion of CKD stage 3a (eGFR 60–45 mL/min/1.73 m2) was 20.6% as calculated by the CG equation, 29.6% as calculated by the BIS1 equation, 5.7% as calculated by the mMDRD equation, 8.5% as calculated by the MDRD equation, 10.1% as calculated by the CKD-EPI equation, 8.0% as calculated by the CKD-EPI-Asia equation, and 10.0% as calculated by the Ruijin equation. The proportion of CKD stage (3b–5, eGFR <45 mL/min/1.73 m2) was 8.3%, 9.5%, 2.7%, 3.3%, 4.2%, 3.5%, and 2.7% as calculated by the eGFRCG, eGFRBIS1, eGFRmMDRD, eGFRMDRD, eGFRCKD-EPI, eGFRCKD-EPI-Asia, and eGFRRuijin equations, respectively. Similar proportions of stage 2 (22.9%, 20.0%) and stage 3a (10.1%, 10.0%) CKD were identified by the CKD-EPI and Ruijin equations (Table 4).
Table 4.
Proportions of stages of diabetic kidney disease according to the various estimating equations (n=21,723)
| eGFR in mL/min/1.73 m2 | CG | BIS1 | mMDRD | MDRD | CKD-EPI | CKD-EPI-Asia | Ruijin |
|---|---|---|---|---|---|---|---|
| ≥60 | 10,605 (48.8) | 9,100 (41.9) | 13,313 (61.3) | 12,909 (59.4) | 12,596 (58.0) | 12,948 (59.6) | 12,793 (58.9) |
| No kidney damage with normal or mildly decreased eGFR | |||||||
| ≥90 (stage 1) | 841 (3.9) | 149 (0.7) | 3,430 (15.8) | 2,380 (11.0) | 1,675 (7.7) | 2,569 (11.8) | 1,197 (5.5) |
| Kidney damage with normal eGFR | |||||||
| 60–89 (stage 2) | 3,999 (18.4) | 39,791 (8.3) | 3,164 (14.6) | 3,876 (17.8) | 4,342 (20.0) | 3,703 (17.0) | 4,964 (22.9) |
| Kidney damage with mildly decreased eGFR | |||||||
| 45–60 (stage 3a) | 4,478 (20.6) | 6,427 (29.6) | 1,228 (5.7) | 1,847 (8.5) | 2,194 (10.1) | 1,748 (8.0) | 2,173 (10.0) |
| Mild to moderately decreased eGFR | |||||||
| 30–45 (stage 3b) | 1,491 (6.9) | 1,801 (8.3) | 432 (2.0) | 550 (2.5) | 701 (3.2) | 572 (2.6) | 486 (2.2) |
| Moderate to severely decreased eGFR | |||||||
| 15–29 (stage 4) | 272 (1.3) | 248 (1.1) | 121 (0.5) | 126 (0.6) | 171 (0.8) | 142 (0.7) | 100 (0.5) |
| Severely decreased eGFR | |||||||
| <15 (stage 5) | 37 (0.2) | 19 (0.1) | 35 (0.2) | 35 (0.2) | 44 (0.2) | 41 (0.2) | 10 (0.0) |
| Kidney failure | |||||||
| <45 (stage 3b–5) | 1,800 (8.3) | 2,068 (9.5) | 588 (2.7) | 711 (3.3) | 916 (4.2) | 755 (3.5) | 596 (2.7) |
| Total CKD stage 1–5 | 11,118 (51.2) | 12,623 (58.1) | 8,410 (38.7) | 8,814 (40.6) | 9,127 (42.0) | 8,775 (40.4) | 8,930 (41.1) |
Note: Data presented as n (%).
Abbreviations: BIS1, Berlin initiative study 1 equation; CG, Cockcroft-Gault equation; CKD, chronic kidney disease; CKD-EPI, chronic kidney disease epidemiology collaboration; CKD-EPI-Asia, CKD-EPI equation in Asians; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease equation; mMDRD, MDRD modified for Chinese equation; Ruijin, Chinese Ruijin equation.
