Abstract
Staphylococcal species are a leading cause of community- and nosocomial-acquired infections, where the placement of foreign materials increases infection risk. Indwelling medical devices and prosthetic implants are targets for staphylococcal cell adherence and biofilm formation. Biofilm products actively suppress proinflammatory microbicidal responses, as evident by macrophage polarization toward an anti-inflammatory phenotype and the recruitment of myeloid-derived suppressor cells. With the rise in prosthetic hip and knee replacement procedures, together with the recalcitrance of biofilm infections to antibiotic therapy, it is imperative to better understand mechanisms of crosstalk between biofilm-associated bacteria and host immune cells. This review describes the current understanding of how staphylococcal biofilms evade immune-mediated clearance to establish persistent infections. The findings described herein may facilitate the identification of novel treatments for these devastating biofilm-mediated infections.
INTRODUCTION
Skin and nasal colonization with Staphylococcus epidermidis or S. aureus are known risk factors for invasive infections [1–3]. Prosthetic joint infections (PJIs) likely originate from colonization with small numbers of bacteria, which may provide a window of opportunity for survival if the pathogen does not elicit an initial vigorous immune response to mediate clearance. Staphylococcal invasion at the surgical site followed by adherence to the prosthesis frequently results in biofilm formation [4–6]; a community of bacterial cells encased within a self-produced matrix composed of proteins, polysaccharides and extracellular DNA [5,7]. The biofilm matrix supports the three-dimensional organization of bacteria while also acting as a barrier against host immune cell invasion. Biofilm development in vitro is a well-characterized process involving an array of proteins required for attachment, maturation, and dispersal [8,9]; however, less is understood about staphylococcal biofilm development in vivo. In addition to limited treatment options due to genetically-acquired antibiotic resistance, staphylococcal biofilm infections are characterized by inherent antibiotic tolerance due to their dampened metabolic state and decreased susceptibility to phagocytosis [10]. As such, biofilm-mediated PJIs often require surgical revision and replacement of a new prosthesis, which is associated with an increased frequency of infection recurrence and significant morbidity [11].
New approaches to prevent and treat biofilm-associated infections include anti-adhesive medical device coatings, therapies which disrupt the biofilm matrix, and antimicrobials targeting biofilm-specific bacterial processes [12]. Until recently, the immune response to staphylococcal biofilm has remained largely unexplored. This scope of this review includes recent advances in our understanding of the effects of staphylococcal biofilm on immune cell function, with particular consideration of macrophage dysfunction and preferential recruitment of myeloid-derived suppressor cells (MDSCs) [13–17]. In addition, staphylococcal biofilm products responsible for modifying immune cell function will be discussed. Findings from these studies may unveil a potential two-pronged strategy that limits bacterial biofilm development and coordinates a productive immune response to these infections. Once such strategies become available, there is promise that the rate of biofilm-mediated infections will be abated.
1. IMMUNE RESPONSE TO STAPHYLOCOCCAL BIOFILMS
With the rise in acquired antibiotic resistance by staphylococcal species leaving few treatment options, deciphering the innate immune response during device-associated biofilm infections could lead to novel therapeutic strategies. Staphylococcal biofilm infections have recently been described to induce an anti-inflammatory response, characterized by the recruitment of alternatively-activated macrophages and MDSC expansion. These two events, described in detail below, polarize the local environment toward an anti-inflammatory and pro-fibrotic milieu, thereby contributing to the chronic nature of biofilm infections. The findings described below raise the possibility of future approaches aimed at targeting immune cell activity to a mount a productive microbicidal response to clear PJIs.