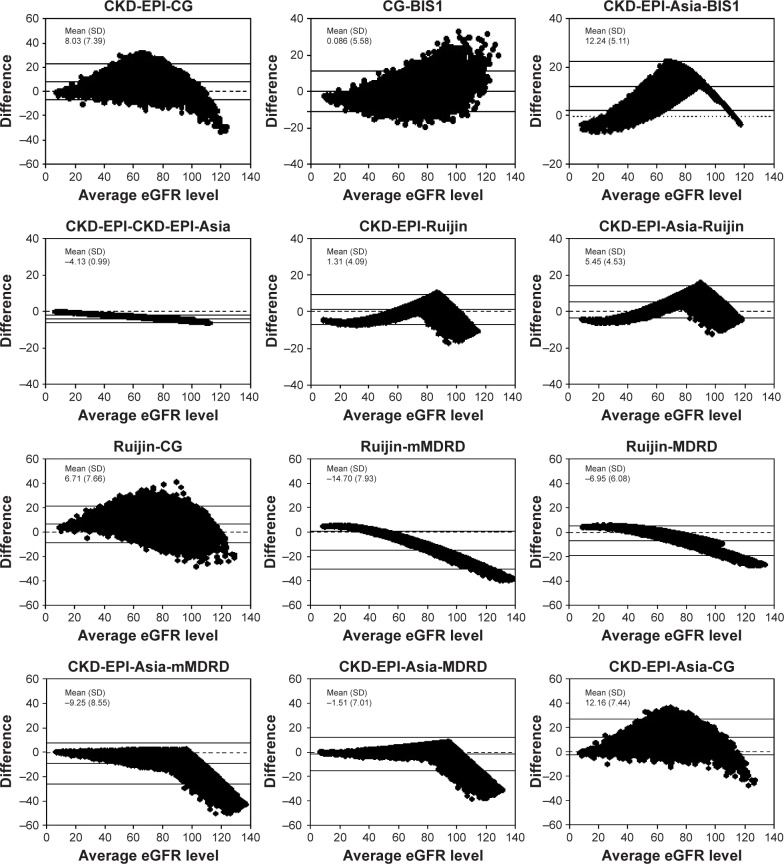
In general, Table 5 reveals good concordance among these creatinine-based equations in classifying CKD stages. High concordance was observed among the MDRD, CKD-EPI-Asia, CKD-EPI, and Ruijin equations. The lowest concordance was observed between the BIS1 and mMDRD equations. The CKD-EPI-Asia equation exhibited excellent concordance with the MDRD (ICC: 0.963), Ruijin (ICC: 0.956), CKD-EPI (ICC: 0.931), and mMDRD (ICC: 0.892) equations, and the Ruijin equation exhibited high concordance with the CKD-EPI equation (ICC: 0.950) (Table 5). In addition, the BIS1 equation exhibited good concordance with the modified CG equation (ICC: 0.809). These results are consistent with the results of the Bland–Altman plot shown in Figure 2. Increasing scatter of the differences with increasing values of eGFR was observed among the equations, especially with eGFR values of ≥90 mL/min/1.73 m2 (Figure 2).
Table 5.
Intraclass-correlation coefficients for 5 stages of diabetic kidney disease according to different equations
| eGFR (mL/min/1.73 m2) | CG | BIS1 | mMDRD | MDRD | CKD-EPI | CKD-EPI-Asia |
|---|---|---|---|---|---|---|
| B1S1 | 0.809 (0.804–0.814) | |||||
| mMDRD | 0.552 (0.540–0.564) | 0.462 (0.447–0.476) | ||||
| MDRD | 0.662 (0.653–0.671) | 0.562 (0.550–0.574) | 0.897 (0.894–0.900) | |||
| CKD-EPI | 0.736 (0.729–0.743) | 0.635 (0.626–0.645) | 0.823 (0.819–0.828) | 0.932 (0.930–0.934) | ||
| CKD-EPI-Asia | 0.667 (0.658–0.676) | 0.566 (0.555–0.578) | 0.892 (0.889–0.895) | 0.963 (0.962–0.964) | 0.931 (0.929–0.932) | |
| Ruijin | 0.688 (0.680–0.697) | 0.590 (0.579–0.601) | 0.868 (0.864–0.871) | 0.953 (0.952–0.954) | 0.950 (0.949–0.951) | 0.956 (0.955–0.957) |
Note: Data presented as intraclass-correlation coefficient (95% confidence interval).
Abbreviations: BIS1, Berlin initiative study 1 equation; CG, Cockcroft–Gault equation; CKD-EPI, chronic kidney disease epidemiology collaboration; CKD-EPI-Asia, CKD-EPI equation in Asians; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease equation; mMDRD, MDRD modified for Chinese equation; Ruijin, Chinese Ruijin equation.
Figure 2.
The Bland–Altman plots showing the comparisons between different eGFR equations.
Notes: The average eGFR level between the two methods in mL/min/1.73 m2 (x-axis) is plotted against their difference (y-axis). Mean and SD of the difference are reported to quantify the extent of the bias.
Abbreviations: BIS1, Berlin initiative study 1 equation; CG, Cockcroft–Gault; CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; MDRD, modification of diet in renal disease; mMDRD, MDRD modified for Chinese populations; Ruijin, Chinese Ruijin equation.
Discussion
This study included nearly all commonly used GFR estimating equations used for CKD in China. For the first time, high concordances were observed between the CKD-EPI-Asia equation and the MDRD, Ruijin, CKD-EPI, and mMDRD equations for the categorization of CKD stages after considering albuminuria in a large community sample of older diabetic patients. Good concordance was also observed between the BIS1 and modified CG equations.
This study found that the great majority of older diabetic patients (over 20.0% according to the various equations) had mild kidney damage (albuminuria with eGFR ≥60 mL/min/1.73 m2); this proportion was higher than that found in the UK study of general T2DM patients (~12.6%) and in the US study of patients ≥65 years of age (~14.7%) using the CKD-EPI equation.36,37 This result was consistent with our previous study, which found that ethnic Chinese people may be prone to albuminuric diabetic kidney disease.27 The Ruijin and CKD-EPI equations had similar predictive values (~10.0%) for the early stage of reduced renal function (CKD stage 3a).
The low concordance among all equations regarding the eGFR value of ≥90 mL/min/1.73 m2 was observed by both the CKD stage classification shown in Table 4 and the mean differences shown in Figure 2 (the Bland–Altman plot). This result is consistent with the report by the ADA consensus conference, which stated that the existing estimation equations had low precision at higher values of GFR.29,38 The question of whether the early course of diabetic kidney disease is associated with hyperfiltration in older patients with T2DM still needs further study.
In this large population of 21,723 older diabetic patients, it was not surprising that eGFR declined with age according to all these creatinine-based estimation equations. The decline found using the CG formula was much greater with advanced age. The finding that the CG formula identified a lower level of eGFR and a higher rate of CKD than the other equations was also reported by other studies.23,39,40 This discrepancy may due to structural differences between the equations. The CG equation was originally based on the urinary creatinine excretion of hospitalized Caucasian men aged 18–92 years and with normal renal function.41 The estimation of GFR by the CG formula is proportional to body weight or BMI, as was verified by our study and other studies.42,43 The high rate of reduced GFR was identified by the CG formula and the BIS1 equation, possibly due to a systematic bias in terms of an overdiagnosis of CKD due to the (on average) lower body weight and decreased muscle mass in older diabetic Chinese patients compared with Caucasians. The mMDRD and Ruijin equations were both adapted from the four-variable MDRD equation, and the gold standard was 99mTc-DTPA plasma clearance.7,25 The mMDRD equation predicted a higher level of GFR and a relatively low CKD rate compared to the other equations, possibly due to the different average reference GFR in the collected sample. In total, 454 patients were randomly selected from 684 patients and used for the training model, and the remaining 230 patients were used to test the performance of the modified MDRD Chinese equation. Only 37 (5.4%) of diabetic nephropathy was diagnosed in the entire sample. The reference GFR in mMDRD was 55.1±35.1 (median 49.9) mL/min/1.73 m2.7 For comparison, the Ruijin formula was based on 670 people randomly selected from 760 CKD patients, and the remaining 90 cases were used to test the modified equations. The reference GFR used in the Chinese Ruijin formula was (51.26±30.49) mL/min/1.73 m2 for males and (54.36±34.94) mL/min/1.73 m2 for females. Furthermore, 67 (8.8%) of diabetic patients were included in this study.25