Alternatively-activated macrophages
Considered among the first lines of innate immune cellular defense against bacterial infections, resident tissue macrophages can be classically-activated (pro-inflammatory) or alternatively-activated (anti-inflammatory) depending on the inflammatory milieu. We will not utilize the M1/M2 nomenclature in this review to describe macrophage activation states, since the original intent of the M1/M2 dichotomy was to describe macrophage responses to well-controlled in vitro conditions [18], which is clearly not the case in vivo. This is reflected by many reports demonstrating that macrophages can simultaneously possess gene expression profiles indicative of both M1 and M2 states [19,20]. Instead, we will refer to general functional attributes of macrophages in response to biofilm-associated bacteria, keeping in mind that responses are likely more complicated. Classically-activated macrophages are essential effectors during planktonic bacterial infection, in part, through their robust pro-inflammatory cytokine production, phagocytosis, and killing by generation of reactive oxygen and nitrogen species. The pro-inflammatory macrophage response is characterized by iNOS, TNF-α, IL-1β, and IFN-γ expression, which promote bacterial clearance, whereas anti-inflammatory mediators, such as arginase-1 (Arg-1), IL-4, and IL-10 attenuate macrophage microbicidal activity and promote a pro-fibrotic environment, facilitating bacterial persistence. Recent studies by our laboratory and others have shown that staphylococcal biofilms evade Toll-like receptor 2 (TLR2) and TLR9 recognition and skew host leukocytes toward an anti-inflammatory, pro-fibrotic response, evidenced by increased Arg-1 and decreased iNOS expression [21–24]. This alternatively-activated response induces robust fibrosis surrounding the biofilm, effectively preventing macrophage invasion and phagocytosis of biofilm-associated bacteria, favoring biofilm persistence [15,25,26]. Although proinflammatory cytokine production is detected during S. aureus biofilm infections, this response is clearly not sufficient to mitigate biofilm growth or survival [13–15,27]. This suggests a primary defect in the phagocytes that normally clear bacteria, which is supported by the preferential recruitment of MDSCs into staphylococcal biofilms that possess anti-inflammatory properties by preventing macrophage proinflammatory activity and T cell activation.
To circumnavigate the anti-inflammatory milieu induced by S. aureus biofilms, an activated macrophage adoptive transfer strategy was employed by Hanke et al. in a catheter-associated model of S. aureus biofilm infection [25]. Since earlier work revealed the inability of resident tissue macrophages to invade the biofilm proper, this approach addressed whether the direct injection of proinflammatory activated macrophages (IFN-γ + peptidoglycan) into biofilms would transform the inflammatory milieu to facilitate biofilm clearance. Injection of activated macrophages during early biofilm formation augmented proinflammatory cytokine production, reduced macrophage Arg-1 expression, and limited S. aureus biofilm development. Proinflammatory macrophage polarization was also shown to increase phagocytosis and killing of S. aureus biofilms in vitro. Furthermore, treatment of mice with the C5a receptor (CD88) agonist EP67, which invokes macrophage proinflammatory activity, augmented macrophage infiltration and proinflammatory mediator expression in biofilm-infected tissues, which translated into reduced S. aureus biofilm growth [25]. Taken together, these findings demonstrate that S. aureus biofilms interfere with antibacterial effector mechanisms of resident tissue macrophages, which is an essential step for biofilm development. However, if proinflammatory macrophages gain access to sites of S. aureus biofilm (i.e. by direct inoculation), they are capable of exerting antibacterial activity that manifests as improved biofilm clearance. This supports an important role for S. aureus in thwarting early macrophage activation to establish chronic S. aureus biofilm infections.
Myeloid-Derived Suppressor Cells (MDSCs)
Suppression of proinflammatory responses by MDSCs have been demonstrated in a number of cancer and bacterial infection models [28–31]. MDSCs are a heterogeneous population of immature monocytes and granulocytes and are functionally characterized by their ability to suppress T cell activation in an antigen-dependent or -independent manner (reviewed in [32]). Depending on the local tissue microenvironment, infiltrating MDSCs can either maintain their suppressive properties or differentiate into mature neutrophils, macrophages, or dendritic cells. Our laboratory was the first to report MDSC recruitment (CD11b+Ly-6G+Ly-6Chigh) in a mouse model of S. aureus PJI, which was confirmed by the identification of MDSC-like cell populations in human PJIs, including those caused by S. epidermidis [13,15]. Subsequently, other groups have reported MDSC recruitment together with immunosuppressive activity in mouse models of S. aureus cutaneous infection [33] and to the kidneys following S. aureus sepsis [17].