The four-variable MDRD equation was originally developed based on data from a study entitled Modification of Diet in Renal Disease, which included 1,628 mostly white CKD patients. Among these patients, 1,070 were randomly selected as the training sample, and the remaining 558 were used for validation. Only 99 patients (6.1%) in the sample had diabetes. The gold standard used to develop the MDRD equation was 125I-iothalamate clearance. The mean GFR for the entire population was 39.8 mL/min/1.73 m2.5 The CKD-EPI equation used the same four variables adopted by the MDRD equation and was based on a cohort study that included 8,254 participants with and without CKD. Among the participants, 5,504 subjects were randomly selected for development, and the remaining subjects were used for validation. Patients with diabetes comprised almost 30% of the cohort. The mean GFR was 68 mL/min/1.73 m2.8 The CKD-EPI-Asia equation is based on the CKD-EPI data source, and the mean GFR was 57 mL/min/1.73 m2 in Asians. The equations were validated in different racial-ethnic groups (White and other, Black, Asian, Native American, and Hispanic groups), including studies conducted in China (N=675) and Japan (N=248). The overall number of patients with diabetes was 2,406 (29%).12 Therefore, there is no standardized protocol for measuring GFR, and the diversity of the mean level of GFR and diabetic status found using different equations will influence the accuracy of the equations in predicting CKD in Chinese diabetic populations. The different standard methods of estimating GFR may influence the concordance. The Jaffe kinetic method was used in this study for creatinine measurement, and this may differ from other methods. Clinicians must understand that for populations such as the elderly, the use of the CG and other equations to estimate kidney function may yield different results. CG tends to overestimate reduced kidney function, which may result in over-diagnosis and unnecessary disease-labeling, especially in elderly Chinese patients.
Approximately only 4.8%–12.9% of diabetic patients with both albuminuria and reduced renal function (eGFR <60 mL/min/1.73 m2) were identified by the equations examined in this study. The low concordance between albuminuria and reduced renal function indicated the importance of adding albuminuria into CKD staging systems based on eGFR, especially for diabetic patients. Albuminuria has been considered a marker of impaired endothelial function and underlying damage to glomerular podocytes and an early clinical indicator of diabetic kidney disease.44,45 For older patients, debate continues regarding the influence of age on GFR. With aging, a wide variability of progressive decreases in GFR and renal blood flow was found among individuals.46 A 3-year prospective study on the CKD stage progression of people aged ≥65 years revealed that older people with CKD exhibit a low progression of renal disease but are at higher risk for comorbidities related to CKD than for progression to ESRD.47 In addition, recent studies in both type 1 and 2 diabetes demonstrated that only some patients progress from microalbuminuria to macroalbuminuria and then further to ESRD.48–50 Bakris and Molitch suggested that only a subgroup of 25%–30% of diabetic patients with microalbuminuria will likely progress to more advanced stages of CKD. The presence of microalbuminuria alone is not predictive of CKD progression.51 Albuminuria may also be increased in some situations, including episodic hyperglycemia, high blood pressure, high protein diet, exercise, fever, urinary tract infection, and congestive heart failure.29 Thus, the measurement of kidney function is methodologically difficult due to the several different interlined functions of the kidney, which include the regulation of water and electrolyte levels, the excretion of waste products, acid–base homeostasis, and hormone secretion.52 The combined consideration and integrated management of malnutrition (undernutrition and overnutrition), hyperglycemia, hypoglycemia, hypertension, hyperuricemia,53 lipoprotein metabolism, systemic inflammation, anemia, and disordered mineral metabolism may alter the risks and benefit the progression of CKD and cardiovascular diseases in older diabetic patients.
In recent years, cystatin C has been proposed as a new endogenous marker of GFR, and several serum cystatin C-based equations have been developed and proposed for estimating the GFR as alternatives to Scr-based equations. Compared to creatinine-based measurement, cystatin C was considered to be less biased by age, gender, race and body weight.54 One proposed equation is the CKD-EPI creatinine and cystatin formula, which uses both Scr and serum cystatin levels for estimating kidney function.55,56 However, the high cost of the cystatin C assay and the lack of specificity for CKD have limited the use of cystatin C as the first-line measure of kidney function.57 To avoid misclassification and mistreatment, some studies have suggested using cystatin C as a supplement to Scr for estimating the risk of adverse outcomes and the diagnosis of mild-to-moderate decreases in GFR (eGFR: 45–59 mL/min/1.73 m2).58,59 The use of albuminuria combined with cystatin C may benefit the risk stratification and management of early stage diabetic kidney disease (GFR values between 60 and 90 mL/min/1.73 m2), where changes in Scr are not observed.
One of the strengths of the study was the large sample size from a community of elderly Chinese diabetic patients based on the EHR information system. This was the first time that the concordance of different creatinine-based GFR estimation equations was evaluated and analyzed in such a large sample of diabetic patients. Also, the selection bias may have been reduced by deleting the data for patients with highly abnormal Scr levels. The data were obtained from one southern district of Shanghai, and the results need to be verified in other districts or regions of China. In addition, prospective studies evaluating the ESRD, mortality, and cardiovascular disease outcomes based on different eGFR equations may provide more valid evidence for accurately identifying patients with early stage diabetic kidney diseases. The main limitation of the study was the unavailability of gold standard isotopic GFR measurements for comparison; therefore, the accuracy and precision of these formulae are not known. However, it has been suggested that the CKD-EPI equation predicts a reasonable distribution of eGFR values in healthy Chinese adult populations compared with the MDRD equation.12 Prospective studies are needed to verify these equations. Recently, a practical method of measuring GFR by iohexol clearance using dried capillary blood spots was developed and has been suggested to be a convenient method for accurately evaluating renal function.60,61 Improved methods and individualized considerations for measuring or estimating GFR will lead to a better ability to accurately identify early changes in GFR and to track GFR changes over time in patients with diabetes in clinical practice.
Conclusion
The CKD-EPI-Asia equation resulted in excellent concordance with the CKD-EPI, MDRD, mMDRD, and Ruijin equations for the classification of CKD stages after considering albuminuria in a large community sample of older diabetic patients, whereas the BIS1 equation exhibited good concordance with the CG equation. In addition, significant differences were observed for stage 1 CKD among the studied equations.
Low concordance between albuminuria and reduced renal function was observed for all creatinine-based equations. Accurate GFR estimates are difficult to obtain using creatinine-based equations in older diabetic patients. Kidney function is complex, and staff need to be aware of the individualized consideration and management of other risk factors or markers of reduced renal function in clinical practice.
Acknowledgments
The study was supported by postdoctoral program of Fudan university, grants from the Key Disease Project of Integrated Traditional and Western Medicine of Shanghai Municipality (zxbz2012-19, 2009MW02, 2009MHZ111), the Biobank of the Fifth People’s Hospital of Shanghai, Fudan University, and by the Natural Science Foundation of Minhang District (2012MHZ016). The funding agencies were not involved in the study design, interpretation of the data, or writing the paper. The authors thank the members of Shanghai Minhang CDC who helped with the data collection process and with the quality control of the data.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Liu ZH. Nephrology in china. Nat Rev Nephrol. 2013;9(9):523–528. doi: 10.1038/nrneph.2013.146. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Li W, Yang G, Liu Y, Li X. Diabetes and hypertension have become leading causes of CKD in Chinese elderly patients: a comparison between 1990–1991 and 2009–2010. Int Urol Nephrol. 2012;44(4):1269–1276. doi: 10.1007/s11255-012-0194-0. [DOI] [PubMed] [Google Scholar]
- 3.Lin J, Knight EL, Hogan ML, Singh AK. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol. 2003;14(10):2573–2580. doi: 10.1097/01.asn.0000088721.98173.4b. [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft DW, Gault M. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 5.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 6.Levey A, Greene T, Kusek J. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000 155A:Abstract. [Google Scholar]