In assessing the functional role of MDSCs in establishing the suppressive environment surrounding S. aureus biofilms, an antibody-mediated depletion strategy was used to target Ly-6G+ cells. Any responses would be attributed MDSC activity, since although Ly6G is also expressed on neutrophils, our prior work has shown that few neutrophils are recruited to S. aureus biofilms. Importantly, Ly6G treatment would leave CD11b+Ly-6Chigh Ly-6G− monocytes intact, which was predicted to enhance monocyte/macrophage proinflammatory and bactericidal activity in the context of reduced MDSC inhibitory signals. This was indeed the case, where Ly6G-depleted animals displayed significantly increased proinflammatory mediator production (i.e. IL-1β, G-CSF) that translated into reduced S. aureus burdens in a mouse PJI model [13]. To directly demonstrate enhanced monocyte/macrophage microbicidal activity in the context of MDSC depletion, mice were treated with Gr-1 antibody, which targets both Ly-6G+Ly-6Chigh MDSCs and Ly-6G−Ly-6Chigh monocytes. This approach resulted in significantly higher S. aureus biofilm burdens, which was attributed to the fact that although MDSC inhibition was removed, effector phagocytes (i.e. monocytes/macrophages) were also reduced, leading to unchecked biofilm expansion [13]. These data demonstrate that MDSCs actively suppress monocyte/macrophage proinflammatory activity, which is important for biofilm persistence. Interestingly, Gr-1+ depletion in a S. aureus sepsis model had no effect on bacterial burdens [17]; however, this is not a biofilm infection and mature macrophages are not present in the bloodstream, and as such, the modes of bacterial clearance differ.
A role for both IL-12 and IL-10 signaling has been reported to shape the anti-inflammatory biofilm milieu by promoting MDSC recruitment; however, the kinetics of cytokine involvement differ [14,15]. Namely, IL-12 deficiency leads to an early reduction in MDSCs, whereas IL-10 affects later MDSC recruitment into S. aureus biofilms (Figure 1). As a result of reduced MDSC infiltrates, monocyte influx was increased in both IL-12 and IL-10 knockout (KO) mice and these monocytes displayed increased proinflammatory activity that translated into decreased S. aureus biofilm burdens. Importantly, adoptive transfer of wild-type MDSCs into IL-12 and IL-10 KO animals returned biofilm growth to levels seen in wild-type animals [14,15]. Adoptive transfer of MDSCs also exacerbated infection outcome in a mouse model of S. aureus sepsis, again supporting the functional role of MDSCs in promoting bacterial expansion [33].
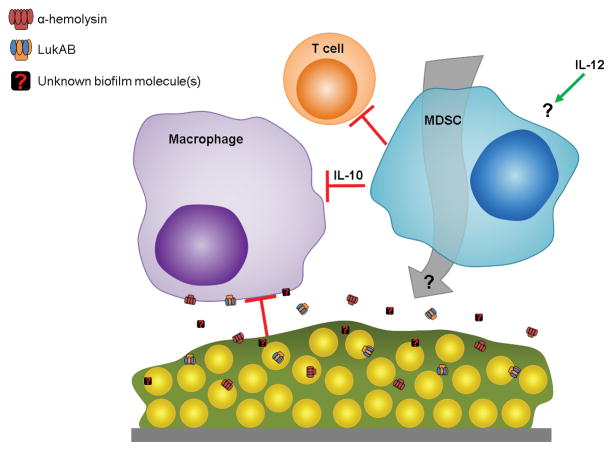
Figure 1. Model for S. aureus biofilm immune evasion.
Toxins secreted from S. aureus biofilms, including α-toxin (Hla) and LukAB, inhibit macrophage microbicidal function and induce cell death. Additional unidentified molecules released from staphylococcal biofilms, either via active secretion or following bacterial cell lysis, likely contribute to maximize inhibition of host antimicrobial activity. MDSC recruitment by a yet unknown mechanism(s) that requires IL-12 suppresses T cell activity and induces a local anti-inflammatory milieu characterized by IL-10 production. Biofilm-mediated immune polarization results in biofilm persistence and chronic disease.
T cells
The role of T cells in mediating staphylococcal biofilm PJIs remains unclear. Our laboratory has found minimal T cell infiltrates in a mouse S. aureus PJI model [14,15] and T cells were undetectable in human PJIs compared to tissues recovered from aseptic orthopedic revisions, where a prominent T cell population was observed [15]. The relative paucity of T cells in both human PJIs and our mouse PJI model could be explained by the robust MDSC infiltrate associated with these infections based on their ability to inhibit T cell proliferation/activation [13]. In contrast to our studies, other work has reported a role for Th1 and Th17 cells in response to S. aureus biofilms [34]. These discrepancies may be explained by the distinct model systems employed; namely the use of a tibial implant pre-coated in vitro with an established biofilm compared to our approach where a sterile orthopedic implant is infected in vivo using a 200-fold lower bacterial dose. Indeed, we recently reported that increasing the infectious inoculum from 103 to 105 CFU in the mouse PJI model altered leukocyte recruitment and inflammatory mediator production as well as biofilm growth/clearance [27]. This highlights the need to carefully consider infectious doses when examining inflammatory attributes of biofilm infection, in particular to their relevance in terms of modeling events that might occur during native PJI in humans.