- 7.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43(6):748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaman T, Filipowicz R, Beddhu S. Implications and importance of skeletal muscle mass in estimating glomerular filtration rate at dialysis initiation. J Ren Nutr. 2013;23(3):233–236. doi: 10.1053/j.jrn.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3(3):141–146. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 12.Stevens LA, Claybon MA, Schmid CH, et al. Evaluation of the chronic kidney disease epidemiology collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int. 2011;79(5):555–562. doi: 10.1038/ki.2010.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association Standards of medical care in diabetes–2015: summary of revisions. Diabetes Care. 2015;38(Suppl 1):S4. doi: 10.2337/dc15-S003. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Katzmarzyk PT, Horswell R, Zhao W, Johnson J, Hu G. Comparison of the heart failure risk stratification performance of the CKD-EPI equation and the MDRD equation for estimated glomerular filtration rate in patients with Type 2 diabetes. Diabet Med. 2016;33(5):609–620. doi: 10.1111/dme.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Targher G, Zoppini G, Mantovani W, et al. Comparison of two creatinine-based estimating equations in predicting all-cause and cardiovascular mortality in patients with type 2 diabetes. Diabetes Care. 2012;35(11):2347–2353. doi: 10.2337/dc12-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossing P, Rossing K, Gaede P, Pedersen O, Parving HH. Monitoring kidney function in type 2 diabetic patients with incipient and overt diabetic nephropathy. Diabetes Care. 2006;29(5):1024–1030. doi: 10.2337/diacare.2951024. [DOI] [PubMed] [Google Scholar]
- 18.Lee EY, Lee YM, Choi KH, Lee HC, Lee BW, Kim BS. Comparison of two creatinine-based equations for predicting decline in renal function in type 2 diabetic patients with nephropathy in a Korean population. Int J Endocrinol. 2013;2013:848963. doi: 10.1155/2013/848963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Maqbali SR, Mula-Abed WA. Comparison between three different equations for the estimation of glomerular filtration rate in omani patients with type 2 diabetes mellitus. Sultan Qaboos Univ Med J. 2014;14(2):e197–e203. [PMC free article] [PubMed] [Google Scholar]
- 20.Rognant N, Lemoine S, Laville M, Hadj-Aissa A, Dubourg L. Performance of the chronic kidney disease epidemiology collaboration equation to estimate glomerular filtration rate in diabetic patients. Diabetes Care. 2011;34(11):1320–1322. doi: 10.2337/dc11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Tan M, Chang H. Clinical application of glomerular filtration rate estimation equation in elderly patients with diabetes mellitus. Acta Universitatis Medicinalis Anhui. 2012;1:89–93. [Google Scholar]
- 22.Chang H, Ye S. Application of modified glomerular filtration rate estimation equations in chinese diabetic patients with chronic kidney diseases. West Indian Med J. 2015;64(3):209. doi: 10.7727/wimjopen.2014.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie YY, Zhou LP, Yu YR. A comparison of different estimation equations for glomerular filtration rate in Chinese diabetic patients. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45(4):685–690. [PubMed] [Google Scholar]
- 24.Sun K, Jing C, Liu Y, et al. Application of estimated glomerular filtration rate formulae for different stages of chronic kidney disease in diabetes. Chin J Dis Control Prev. 2015;3:313–315. [Google Scholar]
- 25.Shi H, Chen N, Zhang W, et al. Evaluating and refitting the simplified equation of MDRD to predict glomerular filtration rate in Chinese patients with chronic kidney disease. Chin J Pract Int Med. 2006;26(9):665–669. [Google Scholar]
- 26.Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med. 2012;157(7):471–481. doi: 10.7326/0003-4819-157-7-201210020-00003. [DOI] [PubMed] [Google Scholar]
- 27.Guo M, Niu JY, Li SR, et al. Gender differences in the association between hyperuricemia and diabetic kidney disease in community elderly patients. J Diabetes Complications. 2015;29(8):1042–1049. doi: 10.1016/j.jdiacomp.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Rossing P, Rossing K, Gaede P, Pedersen O, Parving HH. Monitoring kidney function in type 2 diabetic patients with incipient and overt diabetic nephropathy. Diabetes Care. 2006;29(5):1024–1030. doi: 10.2337/diacare.2951024. [DOI] [PubMed] [Google Scholar]
- 29.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37(10):2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 31.Chen C, Lu FC. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1–36. [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 33.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 34.Hallgren KA. Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol. 2012;8(1):23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 36.Hill CJ, Cardwell CR, Patterson CC, et al. Chronic kidney disease and diabetes in the national health service: a cross-sectional survey of the U.K. National diabetes audit. Diabet Med. 2014;3(4):448–454. doi: 10.1111/dme.12312. [DOI] [PubMed] [Google Scholar]