Part 1 Conclusions
In contrast to planktonic bacteria, the immune response to staphylococcal biofilms is suppressive and polarizes macrophages toward an anti-inflammatory state. This is due, in part, to MDSC recruitment that attenuates monocyte/macrophage activation. Importantly, similar leukocyte infiltration patterns are observed between the mouse PJI model and human PJI tissues, including increased MDSC-like cells and few T cells. Limiting MDSC influx and/or their immunosuppressive action may offer a new therapeutic strategy to thwart chronic staphylococcal biofilm infections.
While biofilm invasion and phagocytosis by neutrophils appear to be less affected than macrophages [21,35,36], studies have suggested that neutrophils recovered from human implant/endoprosthesis infections are less phagocytic and contribute to infection persistence [37]. This may result from inhibition of opsonophagocytic killing of S. epidermidis biofilms compared to planktonic cells [38]. In the S. epidermidis biofilm matrix, polysaccharide intracellular adhesion (PIA) has been shown to play an important role in preventing macrophage [23] and neutrophil phagocytosis [39]. Another model of S. epidermidis biofilm formation on peritoneal dialysis catheters reported defective macrophage function that was linked to reduced IFN-γ production 40]. With regard to S. aureus, it is interesting that the biofilm transcriptome remained relatively unaffected in the face of neutrophil challenge, whereas a large percentage of genes (~ 95%) were significantly downregulated upon macrophage exposure [36]. These experiments demonstrate that S. aureus biofilms differentially modify their gene expression patterns depending on the leukocyte subset encountered. The finding that biofilms were more responsive following macrophage addition rather than neutrophils is in agreement with the preferential recruitment of macrophages but minimal neutrophils during biofilm formation [13–16,21,25–27]. While the anti-inflammatory response appears to be driven, in part, by biofilm products, the specific effectors and their mechanism(s) of action have yet to be fully elucidated.
2. BIOFILM EFFECTORS OF IMMUNE CELL DYSFUNCTION
In general, PJIs associated with more virulent organisms, such as S. aureus, often present within the first 3 months after surgery, whereas complications triggered by less virulent organisms, such as S. epidermidis, can manifest as chronic infections months or years post-surgery [11,41]. This dichotomy may reflect a passive defense strategy in S. epidermidis rather than the broad arsenal of toxins and other virulence determinants that S. aureus possesses [42]. Interestingly, as mentioned above, co-culture of macrophages with S. aureus biofilm resulted in a global suppression of S. aureus transcription, whereas only a few genes were up-regulated [36]. This suggests that S. aureus may “hide” from the immune system by globally repressing gene expression [43]; however it is clear that proteins and other molecules released from S. aureus biofilm also effect macrophage function [16]. Identification of candidate molecules responsible for inhibiting macrophage proinflammatory properties represents an essential step in understanding host-pathogen interactions during PJI. The second part of this review focuses on secreted factors that induce immune cell dysfunction, with a particular focus on macrophages.
Toxins
S. aureus produces a wide array of cell wall-associated and secreted virulence factors that interfere with antimicrobial effectors of the immune system [44,45]. Two well-studied toxins include α-toxin (Hla), which acts on red blood cells and leukocytes by binding to ADAM10 [46,47] and the bi-component leukocidins LukAB [48], LukED [49], and Panton-Valentine leukocidin (PVL, consisting of LukFS) [50], which bind to specific leukocyte surface receptors. S. aureus also produces enterotoxins, such as toxic shock syndrome toxin (TSST); however, they will not be discussed in this review. As mentioned above, S. epidermidis does not produce many toxins [51], but rather relies on a thick biofilm matrix and extensive polysaccharide network for immune evasion. For this reason, the remainder of this section will focus on S. aureus biofilm components and their effects on antimicrobial immune mechanisms.
While biofilm size and matrix density represent physical obstacles for macrophage invasion, our laboratory recently demonstrated macrophage phagocytosis was significantly attenuated following exposure to S. aureus biofilm-conditioned medium [16]. Restoration of phagocytosis was achieved following the treatment of biofilm supernatant with proteinase K, indicating that a secreted proteinaceous factor(s) actively inhibits macrophage phagocytosis of S. aureus biofilm. Mutation in the accessory gene regulator (agr) locus also alleviated the macrophage phagocytic block, suggesting that the putative proteins were regulated in an agr-dependent manner [16]. Subsequent experiments demonstrated that LukAB and α-toxin act in a synergistic manner to prevent macrophage phagocytosis and are also important for inhibiting macrophage invasion in vivo in a mouse PJI model, which translates into biofilm persistence [16]. However, it is clear that additional extracellular factors released from S. aureus biofilms play a role in thwarting macrophage activation, since the phagocytic block was not completely reversed following simultaneous inhibition of α-toxin/LukAB activity [16]. This is not unexpected, given the extensive arsenal of virulence determinants expressed by S. aureus, which may represent viable therapeutic targets to augment macrophage microbicidal activity.
Intracellular components
Cell death and lysis is an essential mechanism in staphylococcal biofilm development, as demonstrated by mutation in genes responsible for autolysis [52,53] or treatment with an inhibitor of autolysis, polyanethol sulfonate (PAS) [54,55]. Intracellular molecules, such as DNA, are important components of the biofilm matrix [5], and while this represents a potential pathogen-associated molecular pattern (PAMP) to trigger proinflammatory activity via TLR9, we have previously shown that S. aureus biofilms evade TLR9-mediated recognition of bacterial DNA [21]. The potential of secreted molecules from S. aureus biofilm to induce macrophage dysfunction was recently addressed by Scherr et al [16]. Namely, treatment of macrophages with conditioned medium from mature S. aureus biofilms prevented macrophage phagocytosis [21]. S. aureus biofilms treated with lysostaphin were no longer able to inhibit macrophage phagocytosis, suggesting that the bioactive molecule(s) was not released following cell lysis. This was confirmed by the fact that conditioned medium from PAS-treated biofilms was still capable of blocking macrophage phagocytosis and was proteinase K sensitive, indicating that secreted proteins are responsible for the observed macrophage dysfunction [16]. However, unpublished observations from our laboratory also suggest that small molecules released via autolysis can escape the biofilm matrix and effect immune cell activity, since conditioned medium from PAS-treated biofilms can also alter macrophage gene expression profiles. Together, these findings suggest that large bacterial PAMPs capable of eliciting robust proinflammatory activity (i.e. eDNA released during autolysis) are buried within the biofilm matrix and are inaccessible to macrophages based on their inability to invade the biofilm, whereas smaller molecules diffuse from the biofilm and, as such, are capable of interacting with proximal immune cells to trigger a non-productive immune response typified by phagocytic impairments and cell death. Therefore, therapeutics targeting biofilm matrix disruption hold interesting potential for augmenting leukocyte microbicidal activity and biofilm clearance.
Part 2 Conclusions
S. aureus biofilms interfere with microbicidal immune responses, in part, by polarizing macrophages toward an anti-inflammatory, pro-fibrotic phenotype. While gene expression profiles in S. aureus biofilms are transiently, but globally repressed following macrophage exposure [36], secreted proteins enriched during S. aureus biofilm growtn, including α-toxin and LukAB, are capable of inducing macrophage dysfunction [16]. Other potential proteins or molecules responsible for inhibiting macrophage anti-biofilm activity remain to be identified. Additionally, further investigation into the role of biofilm molecules that are actively secreted or released following bacterial lysis that promote MDSC recruitment/activity will shed light on critical biofilm processes that contribute to the establishment of chronic PJIs.
FINAL REMARKS
The work discussed in this review details recent advances in the molecular crosstalk between staphylococcal biofilms and host immune cells (Figure 1). Microbial-immune cell interactions have proven to be important factors during biofilm infections, and these studies help advance the knowledge of how bacteria manipulate host immune responses. Staphylococcal biofilms have evolved effective mechanisms to establish chronic infections, in part, by actively preventing macrophage phagocytosis and proinflammatory activity. Evidence suggests that this is due to the action of MDSCs recruited to the site of staphylococcal PJI, which inhibit monocyte/macrophage proinflammatory action and biofilm clearance. The role of MDSCs on mediating T cell suppression during biofilm formation remains unclear; however, they may play a significant role in the failure to induce protective adaptive immunity during staphylococcal PJIs. Preventing the suppressive activity of infiltrating MDSCs may prove to be a novel therapeutic strategy to thwart PJIs; however it remains unclear whether biofilm products directly contribute to MDSC accumulation by arresting their maturation or if staphylococcal biofilms cooperate with MDSCs to inhibit immune effector function. These questions represent areas of active investigation in our laboratory.