- 37.Bailey RA, Wang Y, Zhu V, Rupnow MF. Chronic kidney disease in US adults with type 2 diabetes: an updated national estimate of prevalence based on kidney disease: improving global outcomes (KDIGO) staging. BMC Res Notes. 2014;7:415. doi: 10.1186/1756-0500-7-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52(4):691–697. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 39.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–1009. doi: 10.2215/CJN.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurtal H, Schwenger V, Azzaro M, et al. Clinical value of automatic reporting of estimated glomerular filtration rate in geriatrics. Gerontology. 2009;55(3):288–295. doi: 10.1159/000172982. [DOI] [PubMed] [Google Scholar]
- 41.Gault MH, Longerich LL, Harnett JD, Wesolowski C. Predicting glomerular function from adjusted serum creatinine. Nephron. 1992;62:249–256. doi: 10.1159/000187054. [DOI] [PubMed] [Google Scholar]
- 42.Rigalleau V, Lasseur C, Perlemoine C, et al. Cockcroft-Gault formula is biased by body weight in diabetic patients with renal impairment. Metabolism. 2006;55(1):108–112. doi: 10.1016/j.metabol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 43.Rigalleau V, Lasseur C, Perlemoine C, et al. Estimation of glomerular filtration rate in diabetic subjects: cockcroft formula or modification of diet in renal disease study equation? Diabetes Care. 2005;28(4):838–843. doi: 10.2337/diacare.28.4.838. [DOI] [PubMed] [Google Scholar]
- 44.Siddiqi FS, Advani A. Endothelial-podocyte crosstalk: the missing link between endothelial dysfunction and albuminuria in diabetes. Diabetes. 2013;62(11):3647–3655. doi: 10.2337/db13-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakris GL, Molitch M. Microalbuminuria as a risk predictor in diabetes: the continuing saga. Diabetes Care. 2014;37(3):867–875. doi: 10.2337/dc13-1870. [DOI] [PubMed] [Google Scholar]
- 46.Weinstein JR, Anderson S. The aging kidney: physiological changes. Adv Chronic Kidney Dis. 2010;17(4):302–307. doi: 10.1053/j.ackd.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giannelli SV, Graf CE, Herrmann FR, et al. Natural history of older adults with impaired kidney function: the InCHIANTI study. Rejuvenation Res. 2011;14(5):513–523. doi: 10.1089/rej.2011.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Boer IH, Rue TC, Cleary PA, et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the diabetes control and complications trial/epidemiology of diabetes interventions and complications cohort. Arch Intern Med. 2011;171(5):412–420. doi: 10.1001/archinternmed.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molitch ME, Steffes M, Sun W, et al. Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care. 2010;33(7):1536–1543. doi: 10.2337/dc09-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64) Kidney Int. 2003;63(1):225–232. doi: 10.1046/j.1523-1755.2003.00712.x. [DOI] [PubMed] [Google Scholar]
- 51.Fox CS, Gona P, Larson MG, et al. A multi-marker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol. 2010;21(12):2143–2149. doi: 10.1681/ASN.2010010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sandilands EA, Dhaun N, Dear JW, Webb DJ. Measurement of renal function in patients with chronic kidney disease. Br J Clin Pharmacol. 2013;76(4):504–515. doi: 10.1111/bcp.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lytvyn Y, Perkins BA, Cherney DZ. Uric acid as a biomarker and a therapeutic target in diabetes. Can J Diabetes. 2015;39(3):239–246. doi: 10.1016/j.jcjd.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A. Cystatin C as a marker of GFR – history, indications, and future research. Clin Biochem. 2005;38(1):1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 55.Inker LA, Schmid CH, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye X, Liu X, Song D, et al. Estimating glomerular filtration rate (GFR) by serum creatinine or/and cystatin C equations: an analysis of multi-center Chinese subjects. Nephrology (Carlton) 2016;21(5):372–378. doi: 10.1111/nep.12636. [DOI] [PubMed] [Google Scholar]
- 57.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST. Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int. 2013;83(6):1169–1176. doi: 10.1038/ki.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. 2015;24(3):295–300. doi: 10.1097/MNH.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 59.Rule AD, Glassock RJ. GFR estimating equations: getting closer to the truth? Clin J Am Soc Nephrol. 2013;8(8):1414–1420. doi: 10.2215/CJN.01240213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mafham MM, Niculescu-Duvaz I, Barron J, et al. A practical method of measuring glomerular filtration rate by iohexol clearance using dried capillary blood spots. Nephron Clin Pract. 2007;106(3):c104–c112. doi: 10.1159/000102997. [DOI] [PubMed] [Google Scholar]
- 61.Maahs DM, Bushman L, Kerr B, et al. A practical method to measure GFR in people with type 1 diabetes. J Diabetes Complications. 2014;28(5):667–673. doi: 10.1016/j.jdiacomp.2014.06.001. [DOI] [PubMed] [Google Scholar]