Acknowledgments
This work was supported by the National Institutes of Health P01 AI083211 (Project 4 to T.K.).
References
- 1.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rupp ME, Archer GL. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–243. doi: 10.1093/clinids/19.2.231. quiz 244–235. [DOI] [PubMed] [Google Scholar]
- 3.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74:470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moormeier DE, Bose JL, Horswill AR, Bayles KW. Temporal and stochastic control of Staphylococcus aureus biofilm development. MBio. 2014;5:e01341–01314. doi: 10.1128/mBio.01341-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttner H, Mack D, Rohde H. Structural basis of Staphylococcus epidermidis biofilm formation: mechanisms and molecular interactions. Front Cell Infect Microbiol. 2015;5:14. doi: 10.3389/fcimb.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 11.Del Pozo JL, Patel R. Clinical practice. Infection associated with prosthetic joints. N Engl J Med. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Yu Q, Sun H. Novel strategies for the prevention and treatment of biofilm related infections. Int J Mol Sci. 2013;14:18488–18501. doi: 10.3390/ijms140918488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, et al. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol. 2014;192:3778–3792. doi: 10.4049/jimmunol.1303408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heim CE, Vidlak D, Kielian T. Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J Leukoc Biol. 2015;98:1003–1013. doi: 10.1189/jlb.4VMA0315-125RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heim CE, Vidlak D, Scherr TD, Hartman CW, Garvin KL, et al. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J Immunol. 2015;194:3861–3872. doi: 10.4049/jimmunol.1402689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherr TD, Hanke ML, Huang O, James DB, Horswill AR, et al. Staphylococcus aureus Biofilms Induce Macrophage Dysfunction Through Leukocidin AB and Alpha-Toxin. MBio. 2015:6. doi: 10.1128/mBio.01021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tebartz C, Horst SA, Sparwasser T, Huehn J, Beineke A, et al. A major role for myeloid-derived suppressor cells and a minor role for regulatory T cells in immunosuppression during Staphylococcus aureus infection. J Immunol. 2015;194:1100–1111. doi: 10.4049/jimmunol.1400196. [DOI] [PubMed] [Google Scholar]
- 18.Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. [Google Scholar]
- 19.Cheatle J, Aldrich A, Thorell WE, Boska MD, Kielian T. Compartmentalization of immune responses during Staphylococcus aureus cranial bone flap infection. Am J Pathol. 2013;183:450–458. doi: 10.1016/j.ajpath.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol. 2011;186:6585–6596. doi: 10.4049/jimmunol.1002794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snowden JN, Beaver M, Beenken K, Smeltzer M, Horswill AR, et al. Staphylococcus aureus sarA regulates inflammation and colonization during central nervous system biofilm formation. PLoS One. 2013;8:e84089. doi: 10.1371/journal.pone.0084089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schommer NN, Christner M, Hentschke M, Ruckdeschel K, Aepfelbacher M, et al. Staphylococcus epidermidis uses distinct mechanisms of biofilm formation to interfere with phagocytosis and activation of mouse macrophage-like cells 774A.1. Infect Immun. 2011;79:2267–2276. doi: 10.1128/IAI.01142-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiliopoulou AI, Kolonitsiou F, Krevvata MI, Leontsinidis M, Wilkinson TS, et al. Bacterial adhesion, intracellular survival and cytokine induction upon stimulation of mononuclear cells with planktonic or biofilm phase Staphylococcus epidermidis. FEMS Microbiol Lett. 2012;330:56–65. doi: 10.1111/j.1574-6968.2012.02533.x. [DOI] [PubMed] [Google Scholar]
- 25.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T. Targeting macrophage activation for the prevention and treatment of Staphylococcus aureus biofilm infections. J Immunol. 2013;190:2159–2168. doi: 10.4049/jimmunol.1202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanke ML, Angle A, Kielian T. MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection. PLoS One. 2012;7:e42476. doi: 10.1371/journal.pone.0042476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidlak D, Kielian T. Infectious dose dictates the host response during S. aureus orthopedic biofilm infection. Infect Immun. 2016 doi: 10.1128/IAI.00117-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 29.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 31.Rieber N, Brand A, Hector A, Graepler-Mainka U, Ost M, et al. Flagellin induces myeloid-derived suppressor cells: implications for Pseudomonas aeruginosa infection in cystic fibrosis lung disease. J Immunol. 2013;190:1276–1284. doi: 10.4049/jimmunol.1202144. [DOI] [PubMed] [Google Scholar]
- 32.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skabytska Y, Wolbing F, Gunther C, Koberle M, Kaesler S, et al. Cutaneous innate immune sensing of Toll-like receptor 2–6 ligands suppresses T cell immunity by inducing myeloid-derived suppressor cells. Immunity. 2014;41:762–775. doi: 10.1016/j.immuni.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Prabhakara R, Harro JM, Leid JG, Harris M, Shirtliff ME. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect Immun. 2011;79:1789–1796. doi: 10.1128/IAI.01386-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leid JG, Shirtliff ME, Costerton JW, Stoodley P. Human leukocytes adhere to, penetrate, and respond to Staphylococcus aureus biofilms. Infect Immun. 2002;70:6339–6345. doi: 10.1128/IAI.70.11.6339-6345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherr TD, Roux CM, Hanke ML, Angle A, Dunman PM, et al. Global transcriptome analysis of Staphylococcus aureus biofilms in response to innate immune cells. Infect Immun. 2013;81:4363–4376. doi: 10.1128/IAI.00819-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner C, Kondella K, Bernschneider T, Heppert V, Wentzensen A, et al. Post-traumatic osteomyelitis: analysis of inflammatory cells recruited into the site of infection. Shock. 2003;20:503–510. doi: 10.1097/01.shk.0000093542.78705.e3. [DOI] [PubMed] [Google Scholar]
- 38.Cerca N, Jefferson KK, Oliveira R, Pier GB, Azeredo J. Comparative antibody-mediated phagocytosis of Staphylococcus epidermidis cells grown in a biofilm or in the planktonic state. Infect Immun. 2006;74:4849–4855. doi: 10.1128/IAI.00230-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, et al. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol. 2004;6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 40.Dasgupta MK. Biofilm causes decreased production of interferon-gamma. J Am Soc Nephrol. 1996;7:877–882. doi: 10.1681/ASN.V76877. [DOI] [PubMed] [Google Scholar]
- 41.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014;27:302–345. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheung GY, Rigby K, Wang R, Queck SY, Braughton KR, et al. Staphylococcus epidermidis strategies to avoid killing by human neutrophils. PLoS Pathog. 2010;6:e1001133. doi: 10.1371/journal.ppat.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scherr TD, Heim CE, Morrison JM, Kielian T. Hiding in Plain Sight: Interplay between Staphylococcal Biofilms and Host Immunity. Front Immunol. 2014;5:37. doi: 10.3389/fimmu.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otto M. Staphylococcus aureus toxins. Curr Opin Microbiol. 2014;17:32–37. doi: 10.1016/j.mib.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins (Basel) 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, et al. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat Med. 2011;17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DuMont AL, Yoong P, Day CJ, Alonzo F, 3rd, McDonald WH, et al. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A. 2013;110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alonzo F, 3rd, Kozhaya L, Rawlings SA, Reyes-Robles T, DuMont AL, et al. CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature. 2013;493:51–55. doi: 10.1038/nature11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spaan AN, Henry T, van Rooijen WJ, Perret M, Badiou C, et al. The staphylococcal toxin Panton-Valentine Leukocidin targets human C5a receptors. Cell Host Microbe. 2013;13:584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Vuong C, Otto M. Staphylococcus epidermidis infections. Microbes Infect. 2002;4:481–489. doi: 10.1016/s1286-4579(02)01563-0. [DOI] [PubMed] [Google Scholar]
- 52.Bose JL, Lehman MK, Fey PD, Bayles KW. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One. 2012;7:e42244. doi: 10.1371/journal.pone.0042244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houston P, Rowe SE, Pozzi C, Waters EM, O’Gara JP. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect Immun. 2011;79:1153–1165. doi: 10.1128/IAI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, et al. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2007;104:8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, et al. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One. 2009;4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]